Abstract
Background:
Diabetes mellitus and central obesity are more common among South Asian populations than among White British people. This study explores the differences in diabetes and obesity in South Asians with stroke living in the United Kingdom, India, and Qatar compared with White British stroke patients.
Methods:
The study included the UK, Indian, and Qatari arms of the ongoing large Bio-Repository of DNA in Stroke (BRAINS) international prospective hospital-based study for South Asian stroke. BRAINS includes 4580 South Asian and White British recruits from UK, Indian, and Qatar sites with first-ever ischemic stroke.
Results:
The study population comprises 1751 White British (WB) UK residents, 1165 British South Asians (BSA), 1096 South Asians in India (ISA), and 568 South Asians in Qatar (QSA). ISA, BSA, and QSA South Asians suffered from higher prevalence of diabetes compared with WB by 14.5% (ISA: 95% confidence interval (CI) = 18.6–33.0, p < 0.001), 31.7% (BSA: 95% CI = 35.1–50.2, p < 0.001), and 32.7% (QSA: 95% CI = 28.1–37.3, p < 0.001), respectively. Although WB had the highest prevalence of body mass index (BMI) above 27 kg/m2 compared with South Asian patients (37% vs 21%, p < 0.001), South Asian patients had a higher waist circumference than WB (94.8 cm vs 90.8 cm, p < 0.001). Adjusting for traditional stroke risk factors, ISA, BSA, and QSA continued to display an increased risk of diabetes compared with WB by 3.28 (95% CI: 2.53–4.25, p < 0.001), 3.61 (95% CI: 2.90–4.51, p < 0.001), and 5.24 (95% CI: 3.93–7.00, p < 0.001), respectively.
Conclusion:
South Asian ischemic stroke patients living in Britain and Qatar have a near 3.5-fold risk of diabetes compared with White British stroke patients. Their body composition may partly help explain that increased risk. These findings have important implications for public health policymakers in nations with large South Asian populations.
Keywords: Stroke, diabetes, obesity, ethnicity
Introduction
One in five strokes in Britain are caused by type 2 diabetes which doubles the risk of stroke within the first 5 years of diagnosis. 1 In the United Kingdom, 3.6 million persons, around 5% of the population, have been diagnosed with diabetes, with diabetes mellitus type 2 accounting for around 90% of cases, 2 while an additional million people are thought to suffer from undiagnosed diabetes. Diabetes and central obesity are twice as common among South Asians from India, Pakistan, and Bangladesh living in the United Kingdom than among White British people 3 often striking them earlier in life. 4
South Asian populations have a 15–27% greater prevalence of diabetes mellitus than White British populations. 3 Gender does not appear to influence differences between these two ethnic groups. 5 Moreover, this elevated prevalence is still present in individuals with first-onset stroke. 6 Diabetes is 80 times more likely to occur in obese adults than in healthy individuals with body mass indices (BMIs) under 22. 7 South Asians generally have a lower prevalence of overweight/obesity than the White British population, men having the largest difference in ethnic prevalence (16.3%) and women having the smallest difference (1%), even though there is little, if any, data on prevalence among those who have ischemic stroke. 5 South Asian infants born in the United Kingdom had lower birth weights, which may have a hereditary basis. 8 It is important to note the differences in fat distribution that exist throughout the South Asian community, particularly in view of the effect that these measures have for the prevalence of obesity. South Asians have greater accumulation of visceral and subcutaneous fat around the abdomen as well as increased skinfold thickness compared with their White British counterparts. 9 It has already been determined that increased central adiposity in South Asians increases ischemic stroke risk factors, such as elevated C-reactive protein (CRP) concentration. 10 Furthermore, it has been hypothesized that South Asian’s atherogenic lipid profile is a result of rising central adiposity. 11
Migration and cultural norms around nutrition and exercise have been presumed to significantly compound the increased prevalence of diabetes mellitus among ethnic South Asians. However, the assumption that South Asians who immigrate to other nations have a higher incidence of diabetes, obesity, and visceral fat location compared with local White populations has not been robustly tested using large and comparative international datasets. Demonstrating differences in disease occurrence following migration is likely to have important public health implications for nations with sizable South Asian populations.
We aimed to investigate the disparities in diabetes mellitus and obesity between South Asians in the United Kingdom, India, and Qatar with a White British population among patients who have had first-time an ischemic stroke using one of the largest such datasets currently available.
