Abstract
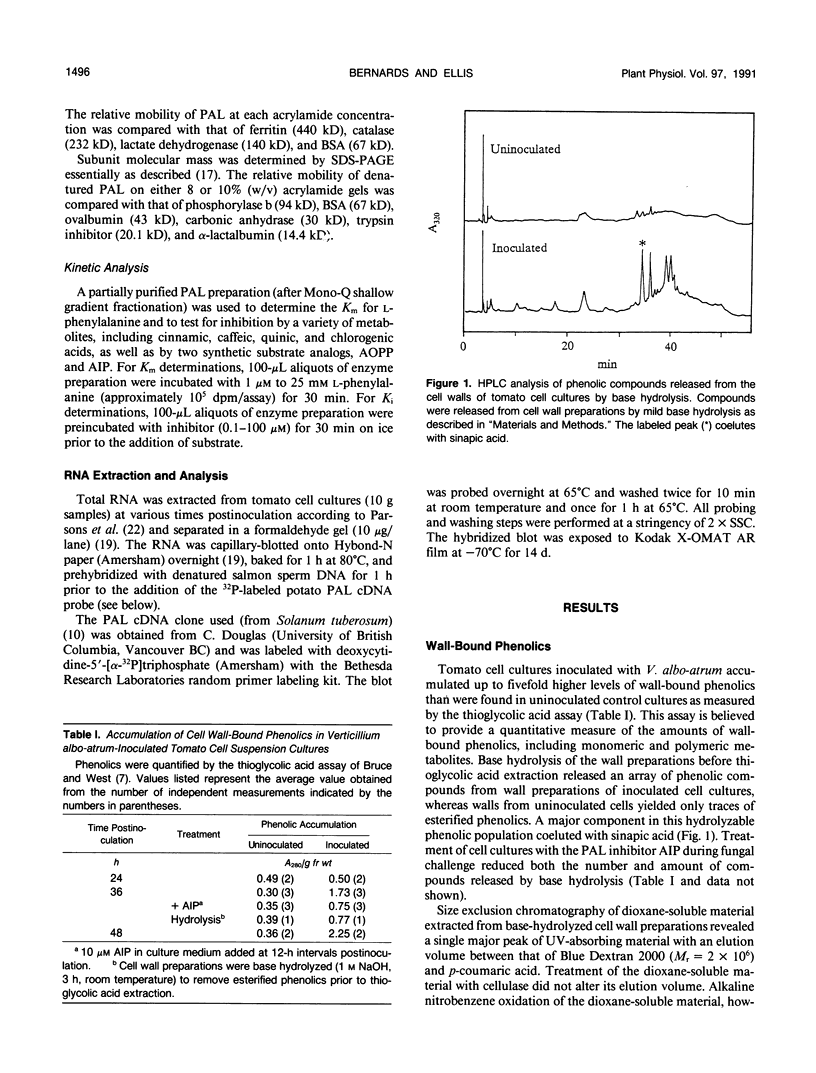
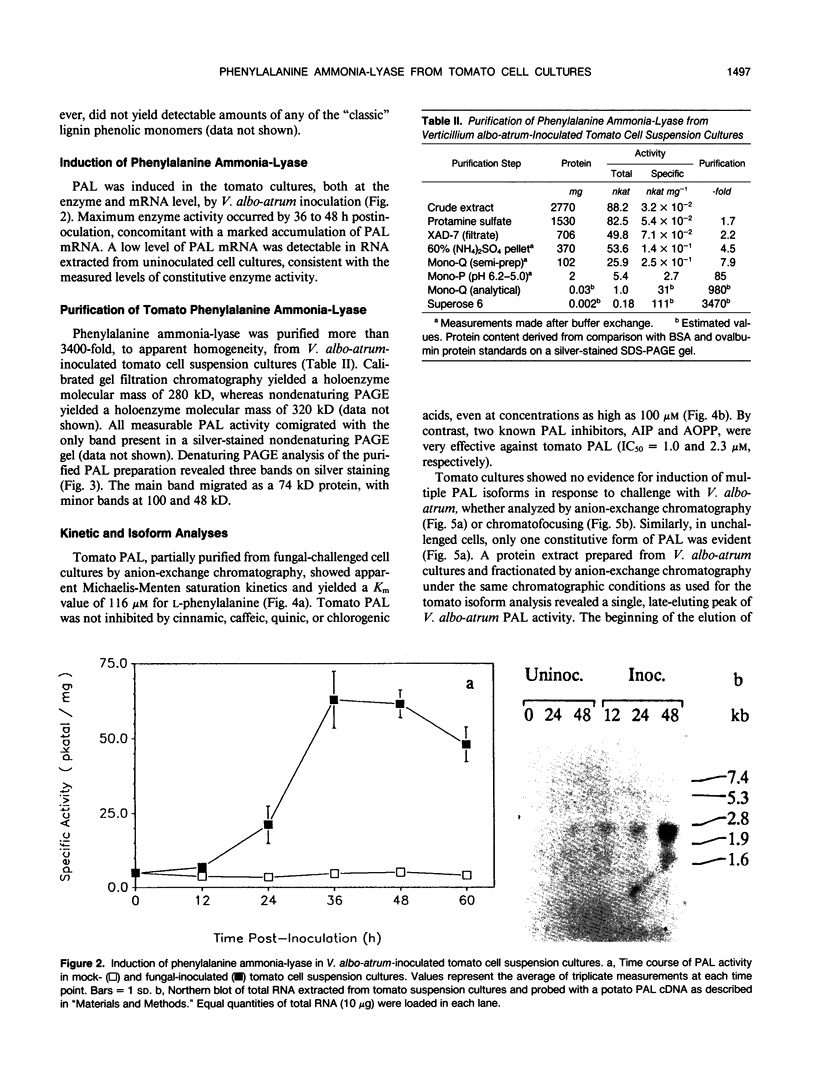
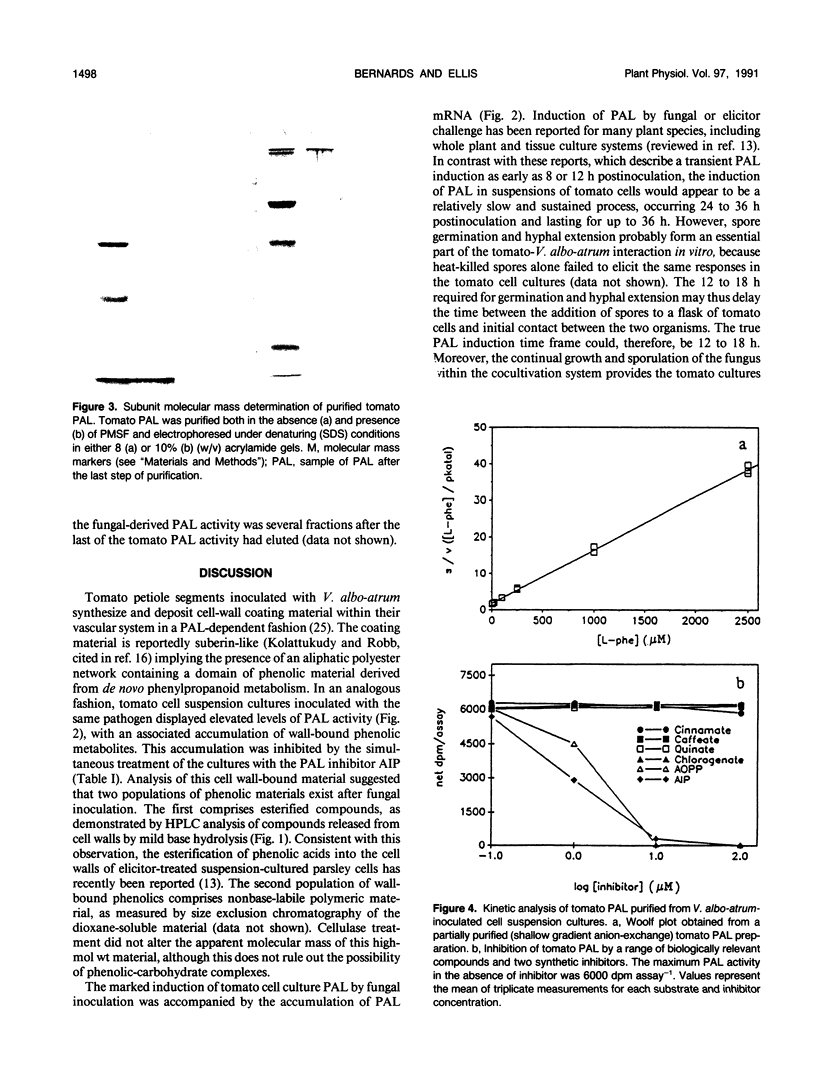
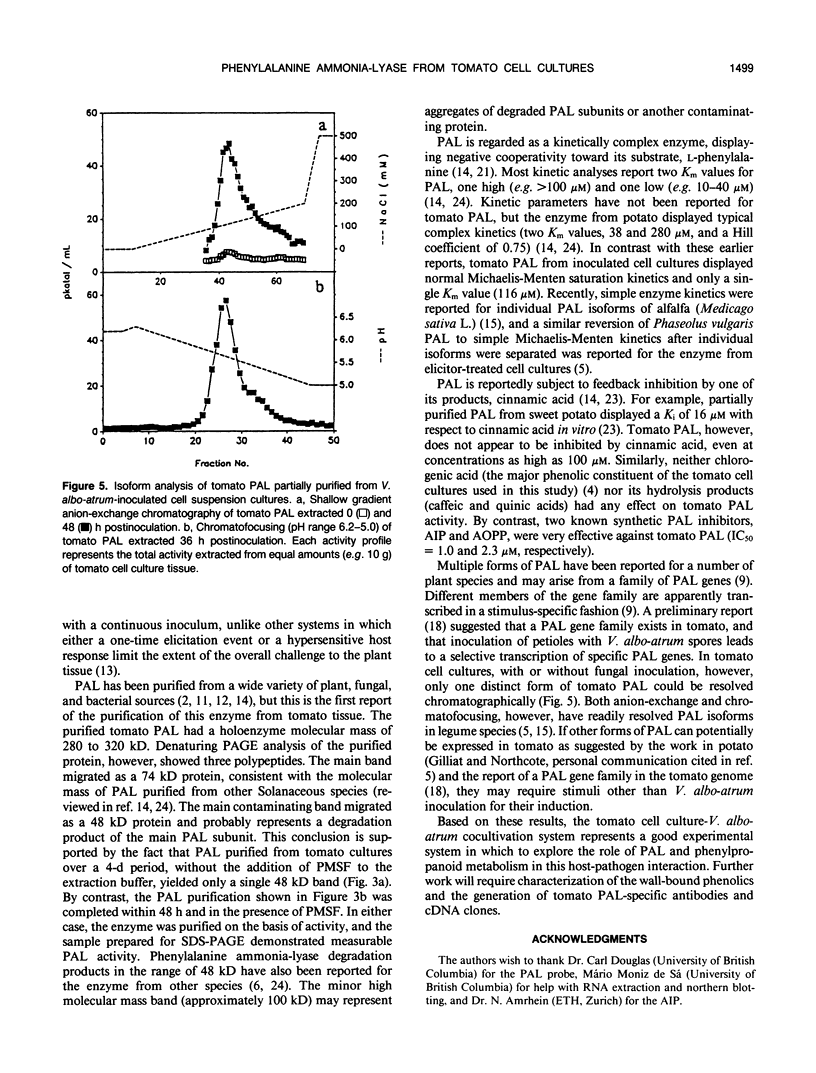
Tomato (Lycopersicon esculentum Mill.) cell suspension cultures accumulated wall-bound phenolic materials in response to inoculation with Verticillium albo-atrum Reinke et Berth. in a fashion analogous to that observed in whole plants. Both monomeric and polymeric materials were recovered. Deposition of phenolics into the cell walls of inoculated tomato cell cultures was inhibited by the phenylalanine ammonia-lyase (PAL) inhibitor, 2-amino-2-indanephosphate. Tomato PAL activity was induced over 12-fold by fungal inoculation, with a concomitant increase in the corresponding mRNA. The enzyme was purified >3400-fold, to apparent homogeneity, by anion-exchange chromatography, chromatofocusing, and gel filtration. The holoenzyme had a molecular mass of 280 to 320 kilodaltons, comprising 74-kilodalton subunits, and