Abstract
Case series summary
Positioning head tilt (PHT) is a dynamic neurological sign in which the head tilts to the opposite side to which it is moving. This sign is triggered in response to head movement and is thought to be due to the lack of inhibition of vestibular nuclei by the cerebellar nodulus and uvula (NU). The occurrence of PHT in animals has been suggested to be an indicator of NU dysfunction. Here, we describe the acute onset of PHT in 14 cats. All the cats were diagnosed with hypokalaemic myopathy caused by a range of pathologies. The PHT resolved along with other signs related to myopathy, such as cervical flexion and generalised weakness, after electrolyte correction in all cats.
Relevance and novel information
Hypokalaemic myopathy was the likely cause of PHT in the present feline cases.
Keywords: Positioning head tilt, hypokalaemic myopathy, hypokalaemia, reversible
Introduction
Positioning head tilt (PHT) is a neurological sign that was first reported in 2016. 1 Animals exhibiting this sign can turn freely in any direction at will. The head is in a level position when the animal looks forward; however, the head tilts to the opposite side when the animal turns its head. As a result, the side of tilting changes every time the animal turns its head.1,2 The cerebellum is responsible for the automatic maintenance of normal head position. Signals from the vestibular system are transmitted to the vestibulocerebellar module (the flocculonodullar lobe, the paraflocculus and the fastigial nucleus) when the head is moved.3–8 Vestibular nuclear projections to the spinal cord are situated in the vestibulospinal tracts. The fibres terminate on interneurons that facilitate ipsilateral extensor and decussate to inhibit contralateral extensor muscle activity. The vestibulospinal tracts facilitate spinal reflexes, especially those involved in maintaining posture and the antigravity/extensor muscles. Turning the head to the left causes a shift in the distribution of body mass to the left. This shift in mass is supported by increased extension on the left side, which is reflexively induced by both myotatic and vestibular reflexes. This also reduces extension on the right side, and thereby minimises weight transfer to the left. The cerebellar nodulus and uvula (NU; lobules of the cerebellar vermis IX and X, respectively) coordinate this system by inhibition of stimulation of vestibular nuclei to maintain a level head position in response to head movement. 1 This inhibition is impaired in animals with NU dysfunction and, consequently, PHT occurs. Thus, PHT can be used to ascertain lesion localisation. 1 To date, PHT has been reported in three dogs with NU hypoplasia, 1 in dogs with five different types of lysosomal storage disease 9 and in a dog with gliomatosis cerebri affecting NU.10–12
Hypokalaemic myopathy is a relatively common myopathy in cats associated with low extracellular potassium concentration. It often occurs in cats with chronic kidney disease (CKD), hyperthyroidism, dietary potassium deficiency, hyperaldosteronism, fluid over-administration, chronic vomiting/diarrhoea and overuse of potassium-wasting diuretics. Diagnosis is based upon signalment, historical and clinical findings, resolution of clinical signs with potassium supplementation and supportive evidence of myopathy from electromyogram (EMG) analysis and muscle biopsy.13,14 A hereditary condition in Burmese kittens is also suspected to cause potassium-losing nephropathy with periodic hypokalaemia and signs of myopathy.15,16 Hypokalaemia leads to hyperpolarisation of the sarcolemma resting membrane potential, making it refractory to depolarisation and subsequent contraction. Feline hypokalemic myopathy is a generalised polymyopathy, which is often associated with observed cervical flexion. In general, the prognosis associated with feline hypokalaemic myopathy is good if potassium concentrations are supplemented and the primary cause of the hypokalaemia is treated.13,14
The current report describes reversible PHT observed in 14 cats with hypokalaemic myopathy. A potential mechanism for the PHT observed in these cats is described and discussed.