Materials and methods
Data source
We used the UK, Indian, and Qatar arms of the ongoing large prospective international Bio-Repository of DNA in Stroke (BRAINS) study, the details of which have been previously published12,13 but are described in detail in the Supplementary material. However, briefly, ischemic stroke patients of South Asian descent were recruited from 21 sites in the United Kingdom, 2 in India (North and South), and 1 in Qatar. Detailed phenotypic and demographic information was documented.12,13 Stroke in all patients was confirmed by computed tomography (CT) and/or magnetic resonance imaging (MRI) of the brain. All patients were reviewed by a stroke or neurology physician.
Statistical analysis
Descriptive statistics summarized data using mean with standard deviation (SD) or median with interquartile range (IQR) for continuous variables, and proportion for categorical variables. For single-factor analysis, chi-square (or Fisher exact test, where appropriate) was used for categorical variables, and independent t-test (or Mann–Whitney U test, where appropriate) for continuous variables. Univariate and multivariate logistic regression estimated the associations of covariates (age, sex, central obesity, smoking history, alcohol consumption, hypertension, hypercholesterolemia, and cardiovascular diseases) with diabetes mellitus status. The reliability (goodness-of-fit) of each model was quantified using the Hosmer and Lemeshow test. Models evaluated using Akaike’s information criterion (AIC) and the likelihood ratio chi-square test. Rather than applying a correction for multiple testing at global significance level, for individual tests of association defined statistical significance was <0.01.
Results
The study population was 4580 individuals identified with first-time ischemic stroke comprising 1751 (men: 980, women: 771) White British (WB), 1165 (men: 752, women: 413) British South Asians (BSA), 1096 (men: 739, women: 359) South Asians in India (ISA), and 568 (men: 543, women: 25) South Asians in Qatar (QSA) patients. The mean age was 62.2 ± 16.0 and 65.8% (95% confidence interval (CI): 64.4–67.2) of the sample were male. The prevalence among first-time ischemic stroke patients of diabetes was 34.1% (95% CI: 32.8–35.5), hypertension 67.8% (95% CI: 66.4–69.1), hypercholesterolemia 39.1% (95% CI: 37.6–40.6), smoking history 18.6% (95% CI: 17.4–19.7), and alcohol consumption 36.0% (95% CI: 34.6–37.5).
Demographic and clinical characteristics by diabetes status and ethnicity are presented in Table 1. Diabetic patients compared with non-diabetic patients suffered from higher adiposity index in all ethnic groups. Diabetic patients had a higher average BMI and waist circumference value than non-diabetic patients by 0.94 kg/m2 (95% CI: 0.59–1.28, p < 0.001) and 6.7 cm (95% CI: 5.32–8.09, p < 0.001), respectively. In addition, diabetic patients had higher prevalence of central obesity by 5.6% (95% CI: 2.6–8.6, p < 0.001) compared with non-diabetic patients. Diabetic patients had an increased prevalence of diabetes family history by 31.2% (95% CI: 27.9–34.5, p < 0.001), hypertension by 23.6% (95% CI: 21.0–26.2, p < 0.001), hypercholesterolemia by 22.0% (95% CI: 18.9–25.1, p < 0.001), and cardiovascular disease by 11.7% (95% CI: 9.1–14.3, p < 0.001) compared with non-diabetic patients. No significant difference was observed in gender between diabetes and non-diabetes patients.
Table 1.
Population characteristics and comorbidities by ethnicity and diabetes status.