displayed an isoelectric point of 5.6 to 5.7. Induced PAL displayed apparent Michaelis-Menten kinetics (Km = 116 micromolar) and was not appreciably inhibited by its product cinnamic acid. Chromatographic analysis did not reveal multiple forms of the enzyme in either inoculated or uninoculated cultures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolwell G. P., Bell J. N., Cramer C. L., Schuch W., Lamb C. J., Dixon R. A. L-Phenylalanine ammonia-lyase from Phaseolus vulgaris. Characterisation and differential induction of multiple forms from elicitor-treated cell suspension cultures. Eur J Biochem. 1985 Jun 3;149(2):411–419. doi: 10.1111/j.1432-1033.1985.tb08941.x. [DOI] [PubMed] [Google Scholar]
- Bruce R. J., West C. A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989 Nov;91(3):889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzemeier K. H., Cretin C., Kombrink E., Rohwer F., Taylor J., Scheel D., Hahlbrock K. Transient Induction of Phenylalanine Ammonia-Lyase and 4-Coumarate: CoA Ligase mRNAs in Potato Leaves Infected with Virulent or Avirulent Races of Phytophthora infestans. Plant Physiol. 1987 Sep;85(1):34–41. doi: 10.1104/pp.85.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Acton G. J. Purification to homogeneity and some properties of L-phenylalanine ammonia-lyase of irradiated mustard (Sinapis alba L.) cotyledons. Biochim Biophys Acta. 1979 Sep 12;570(1):187–197. doi: 10.1016/0005-2744(79)90213-4. [DOI] [PubMed] [Google Scholar]
- Jorrin J., Dixon R. A. Stress Responses in Alfalfa (Medicago sativa L.): II. Purification, Characterization, and Induction of Phenylalanine Ammonia-Lyase Isoforms from Elicitor-Treated Cell Suspension Cultures. Plant Physiol. 1990 Feb;92(2):447–455. doi: 10.1104/pp.92.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mohan R., Kolattukudy P. E. Differential Activation of Expression of a Suberization-Associated Anionic Peroxidase Gene in Near-Isogenic Resistant and Susceptible Tomato Lines by Elicitors of Verticillium albo-atratrum. Plant Physiol. 1990 Jan;92(1):276–280. doi: 10.1104/pp.92.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nari J., Mouttet C., Fouchier F., Ricard J. Subunit interactions in enzyme catalysis. Kinetic analysis of subunit interactions in the enzyme L-phenylalanine ammonia-lyase. Eur J Biochem. 1974 Feb 1;41(3):499–515. doi: 10.1111/j.1432-1033.1974.tb03291.x. [DOI] [PubMed] [Google Scholar]
- Parsons T. J., Bradshaw H. D., Jr, Gordon M. P. Systemic accumulation of specific mRNAs in response to wounding in poplar trees. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7895–7899. doi: 10.1073/pnas.86.20.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N. M., Bolwell G. P., Smith C. Wound-induced phenylalanine ammonia-lyase in potato (Solanum tuberosum) tuber discs. Significance of glycosylation and immunolocalization of enzyme subunits. Biochem J. 1990 Apr 1;267(1):163–170. doi: 10.1042/bj2670163. [DOI] [PMC free article] [PubMed] [Google Scholar]