Case series description
Cases and data collection
A 5-year-old neutered male domestic shorthair cat (case 1) was presented to Neuro Vets Animal Neurology Clinic with a history of head tilt and cervical flexion. A general physical examination revealed no abnormalities. A skin turgor test revealed <5% dehydration. On neurological examination, cervical flexion and PHT was clearly observed (Figure 1a; see also Video 1 in the supplementary material). Complete blood count results were within the normal reference intervals. Serum biochemistry revealed azotaemia and hypokalaemia (see file in the supplementary materials). Creatine kinase activity was not examined. Thoracic and abdominal radiographs revealed no abnormalities. Brain MRI (Figure 2) and cerebrospinal fluid analysis on the day of presentation revealed no abnormalities. Temporalis, Masseter, trapezius, clavicular cervicis, posterior sternal, longissimus (cervicis and thoracis), triceps brachii, biceps brachii, extensor carpi radialis, quadriceps femoris, tibialis anterior, gastrocnemius and interdigital muscle were evaluated by EMG. EMG revealed fibrillation potentials and positive sharp waves in the constituent muscles of the whole body, especially in the cervical muscles (Figure 3). Based on these findings, a diagnosis of kidney failure with hypokalaemic myopathy was made. An intravenous infusion of 0.9% saline supplemented with potassium (40 mmol/l) was administered; after this treatment, no further signs of weakness suggestive of myopathy or PHT were observed.
Figure 1.
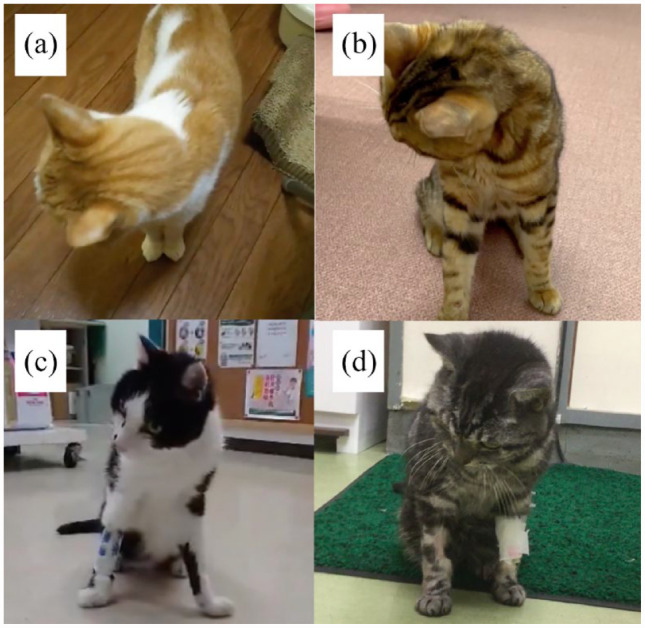
(a–d) Still images captured from videos of cases 1, 7, 10 and 11, respectively. The head is seen tilting to the left when the head of the cats turned to the right; positioning head tilt is observed clearly in cases 1 and 7 and mildly in cases 10 and 11
Figure 2.
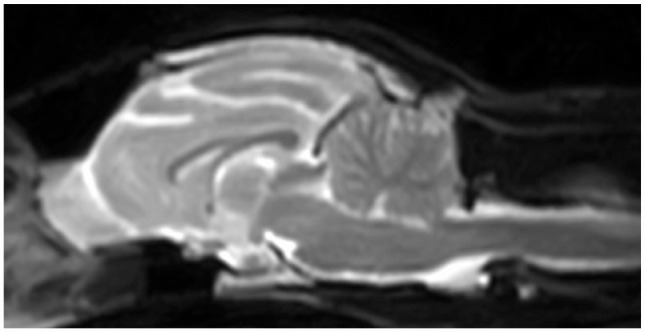
Mid-sagittal brain T2-weighted magnetic resonance image of case 1. No abnormality is evident
Figure 3.