| WB |
BSA |
ISA |
QSA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes (n = 325) Mean (SD) |
Non-diabetes (n = 1426) Mean (SD) |
p-value | Diabetes (n = 585) Mean (SD) |
Non-diabetes (n = 580) Mean (SD) |
p-value | Diabetes (n = 362) Mean (SD) |
Non-diabetes (n = 734) Mean (SD) |
p-value | Diabetes (n = 291) Mean (SD) |
Non-diabetes (n = 277) Mean (SD) |
p-value | |
| Average age of onset (years, SD) | 71.1 (11.6) | 71.4 (13.8) | 0.65 | 65.4 (12.6) | 62.4 (17.2) | <0.001 | 56.3 (9.9) | 49.9 (14.2) | <0.001 | 50.9 (9.7) | 50.9 (11.0) | 0.97 |
| Men, n (%) | 197 (60.6) | 783 (54.9) | 0.071 | 375 (64.1) | 377 (65.0) | 0.80 | 256 (70.7) | 483 (65.8) | 0.12 | 282 (96.9) | 261 (94.2) | 0.18 |
| Smoking history, n (%) | 842 (13.9) | 193 (15.2) | 0.67 | 96 (16.5) | 87 (15.1) | 0.77 | 63 (17.5) | 128 (17.5) | 0.31 | 95 (32.6) | 114 (41.2) | <0.001 |
| Alcohol consumption, n (%) | 93 (33.1) | 583 (49.1) | <0.001 | 115 (20.6) | 139 (25.0) | 0.089 | 149 (41.4) | 309 (42.6) | 0.76 | 60 (20.6) | 77 (27.8) | 0.057 |
| Adiposity index | ||||||||||||
| BMI (kg/m2) | 29.2 (6.1) | 26.7 (6.0) | <0.001 | 26.7 (5.0) | 25.9 (5.1) | 0.007 | 24.7 (3.7) | 23.5 (4.4) | <0.001 | 26.4 (4.1) | 26.2 (3.9) | 0.58 |
| Waist circumference (cm) | 97.4 (20.6) | 89.2 (16.2) | <0.001 | 100.4 (16.7) | 93.8 (14.2) | <0.001 | 101.7 (22.0) | 91.9 (18.0) | <0.001 | 92.2 (11.5) | 91.0 (11.3) | 0.22 |
| Central obesity, n (%) | 116 (51.6) | 336 (34.1) | <0.001 | 193 (37.2) | 138 (27.5) | <0.001 | 52 (16.0) | 56 (9.0) | 0.002 | 41 (14.1) | 41 (14.8) | 0.90 |
| Comorbidities, n (%) | ||||||||||||
| Diabetes family history | 107 (46.1) | 194 (18.7) | <0.001 | 318 (71.5) | 232 (58.6) | <0.001 | 161 (45.6) | 142 (19.7) | <0.001 | 129 (56.6) | 38 (18.2) | <0.001 |
| Hypertension | 257 (79.3) | 834 (58.6) | <0.001 | 510 (88.1) | 338 (58.6) | <0.001 | 296 (82.7) | 423 (59.1) | <0.001 | 229 (79.0) | 192 (69.3) | 0.011 |
| Hypercholesterolemia | 165 (50.8) | 419 (29.8) | <0.001 | 373 (66.1) | 214 (37.8) | <0.001 | 149 (45.0) | 200 (31.6) | <0.001 | 121 (41.9) | 75 (27.4) | <0.001 |
| Cardiovascular diseases | 81 (27.1) | 224 (17.6) | <0.001 | 218 (38.4) | 110 (19.4) | <0.001 | 63 (17.5) | 79 (10.9) | 0.003 | 39 (13.5) | 8 (2.9) | <0.001 |
n: sample size; WB: White British individuals living in the United Kingdom; BSA: British South Asians living in the United Kingdom; ISA: South Asians living in India; QSA: South Asians living in Qatar; SD: standard deviation; BMI: body mass index; MI: myocardial infarction.
Central obesity was defined by waist circumference (men: >102 cm, women: >88 cm) or body mass index (⩾27 kg/m2); cardiovascular diseases was defined by the presence of ischemic heart disease, angina, or MI.
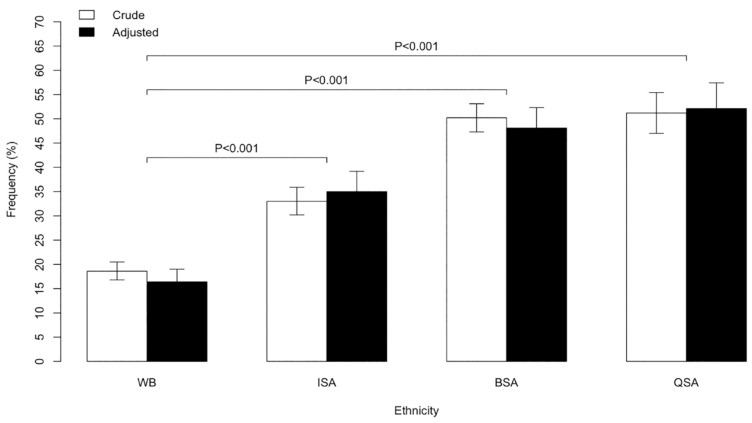
The prevalence of diabetes among WB, ISA, BSA, and QSA is presented in Figure 1. South Asian patients had a higher prevalence of diabetes compared with White British patients among first-time ischemic stroke patients. ISA had higher prevalence of diabetes compared with WB by 14.5% (95% CI: 18.6–33.0, p < 0.001), BSA by 31.7% (95% CI: 35.1–50.2, p < 0.001), and QSA by 32.7% (95% CI: 28.1–37.3, p < 0.001). Migrated South Asian patients BSA and QSA patients suffer from greater prevalence of diabetes compared with ISA patients by 17.2% (95% CI: 13.0–21.2, p < 0.001) and 18.2% (95% CI: 13.1–23.3, p < 0.001), respectively.