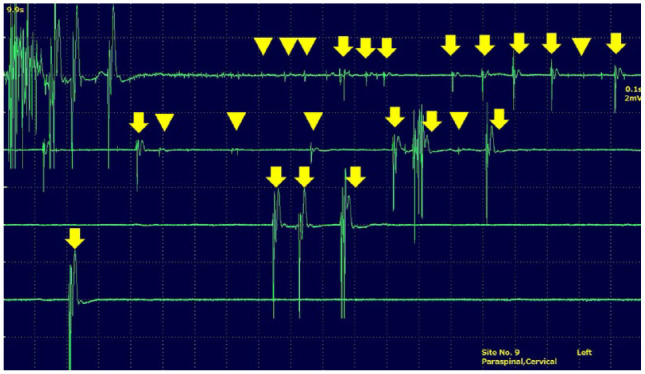
Needle electromyogram of the cervical left paraspinal muscle. Fibrillation potential (arrowheads) and fasciculation potential (arrows) are found in the muscle
To further investigate the relationship of PHT and hypokalaemic myopathy, the authors sought cats with hypokalaemic myopathy using social networking sites (Facebook) and personal emails. The authors obtained videos and clinical information of cats that fulfilled our search criteria. The inclusion criteria were as follows: a cat with hypokalaemia; myopathic signs, such as characteristic posture with cervical flexion and generalised weakness consistent with myopathy; and resolution of weakness after potassium correction. Clinical data included breed, sex, age of onset, diet at onset, complete blood count, blood chemistry and other clinical examination results. MRI, EMG and biopsy were not performed in primary care clinics.
In total, data on 14 feline cases, including case 1, were obtained; the breed, age, sex, cause of hypokalaemic myopathy, serum potassium concentration and serum creatine kinase level data are summarised in Table 1. Other blood chemistry data and haematological data on which a clinical diagnosis was based are summarised in the supplementary material. Twelve domestic shorthair cats, a Munchkin and a Scottish Fold, were included. Nine males, eight of which were castrated, and five spayed females were included in the investigation. The age of one cat was uncertain; the median age at the time of diagnosis for the remainder was 12 years (range 3–20 years).The most common cause of hypokalaemic myopathy was CKD (n = 8); other causes were severe diarrhoea (n = 2), anorexia caused by feline viral rhinotracheitis (n = 1) and suspected primary aldosteronism (n = 1); in two cats, the cause was not diagnosed. Three different instruments were used to measure serum potassium concentration (reference ranges: 2.9–4.5, 3.4–4.6 and 3.5–5.8 mmol/l, respectively) and cases with measurements bellow the lower limit of the reference range were diagnosed as hypokalaemia. The median serum potassium concentration was 2.45 mmol/l (range 1.6–3.0 mmol/l). Serum creatine kinase activity was measured in 11 cases; the median was 720 U/l (range 215–>2000 U/l). In nine cases, activity exceeded the upper reference limit of the measuring instrument. In all 14 cases, myopathic signs such as cervical flexion and generalised weakness resolved after potassium correction.
Table 1.
Data on breed, age, sex, cause of hypokalaemic myopathy, serum potassium concentration, serum creatine kinase level, degree of cervical flexion, degree of positioning head tilt and outcome of 14 feline cases
| Case | Breed | Age (years) | Sex | Diet at onset | Cause of HM | K (mmol/l) | CK (U/l) | Degree of CF | Degree of PHT | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DSH | 5 | MC | CCF | CKD | 2.4 | NE | Clearly | Clearly | PHT disappeared |
| 2 | Munchkin | 5 | MC | PD for LUTD | CKD | 2.5 | NE | Clearly | Clearly | PHT disappeared |
| 3 | DSH | 3 | MC | PD for liver disease | Unknown | 2.6 | >2000 | Clearly | Clearly | PHT disappeared |
| 4 | DSH | 14 | FS | CCF | CKD | 3 | 672 | Clearly | Clearly | PHT disappeared |
| 5 | DSH | 9 | FS | CCF | CKD | 2.4 | 267 | Clearly | Clearly | PHT disappeared |
| 6 | DSH | 16 | FS | PD for renal disease | Anorexia (FVR) | 2.1 | 839 | Clearly | Clearly | PHT disappeared |
| 7 | DSH | Unknown | M | Unknown (stray cat) | Severe diarrhoea | 1.6 | 1770 | Clearly | Clearly | PHT disappeared |
| 8 | DSH | 8 | MC | PD for allergic dermatitis | CKD | 2.2 | 720 | Clearly | Clearly | PHT disappeared |
| 9 | Scottish Fold | 12 | MC | CCF | Suspected PA | 2.9 | >2000 | Clearly | Clearly | PHT disappeared |
| 10 | DSH | 14 | MC | PD for gastrointestinal disease | Severe diarrhoea | 1.8 | NE | Mild | Mild | PHT disappeared |
| 11 | DSH | 18 | MC | CCF | CKD | 3 | 574 | Mild | Mild | PHT disappeared |
| 12 | DSH | 18 | FS | PD for renal disease | CKD | 2.8 | 215 | Mild | Mild | PHT disappeared |
| 13 | DSH | 11 | MC | PD for LUTD | Unknown | 2.8 | 966 | Mild | Mild | PHT disappeared |
| 14 | DSH | 20 | FS | Jerky, boiled chicken, cheese for cats | CKD | 2 | 536 | Mild | Mild | PHT disappeared |
CCF = commercial cat food; CF = cervical flexion; CK = serum creatine kinase level; CKD = chronic kidney disease; DSH = domestic shorthair; FS = spayed female; FVR = feline viral rhinotracheitis; HM = hypokalaemic myopathy; K = serum potassium concentration; LUTD = lower urinary tract disease; M = male; MC = castrated male; NE = not examined; PA = primary aldosteronism; PD = prescription diet; PHT = positioning head tilt
Evaluation of videos
Most of the cases did not move spontaneously at the time of diagnosis of hypokalaemic myopathy but adopted a recumbent or prone position. In these cases, videos were taken and immediately evaluated when potassium correction enabled them to support themselves on all four limbs. Cervical flexion was clear in nine cases and was mildly present in five cases. PHT was clear in nine cases and mildly present in five cases and corresponded to the observations of cervical flexion. The degree of cervical flexion and PHT and outcome after treatment are summarised in Table 1. The extent of PHT in cats with hypokalaemic myopathy is presented in Figure 1, and in Videos 1–4 in the supplementary material.
PHT after potassium correction
In all cats, PHT resolved along with other signs related to myopathy, such as cervical flexion and generalised weakness, after electrolyte correction.
Discussion
Typical clinical signs, such as cervical flexion and generalised weakness, clinical pathologic data and response to treatment indicate a diagnosis of hypokalaemic myopathy caused by multiple pathologies in the present feline cases. Myopathy of any aetiology, anorexia and non-muscle pathology may also cause creatine kinase elevation. Creatine kinase elevation is considered an indication of muscle damage but significant pathologic changes in muscle have not been reported in cats with hypokalaemic myopathy. 14 Serum creatine kinase activity in two cases did not exceed the reference range of the measuring instrument. However, elevated creatine kinase levels are not observed in all cases of hypokalaemic myopathy. 13
In all the cases reported here, PHT and other signs related to hypokalaemic myopathy resolved after recovery from hypokalaemia. No NU abnormalities (where a lesion would cause PHT1,2) were detected by MRI in case 1. The clinical outcomes in the present cases suggested that PHT was related to hypokalaemic myopathy and was reversible. Mild PHT is present in cats described in the supplementary videos of a review article on hereditary hypokalaemia in Burmese kittens (see Video 3 in Malik et al) 16 and a cat with hypokalaemic myopathy in a previous publication (see Video 5-68 in De Lahunta et al). 17 Weakness in the cervical muscles in these cats, and in cases 10–14 in the present study, is thought to be milder than that of cases 1–9. Mild PHT in cats with mild weakness and clear PHT in cats with severe weakness indicates that the sign is related to myopathy. There is no information on PHT in the two references cited above16,17 as a possible mechanism of PHT had not been postulated and the abnormal head positioning was not reported.