Figure 1.
Prevalence of diabetes among WB, ISA, BSA, and QSA.
WB: White British individuals living in the United Kingdom; BSA: British South Asians living in the United Kingdom; ISA: South Asians living in India; QSA: South Asians living in Qatar.
Adjusted for age and sex.
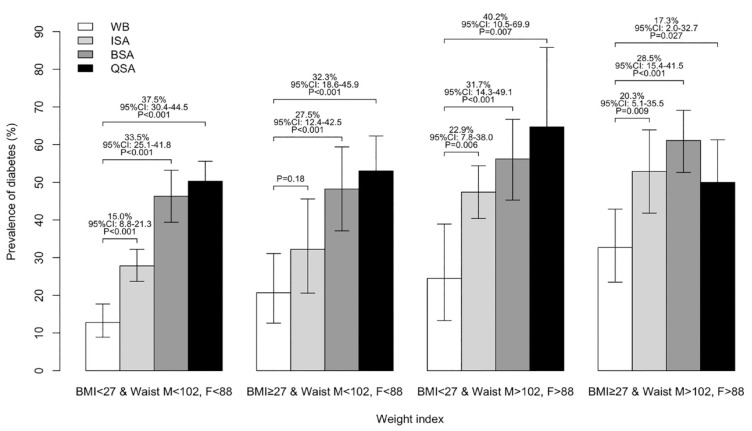
The prevalence of diabetes by BMI above or below 27 kg/m2 and waist circumference above or below 102 cm in male and 88 cm in female are presented in Figure 2. The BSA and QSA had the highest prevalence of diabetes in all four combinations of BMI and waist circumference. This was followed by ISA who had the third highest prevalence.
Figure 2.
Prevalence of diabetes among BMI above or below 27 kg/m2 and waist circumference above or below 102 cm in male and 88 cm in female.
WB: White British individuals living in the United Kingdom; BSA: British South Asians living in the United Kingdom; ISA: South Asians living in India; QSA: South Asians living in Qatar.
The association between diabetes status and ethnic group among ischemic stroke patients was evaluated using logistic regression (Table 2). Univariate analysis showed that south Asian ethnicity (ISA, BSA, QSA) was associated with diabetes status. The odds ratio (OR) for diabetes presence was 3.41 (95% CI: 2.97–3.94, p < 0.001) for South Asians (ISA, BSA, QSA) versus WB. Multivariate logistic regression analysis modeling including traditional stroke risk factors (age, sex, central obesity, smoking history, alcohol consumption, hypertension, hypercholesterolemia, and cardiovascular diseases) showed that ethnicity independently associated with diabetes (Table 2). Multivariate adjusted OR for South Asian ethnicity (ISA, BSA, QSA) compared with WB was 3.68 (95% CI: 2.99–4.53, p < 0.001).
Table 2.
Multivariate logistic regression analysis of diabetes status.
| Model 1 OR (95% CI), p-value |
Model 2 OR (95% CI), p-value |
Model 3 OR (95% CI), p-value |
|
|---|---|---|---|
| WB | Reference group | Reference group | Reference group |
| ISA | 2.16 (1.82–2.58), <0.001 | 2.81 (2.30–3.43), <0.001 | 3.28 (2.53–4.25), <0.001 |
| BSA | 4.43 (3.75–5.23), <0.001 | 4.91 (4.13–5.84), <0.001 | 3.61 (2.90–4.51), <0.001 |
| QSA | 4.61 (3.76–5.66), <0.001 | 5.79 (4.58–7.34), <0.001 | 5.24 (3.93–7.00), <0.001 |
OR: odds ratio; CI: confidence interval; WB: White British individuals living in the United Kingdom; ISA: South Asians living in India; BSA: British South Asians living in the United Kingdom; QSA: South Asians living in Qatar.
Model 1: Unadjusted.