The mechanism of the PHT in the present feline cases is unclear. Most metabolic disorders, such as hypokalaemia, would be expected to cause generalised (and likely bilateral) abnormality. With regard to pathways related to PHT,1,2 we suggest that PHT in the present feline cases with hypokalaemic myopathy could be explained by the following hypothesis. In order to maintain the equilibrium of the head when it is moving, the vestibular nuclei contract the oblique and rectus capitis muscles bilaterally through the vestibulospinal tract based on the information of movement from the vestibular apparatus.1,2 The function of the NU is to inhibit the reflexive excitation of the vestibular nuclei and to initiate appropriate muscle contraction. In order to achieve this function, the NU needs information on how much to inhibit the excitation of the vestibular nuclei. Actual head position is detected by the proprioceptive receptor (muscle spindle) of the obliquus and rectus capitis muscles. The muscle spindles of these muscles transmit information on the changes in lengths of the cervical muscles (ie, the actual tilt of the head) to the NU through the spinocuneocerebellar tract.3–8 Thus, the degree of inhibition of the vestibular nuclei to maintain the equilibrium of the head when it is moving is determined by a comparison of information on head movements from the right and left vestibular apparatus with information on the actual head tilt from the muscle spindles of the oblique and rectus capitis muscles. Therefore, if the muscle spindles malfunction, information on the changes in cervical muscle length is not sent to the NU; consequently, the NU judges that muscle tension is insufficient and does not produce the output to inhibit the vestibular nucleus.
Muscle spindles are highly specialised stretch receptors distributed throughout the skeletal muscles. These spindle-shaped receptors are made of several small skeletal muscle fibres often referred to as intrafusal fibres. For the central nervous system to recognise sensory stimuli, sensory receptors must convert a stimulus into neural activity. 18 It has been reported that reduction and eventual disappearance of the dynamic overshoot of receptor potential occurs in isolated frog muscle spindles in a potassium-free solution.19,20 Dysfunction of muscle spindles in the obliquus and rectus capitis muscles bilaterally, as well as that of the general skeletal muscle fibres, is believed to be characteristic of hypokalaemia (ie, hypokalaemic myopathy). As a result, information on cervical muscle tension is not sent to the NU and, by the above mechanism, the heads of these cats tilt towards the opposite side of their movement. Proprioceptive system deficit owing to muscle spindle dysfunction partially consistent with this hypothesis is suggested in a large number of human muscular dystrophy patients. They have symptoms such as postural instability, sudden falls and poor manual dexterity.21–24
We considered, but rejected, other possible causes for PHT in the present cases. Cerebellar abnormalities, including malformation, degenerative diseases, encephalitis and tumours affecting the NU, were ruled out because of the acute onset of PHT and its disappearance after potassium correction. Cerebellovascular diseases were excluded because there were no other cerebellar signs seen. Cerebellar trauma was ruled out based on the clinical history of the cats. There were no cases of suspected diet-induced thiamine deficiency, although one cat had an unknown dietary history, and all patients improved without thiamine supplementation. Although uremic polyneuropathy in humans is described in the literature, haemodialysis and transplantation are required to reduce the prevalence and severity of peripheral neuropathy in these patients. 25 Therefore, uremic neuropathy was excluded.
Although the anaemia, hypokalaemia, megacolon and diarrhoea observed in the present cases have not been reported to cause cerebellar disorders or neuropathy in humans and animals, we cannot rule out that NU dysfunction or a bilateral spinocuneocerebellar dysfunction caused by an unknown mechanism related to them could be present in the current cases.
The limitations of this study include the small number of cases analysed and the lack of muscle pathological evaluation, including that of intrafusal fibres. A detailed observation of the clinical signs and course in a larger number of cases with similar characteristics to those of the present study is warranted. However, it may be difficult to detect pathological changes in muscle biopsy specimens. Previous studies identified no abnormalities or only mild abnormalities in the muscles of cats with hypokalaemic myopathy.3,4,14 In addition, it is ethically problematic to perform an invasive biopsy requiring anaesthesia on cases diagnosed with hypokalaemic myopathy based upon signalment, historical and clinical findings and resolution of clinical signs with potassium supplementation to validate the conclusions of this study. The possibility cannot be ruled out that weakness of the cervical muscles alone may be the cause of the inability to maintain head equilibrium when the head is turned to the side. Behavioural posturing of the head to increase the visual field of view can also cause head tilt in cats with weakness.
The mechanism of weakness/myopathy in association with hypokalaemia is not yet fully understood. It is unclear whether PHT would be expected to be a clinical feature of other cases of generalised myopathy. Proprioceptive system deficit owing to muscle spindle dysfunction is suggested in human muscular dystrophy patients.21–24 This suggests that PHT may also be seen in dogs and cats with muscular dystrophy. To verify whether PHT is also observed in other muscle diseases, such as muscular dystrophy and myasthenia gravis, more specific observations are warranted.