Model 2: Adjusted for age and sex.
Model 3: Adjusted for age, sex, central obesity, smoking history, alcohol consumption, hypertension, hypercholesterolemia, and cardiovascular diseases.
Discussion
South Asian ISA, BSA, and QSA ischemic stroke patients compared with their WB stroke counterparts suffer from higher prevalence of diabetes by 15%, 32%, and 33%, respectively, following adjustment for traditional risk factors (age, sex, central obesity, smoking history, alcohol consumption, hypertension, hypercholesterolemia, and cardiovascular diseases). Moreover, migrated South Asians present with a greater prevalence of diabetes compared with those who remain in the subcontinent. These results reflect differences in adiposity index, BMI, waist circumference, and central obesity among South Asian patients compared with WB and ISA, likely highlighting differences in lifestyle and environmental factors such as diet and exercise.
The body composition of the South Asian patients may help to explain the increased risk of developing diabetes compared with the WB patients. A high visceral fat percentage has been highly correlated with having a greater waist circumference. South Asians tend to have higher amounts of abdominal adipose tissue, including both subcutaneous and visceral fat than their Caucasian counterparts at similar BMI values, although no differences were found at very high values of BMI. 14 Observational studies have shown that excessive amounts of visceral adipose tissue can increase the risk of developing diabetes due to it being the primary causal factor of insulin resistance. 15
Previous studies indicated that South Asian populations are more likely to have abdominal obesity and more body fat at a given BMI value than Caucasians. 16 Due to this, they are at an increased risk of developing diabetes and other comorbidities, including hypertension, stroke, cardiovascular disease, and hypercholesterolemia. 17 Several studies have demonstrated that South Asians are at risk of developing obesity-related comorbidities at lower BMI levels or smaller waist circumference measures. 18
BMI does not distinguish between muscle and fat. 16 A person with a high proportion of muscle may be considered overweight or even obese since muscle weighs more than fat. Moreover, BMI ignores the distribution of fat, despite that being important for predicting health outcomes. 16 The risk of diabetes and heart disease is higher in people with “apple” shapes who carry fat around their middle but may be quite thin elsewhere. 16 Individuals with this body type could be considered healthy according to the BMI calculation. 16 “Pear” shapes obesity formally classified as “overweight,” despite the fact that fat stored around the hips, bottom, and thighs is more secure. Asian-descent individuals have higher weight-related disease risks at lower BMIs and more likely to develop diabetes and heart disease, due to central fat. 16 This requires separate standards for various ethnic groups. As waist circumference is a stronger predictor of type 2 diabetes risk, regardless of BMI, 19 it presents a powerful argument for a wider adoption of more ethnic-specific criteria. 20
Release of adipokines and their associated metabolic consequences may partly explain why South Asians have a higher risk of diabetes due to an excessive build-up of adipose tissue. A secreted adipokine known as adiponectin is thought to play a role in the modulation of glucose and lipid metabolism in insulin-sensitive tissues with lower levels of blood adiponectin implicated in the pathogenesis of insulin resistance and diabetes. 21 Unlike their Caucasian counterparts, South Asian men have been found to have lower levels of adiponectin despite their body fat content and body fat distribution. 22 A correlation between lower levels of adiponectin and insulin has been previously identified, as well as the identification of lower levels of adiponectin in South Asians with diabetes.21,22 Adipocytes also produce leptin which is known to have insulin-sensitizing effects as well as causing a reduction in a person’s appetite. 23 Obese individuals tend to develop hyperleptinemia which causes them to become leptin resistant which contributes to insulin resistance as it suppresses insulin action. 23 South Asians have been found to have higher levels of leptin in the system than their Caucasian counterparts, despite their body fat distribution or body composition. 21 Leptin levels have been found to be higher in South Asians with diabetes than those with only an impaired glucose tolerance or normal glucose tolerance. 22
The higher prevalence of diabetes in South Asians in the United Kingdom and Qatar compared with Indian South Asians highlights the possible changes in lifestyle and environmental factors, including nutrition and exercise that may occur when people migrate. The traditional South Asian carbohydrates heavy diet of rice and bread is better suited to a physically demanding rural environment. 24 Yet South Asians in the United Kingdom continue to eat a high-carbohydrate diet and engage in less exercise than the WB population. 25 The World Health Organization (WHO) has recommended special standards for determining obesity in South Asians due to a tendency toward visceral adiposity in the truncal region. 20 Another potential element in the variation in diabetes risk is most likely the impact of migration on environmental and lifestyle factors. In migration studies, access to healthcare is a crucial component of diabetes prevention that is frequently ignored. Despite having access to healthcare facilities that can help prevent disease, BSA have demonstrably limited awareness of the risk factors that contribute to disease. South Asian patients in the United Kingdom and Qatar may be less informed of the typical complications linked to diabetes mellitus, the significance of screening clinics, and the necessity of consulting chiropodists. 26 In addition, sociocultural and religious factors might compound this diminished awareness by creating false notions of social stigma and failure to self-care. 27
Diabetes mellitus may be prevented in South Asian communities using programs that promote health-protective lifestyle changes and comprise multi-component interventions that encompass parts of health promotion and behavioral change that are passive (lecture-based), active (activity-based), and individualized (such as targeted counseling). 28 These programs should focus on cultural values related to lifestyle aspects such as food, which reduces carbohydrate intake, and exercise, which raising aerobic exercise at a moderate intensity.
Limitations
As with all studies, a number of limitations need to be noted. The BRAINS project has been ongoing for several years and in that time risk factor thresholds and management approaches have evolved. However, those changes would have led to a non-differential ethnic bias and are unlikely to have an impact on the significance of the findings in this study. The prevalence of comorbidities is recorded at the time of the event. Most of the prevalence data was gathered from treatment regimens documented on the patient’s health record even though we are unable to comment on how long these comorbidities were prevalent before the diabetic event. The large sample size (n = 4580) accurately reflects the current influence of these risk factors on diabetes in the South Asian population, even though this may result in an underestimation of the real effect size on the diabetes event. Grandparent origin defined ethnicity. Although this was self-reported, prior research has shown that this methodology is accurate. 29 Due to the lack of socioeconomic data collection, we are unable to determine how socioeconomic status may affect morbidity and mortality or the occurrence of developing diabetes. Using the larger BRAINS dataset which includes both ischemic and hemorrhagic events, we evaluated how well subjects were represented in our study. We discovered similar stroke subtype prevalence in all four of our study’s groups. 30 In addition, because long-term follow-up was not conducted, we are unable to report on specific migratory effects and how they develop. The recruitment sites in the United Kingdom, India, and Qatar were selected to provide a representative sample. Twenty-one hospital locations with a strong South Asian population were identified in the United Kingdom. All India Institute of Medical Sciences and Sree Chitra Tirunal Institute for Medical Sciences and Technology were chosen as the two hospital locations since they are situated in the north and south of the nation, respectively. Different cultures have different responses and access to seeking emergency healthcare support services. However, all our recruitment sites across three countries provide free access to medical services, making it likely to draw people from a wide range of socioeconomic backgrounds. Nevertheless, this is a hospital-based study and is dependent on recruitment for patients attending hospital.
Conclusion
South Asian ischemic stroke patients in India (ISA), Britain (BSA), and Qatar (ISQ) have higher prevalence of diabetes mellitus compared with White British stroke patients, with migrants being most affected. Body composition of South Asian patients may help to explain the increased risk of developing diabetes. These findings have important implications for public health policymakers in nations with large migrant South Asian populations.
Supplemental Material
Supplemental material, sj-docx-1-wso-10.1177_17474930231203149 for Diabetes mellitus and obesity among South Asians with ischemic stroke across three countries by Gie Ken-Dror, Intisar Ajami, Thang S Han, Taylor Aurelius, Ankita Maheshwari, Hassan Al Hail, Dirk Deleu, Sapna D Sharma, Sageet Amlani, Gunaratnam Gunathilagan, David L Cohen, Chakravarthi Rajkumar, Stuart Maguire, Sissi Ispoglou, Ibrahim Balogun, Anthea Parry, Lakshmanan Sekaran, Hafiz Syed, Enas Lawrence, Ravneeta Singh, Ahamad Hassan, Chris Wharton, Khalid Javaid, Neetish Goorah, Peter Carr, Eman Abdus Sami, Musab Ali, Hassan Al Hussein, Hassan Osman Abuzaid, Khalid Sharif, Shri Ram Sharma, PN Sylaja, Fahmi Yousef Khan, Kameshwar Prasad and Pankaj Sharma in International Journal of Stroke
Acknowledgments
The authors thank Isaac John and Tasmin Patel for the daily operations and database management of the BRAINS study.
Footnotes
Clinical trial registration: URL: http://www.clinicaltrials.gov (Unique identifier: NCT05491824).