Conclusions
This is the fourth study describing this clinical neurological abnormality and there is still very limited published information about this neurological sign. Although PHT has been reported to reflect lesions localised in the NU, hypokalaemic myopathy was suspected as the cause of PHT in the present feline cases. The recognition of these clinical signs and the proposed mechanism for PHT may alert veterinary clinicians to the presence of hypokalaemic myopathy in cats, as well as help neurophysiologists acquire further understanding of the mechanism of equilibration of the head. Further observations and investigation may help in determining more precisely the mechanism for PHT in cats with hypokalaemic myopathy.
Supplemental Material
Summary of blood chemistry data and haematological data
Acknowledgments
The authors thank the following for referring the clinical cases: Naomichi Tamaru, DVM, Ciao Animal Clinic (case 2); Ryo Ogusu, Narita Animal Hospital (case 3); Yuichi Nonaka, DVM, Nonaka Animal Hospital (case 4); Yuki Shoji, DVM, Sanyo Animal Medical Center (cases 5 and 6); Hiroshi Tanaka, DVM, PhD, Nakayama Veterinary Hospital (case 7); Takahisa Hasegawa, DVM, Misasa Animal Hospital (cases 8 and 13); Hiroyuki Yamasaki, DVM, Yamasaki Pet Clinic (case 9); Kazuhiro Itamoto, DVM, Itamoto Animal Hospital (case 10); Junya Sugahara, DVM, Sugahara Veterinary Hospital (case 11); Toshiyuki Suzuki, DVM, Suzuki Animal Hospital (case 12); and Naotami Ueoka, DVM, PhD, Ueoka Animal Hospital (case 14).
Footnotes
Accepted: 27 April 2023
Supplementary material: The following files are available online:
Video 1: Case 1.
Video 2: Case 7.
Video 3: Case 10.
Video 4: Case 11.
File 1: Summary of blood chemistry data and haematological data.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognised high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). For any animals or people individually identifiable within this publication, informed consent (either verbal or written) for their use in the publication was obtained from the people involved.
ORCID iD: Shinji Tamura  https://orcid.org/0000-0002-1005-2598
https://orcid.org/0000-0002-1005-2598
References
- 1. Tamura S, Nakamoto Y, Uemura T, et al. Head tilting elicited by head turning in three dogs with hypoplastic cerebellar nodulus and ventral uvula. Front Vet Sci 2016; 3. DOI: 10.3389/fvets.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tamura S. Commentary: transient postural vestibulo-cerebellar syndrome in three dogs with presumed cerebellar hypoplasia. Front Vet Sci 2021; 8. DOI: 10.3389/fvets.2021.613521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders SG. Cerebellar diseases and tremor syndromes. In: Dewey CW, DaCosta RC. (eds). Practical guide to canine and feline neurology. 3rd ed. Ames, IA: Wiley Blackwell, 2016, pp 299–327. [Google Scholar]
- 4. de Lahunta A, Glass E, Kent M. Cerebellum. In: de Lahunta A, Glass E, Kent M (eds). Veterinary neuroanatomy and clinical neurology. 4th ed. St. Louis, MO: Elsevier, 2015, pp 368–408. [Google Scholar]
- 5. Uemura EE. Cerebellum. In: Uemura EE. (ed). Fundamentals of canine neuroanatomy and neurophysiology. Ames, IA: Wiley Blackwell, 2015, pp 288–306. [Google Scholar]
- 6. Thomson C, Hahn C. The cerebellum. In: Thomson C, Hahn C. (eds). Veterinary neuroanatomy: a clinical approach. St Louis, MO: Elsevier, 2012, pp 67–74. [Google Scholar]
- 7. Thomson C, Hahn C. Vestibular system. In: Thomson C, Hahn C (eds). Veterinary neuroanatomy: a clinical approach. St Louis, MO: Elsevier, 2012, pp 75–83. [Google Scholar]