Contributions: P.S. conceived and overall directed the global study. K.P. was the lead investigator in India. F.Y.K. was the lead investigator in Qatar. G.K.-D. undertook the initial analysis and wrote the first draft. G.K.-D., T.S.H., and P.S. undertook the analysis. S.D.S. assisted with clinical phenotyping. A.M., T.A., H.A.H., D.D., S.A., G.G., D.L.C., C.R., S.M., S.I., I.B., A.P., L.S., H.S., E.L., R.S., A.H., C.W., K.J., N.G., P.C., E.A.S., M.A., H.A.H., H.O.A., K.S., S.R.S., F.Y.K., P.N.S., K.P., and P.S. contributed to recruitment of data. All the authors contributed to the final draft. P.S. accepts overall responsibility for the final work.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The global BRAINS study has received funding from the UK-India Education and Research Initiative (UKUTP2011082), Qatar National Research Fund (NPRP 6-068-3-015) and Henry Smith (UK) foundation. The UK arm of BRAINS has received support from the UK Clinical Research Network (CRN). P.S. was supported by a Department of Health (UK) senior fellowship during part of the BRAINS study.
Consent for publication: Not applicable.
Ethical approval: Ethical approval for this study was obtained from Riverside Research Ethics Committee (04/Q0401/40). Research governance is through the standard Royal Holloway University of London (RHUL) procedures and protocols.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
ORCID iDs: Gie Ken-Dror  https://orcid.org/0000-0003-3747-7112
https://orcid.org/0000-0003-3747-7112
Thang S Han  https://orcid.org/0000-0003-2570-0938
https://orcid.org/0000-0003-2570-0938
Taylor Aurelius  https://orcid.org/0000-0001-5925-1910
https://orcid.org/0000-0001-5925-1910
Supplemental material: Supplemental material for this article is available online.
References
- 1. Royal College of Physicians; Sentinel Stroke National Audit Programme (SSNAP). National clinical audit annual results portfolio March 2016-April 2017. http://bit.ly/1NHYlqH
- 2. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. Lancet 2016; 388: 170–177. [DOI] [PubMed] [Google Scholar]
- 3. Bhopal R, Unwin N, White M, et al. Heterogeneity of coronary heart disease risk factors in Indian, Pakistani, Bangladeshi, and European origin populations: cross sectional study. BMJ 1999; 319: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McFadden E, Luben R, Wareham N, Bingham S, Khaw KT. Occupational social class, risk factors and cardiovascular disease incidence in men and women: a prospective study in the European prospective investigation of cancer and Nutrition in Norfolk (EPIC-Norfolk) cohort. Eur J Epidemiol 2008; 23: 449–458. [DOI] [PubMed] [Google Scholar]
- 5. Becker E, Boreham R, Chaudhury M, et al. Health survey for England – 2004: health of ethnic minorities (Sproston K and Mindell J Eds, Vol. 1). London: The Information Centre, 2004, pp.1–435. [Google Scholar]
- 6. Gunarathne A, Patel JV, Potluri R, Gill PS, Hughes EA, Lip GY. Secular trends in the cardiovascular risk profile and mortality of stroke admissions in an inner city, multiethnic population in the United Kingdom (1997-2005). J Hum Hypertens 2008; 22: 18–23. [DOI] [PubMed] [Google Scholar]
- 7. Diabetes UK. Diabetes and obesity, 2022, https://www.diabetes.co.uk/diabetes-and-obesity.html
- 8. Harding S, Rosato MG, Cruickshank JK. Lack of change in birthweights of infants by generational status among Indian, Pakistani, Bangladeshi, Black Caribbean, and Black African mothers in a British cohort study. Int J Epidemiol 2004; 33: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 9. Nightingale CM, Rudnicka AR, Owen CG, Cook DG, Whincup PH. Patterns of body size and adiposity among UK children of South Asian, black African-Caribbean and white European origin: child heart and health study in England (CHASE Study). Int J Epidemiol 2011; 40: 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forouhi NG, Sattar N, McKeigue PM. Relation of C-reactive protein to body fat distribution and features of the metabolic syndrome in Europeans and South Asians. Int J Obes Relat Metab Disord 2001; 25: 1327–1331. [DOI] [PubMed] [Google Scholar]
- 11. Gunarathne A, Patel JV, Potluri R, et al. Increased 5-year mortality in the migrant South Asian stroke patients with diabetes mellitus in the United Kingdom: the West Birmingham stroke project. Int J Clin Pract 2008; 62: 197–201. [DOI] [PubMed] [Google Scholar]
- 12. Yadav S, Schanz R, Maheshwari A, et al. Bio-repository of DNA in stroke (BRAINS): a study protocol. BMC Med Genet 2011; 12: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cotlarciuc I, Khan MS, Maheshwari A, et al. Bio-repository of DNA in stroke: a study protocol of three ancestral populations. JRSM Cardiovasc Dis 2012; 1: cvd.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr 2007; 86: 353–359. [DOI] [PubMed] [Google Scholar]
- 15. Frayn KN. Visceral fat and insulin resistance–causative or correlative? Br J Nutr 2000; 83: S71–S77. [DOI] [PubMed] [Google Scholar]
- 16. Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 2002; 3: 141–146. [DOI] [PubMed] [Google Scholar]
- 17. Khaodhiar L, McCowen KC, Blackburn GL. Obesity and its comorbid conditions. Clin Cornerstone 1999; 2: 17–31. [DOI] [PubMed] [Google Scholar]
- 18. Bodicoat DH, Gray LJ, Henson J, et al. Body mass index and waist circumference cut-points in multi-ethnic populations from the UK and India: the ADDITION-Leicester, Jaipur heart watch and New Delhi cross-sectional studies. PLoS ONE 2014; 9: e90813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Misra A, Vikram NK. Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: evidence and implications. Nutrition 2004; 20: 482–491. [DOI] [PubMed] [Google Scholar]
- 20. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 21. Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone. Diabetes Care 2003; 26: 2442–2450. [DOI] [PubMed] [Google Scholar]
- 22. Abate N, Chandalia M, Snell PG, Grundy SM. Adipose tissue metabolites and insulin resistance in nondiabetic Asian Indian men. J Clin Endocrinol Metab 2004; 89: 2750–2755. [DOI] [PubMed] [Google Scholar]
- 23. Rabe K, Lehrke M, Parhofer KG, et al. Adipokines and insulin resistance. Mol Med 2008; 14: 741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leung G, Stanner S. Diets of minority ethnic groups in the UK: influence on chronic disease risk and implications for prevention. Nutrition Bulletin 2011; 36: 161–198. [Google Scholar]
- 25. Fischbacher CM, Hunt S, Alexander L. How physically active are South Asians in the United Kingdom? A literature review. J Public Health 2004; 26: 250–258. [DOI] [PubMed] [Google Scholar]
- 26. Hawthorne K. South Asian diabetic patients need more education about their illness. BMJ 1997; 314: 1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel V, Morrissey J, Goenka N, et al. Diabetes care in the Hindu patient: cultural and clinical aspects. Brit J Diabet Vasc Dis 2001; 1: 132–135. [Google Scholar]
- 28. Ali SH, Misra S, Parekh N, Murphy B, DiClemente RJ. Preventing type 2 diabetes among South Asian Americans through community-based lifestyle interventions: a systematic review. Prev Med Rep 2020; 20: 101182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Regenstein M, Sickler D. Race, ethnicity, and language of patients: hospital practices regarding collection of information to address disparities in health care. Washington, DC: National Public Health and Hospital Institute, 2006. [Google Scholar]
- 30. Pandian JD, Sudhan P. Stroke epidemiology and stroke care services in India. J Stroke 2013; 15: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-wso-10.1177_17474930231203149 for Diabetes mellitus and obesity among South Asians with ischemic stroke across three countries by Gie Ken-Dror, Intisar Ajami, Thang S Han, Taylor Aurelius, Ankita Maheshwari, Hassan Al Hail, Dirk Deleu, Sapna D Sharma, Sageet Amlani, Gunaratnam Gunathilagan, David L Cohen, Chakravarthi Rajkumar, Stuart Maguire, Sissi Ispoglou, Ibrahim Balogun, Anthea Parry, Lakshmanan Sekaran, Hafiz Syed, Enas Lawrence, Ravneeta Singh, Ahamad Hassan, Chris Wharton, Khalid Javaid, Neetish Goorah, Peter Carr, Eman Abdus Sami, Musab Ali, Hassan Al Hussein, Hassan Osman Abuzaid, Khalid Sharif, Shri Ram Sharma, PN Sylaja, Fahmi Yousef Khan, Kameshwar Prasad and Pankaj Sharma in International Journal of Stroke