- 8. Thomson C, Hahn C. Reflexes and motor systems. In: Thomson C, Hahn C (eds). Veterinary neuroanatomy: a clinical approach. St Louis, MO: Elsevier, 2012, pp 47–57. [Google Scholar]
- 9. Tamura S, Tamura Y, Nakamoto Y, et al. Positioning head tilt in canine lysosomal storage disease: a retrospective observational descriptive study. Front Vet Sci 2021; 8. DOI: 10.3389/fvets.2021.802668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liatis T, Hammond D, Chapman GE, et al. MRI findings in a young dog with gliomatosis cerebri. J Small Anim Pract 2022; 63: 83. DOI: 10.1111/jsap.13394. [DOI] [PubMed] [Google Scholar]
- 11. Tamura S. Was the ‘alternating head tilt’ a ‘positioning head tilt’? J Small Anim Pract 2022; 63: 84. DOI: 10.1111/jsap.13447. [DOI] [PubMed] [Google Scholar]
- 12. Liatis T, Gutierrez-Quintana R. Response to: Was the ‘alternating head tilt’ a ‘positioning head tilt’? J Small Anim Pract 2022; 63: 85. DOI: 10.1111/jsap.13427. [DOI] [PubMed] [Google Scholar]
- 13. Dewey C, Talarico LR. Myopathies: disorders of skeletal muscle. In: Dewey CW, DaCosta RC. (eds). Practical guide to canine and feline neurology. 3rd ed. Ames, IA: Wiley Blackwell, 2016, pp 481–520. [Google Scholar]
- 14. Dow SW, LeCouteur RA, Fettman MJ, et al. Potassium depletion in cats: hypokalemic polymyopathy. J Am Vet Med Assoc 1987; 191: 1563–1568. [PubMed] [Google Scholar]
- 15. Gandolfi B, Gruffydd-Jones TJ, Malik R, et al. First WNK4-hypokalemia animal model identified by genome-wide association in Burmese cats. PLoS One 2012; 7. DOI: 10.1371/journal.pone.0053173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malik R, Musca FJ, Gunew MN, et al. Periodic hypokalemic polymyopathy in Burmese and closely related cats: a review including the latest genetic data. J Feline Med Surg 2015; 17: 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Lahunta A, Glass E, Kent M. Lower motor neuron: spinal nerve, general somatic efferent system. In: De Lahunta A, Glass E, Kent M. (eds). Veterinary neuroanatomy and clinical neurology. 4th ed. St Louis, MO: Elsevier, 2015, pp 102–161. [Google Scholar]
- 18. Uemura EE. Somatosensory system. In: Uemura EE. (ed). Fundamentals of canine neuroanatomy and neurophysiology. Ames, IA: Wiley Blackwell, 2015, pp 128–155. [Google Scholar]
- 19. Husmark I, Ottoson D. Ionic effects on spindle adaptation. J Physiol 1971; 218: 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Husmark I, Ottoson D. Impulse activity of the isolated spindle in potassium-free solution. Acta Phys Scand 1971; 83: 486–494. [DOI] [PubMed] [Google Scholar]
- 21. Hus JD, Furumasu J. Gait and posture changes in the Duchenne muscular dystrophy child. Clin Orthopaed Rel Res 1993; 288: 122–125. [PubMed] [Google Scholar]
- 22. Mahjneh I, Marconi G, Bushby K, et al. Dysferlinopathy (LGMD2B): a 23-year follow-up study of 10 patients homozygous for the same frameshifting dysferlin mutation. Meuromuscul Disord 2001; 11: 20–26. [DOI] [PubMed] [Google Scholar]
- 23. Pradhan S, Ghosh D, Srivastava NK, et al. Prednisolone in Duchenne muscular dystrophy with imminent loss of ambulation. J Neurol 2006; 253: 1309–1316. [DOI] [PubMed] [Google Scholar]
- 24. Troise D, Yoneyama S, Resende MB, et al. The influence of visual and tactile perception on hand control in children with Duchenne muscular dystrophy. Dev Med Child Neurol 2014; 56: 882–887. [DOI] [PubMed] [Google Scholar]
- 25. Said G. Uremic neuropathy. In: Said G, Krarup C. (eds). Handbook of clinical neurology, vol 115. St Louis, MO: Elsevier, 2013, pp 607–612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of blood chemistry data and haematological data


