Abstract
Objectives
The objective of the study was to compare renal functional biomarkers in cats and in caudal stomatitis (CS) and in age-matched control cats.
Methods
A cross-sectional, case-control study was conducted on 44 client-owned cats with CS that were prospectively enrolled and evaluated for a Comprehensive Oral Health Assessment and Treatment at one of four institutions. Renal function was assessed with measurement of serum creatinine, urea nitrogen, serum symmetric dimethylarginine, urinalysis, urine protein:creatinine ratio and urine protein electrophoresis. Affected gingiva was biopsied to confirm the diagnosis of stomatitis. Renal biochemical analyses from the experimental group were compared with those of 44 age-matched controls without CS enrolled prospectively or retrospectively after presenting to the primary institution for routine healthcare. Control cats were included if they were clinically stable, their chronic illnesses were well managed and minimal dental disease was present on examination. Renal biomarkers were compared between groups using a t-test or the Mann–Whitney U-test. Frequency of azotemia, proteinuria and the clinical diagnosis of renal disease were compared using Fisher’s exact test.
Results
Relative to the control group, cats in the CS group had significantly lower serum creatinine (P <0.001) and albumin concentrations (P <0.001), urine specific gravity (P = 0.024) and hematocrit (P = 0.003), and higher serum phosphorus (P <0.001), potassium (P <0.001) and globulin concentrations (P <0.001), white blood cell count (P <0.001) and urine protein:creatinine ratio (P = 0.009). There were no significant differences in serum symmetric dimethylarginine or urea nitrogen concentrations. No clinically significant findings were noted on urine protein electrophoresis. There were no significant differences in the frequency of azotemia, proteinuria or renal disease categories between the two groups.
Conclusions and relevance
The present study does not demonstrate a significant difference in the frequency of kidney disease between cats with and without CS. Longitudinal evaluation is warranted to investigate the relationship between renal disease and CS.
Keywords: Caudal stomatitis, chronic kidney disease, renal dysfunction, stomatitis
Introduction
Feline chronic gingivostomatitis is an inflammatory syndrome affecting the oral cavity of cats. This disease is also referred to as caudal stomatitis (CS) when occurring primarily in the caudal aspect of the oral cavity, along with a variety of other interchangeable names. 1 This is a painful disease process that can be debilitating, affecting 0.7–13.5% of cats worldwide. 1 This disease is characterized by severe inflammation of the gingiva, mucosa and/or the caudal oral mucosa. 2 Various portions of the mucosa in the oral cavity can be affected, including the palatoglossal folds, pharynx, soft palate, hard palate and tongue. 1 When alveolar and buccal mucosa are affected, the disease is defined as alveolar or rostral stomatitis, while caudal stomatitis occurs in the caudal oropharynx lateral to the palatoglossal folds. 2 Because of the varied locations in which this disease can manifest, clinical signs are varied and can include decreased appetite, dysphagia, generalized oral discomfort, apparent dental disease (with or without tooth resorption), becoming ‘head shy’, vomiting and even dysphonia.
The etiology of CS and its varieties remains uncertain but is likely multifactorial. A variety of factors have been described, including infectious (particularly feline calicivirus, feline immunodeficiency virus, feline herpesvirus and a generalized overreaction of the immune system to anaerobic bacterial species colonizing the teeth), immunological (suppression of the immune system due to other disease processes or immune complex disease), dental disease as a whole, as well as genetic and breed predispositions. 1
Another disease commonly affecting cats is chronic kidney disease (CKD), reported to affect 30–40% of domestic cats aged >10 years. 3 In dogs and people, there have been positive correlations between the development of dental disease and bacteremia as well as diseases at distant sites (particularly cardiac disease, thromboembolic disease and stroke, atherosclerosis, hepatic disease, renal disease and low birth weight).4–12 A study by Pavlica et al, performed in 2008, showed a significant correlation between chronically infected periodontal lesions and the level of morphologic alterations in the internal organs of small-breed dogs. 13 Infectious and inflammatory diseases, including periodontal disease, resulting in chronic low-grade bacteremia have also been associated with deposition of immune complexes in the kidneys of dogs, causing glomerulonephritis. 14
The correlation between renal pathology and periodontal disease in dogs suggests that periodontal disease may contribute to the development of chronic disease in general as a result of persistent low-grade insults. 13 Specifically, it has been reported that for each square centimeter of periodontal disease in dogs, there is a 1.4-fold greater odds of kidney pathology. 13 Common mechanisms by which dental disease may cause kidney injury include production of pro-inflammatory cytokines, endotoxemia and individual immune responses to bacteria. CS is a chronic inflammatory condition that may expose the kidneys of affected cats to many of these factors.
Little is known, however, about the systemic implications of periodontal disease in domestic cats. One study showed a correlation between systemic health indices and the severity of periodontitis in cats, 15 but no such evaluation has yet been performed regarding cats with CS specifically, a disease commonly seen in the general practice setting and affecting up to 12% of the feline population. 16
Although the above-described associations have been made between periodontal disease and CKD in cats, to the authors’ knowledge, no study has previously sought to report the relationship between clinicopathologic evidence of renal disease and feline CS specifically. The aim of the present cross-sectional study was to describe renal biomarker concentrations and the frequency of evidence of renal disease in cats with CS, relative to age-matched cats without CS. The authors hypothesized that cats with CS would have elevated renal biomarker concentrations and a higher frequency of evidence of renal disease compared with the control group.
Materials and methods
Animals
This was a cross-sectional study of client-owned cats. A total of 44 cats with clinical signs of CS presented to referral practices (the University of Georgia Pet Health Center, Washington State College of Veterinary Medicine, Atlanta Veterinary Dental Services and the University of Florida College of Veterinary Medicine) for Comprehensive Oral Health Assessment and Treatment (COHAT) procedures were prospectively enrolled. At the time of the COHAT procedure, comprehensive pre-anesthetic laboratory work was performed (complete blood count [CBC], biochemistry panel, serum thyroxine concentration [total T4], urinalysis and urine protein:creatinine ratio [UPC]). To investigate for evidence of glomerular disease, urine protein electrophoresis was performed in a subset of cats with CS if the level of protein in the urine exceeded IDEXX laboratory’s threshold for urine protein.
An age-matched control group was selected from cats presenting to the primary institution, the University of Georgia Community Practice Clinic, for wellness visits. Data from control cats were collected prospectively (n = 20) or retrospectively (n = 24) through review of medical records of cats presented for wellness examination in the preceding 5 years.
Comprehensive laboratory work, including CBC, biochemistry panel, total T4 if aged >7 years, urinalysis and UPC, was performed on each potential control cat enrolled prospectively. Available demographic and clinicopathologic data were collected from the medical records of cats enrolled retrospectively.
Inclusion criteria
In the experimental group, cats with CS were included if the diagnosis was histologically confirmed via a gingival biopsy sample taken during the COHAT procedure.
In the control group, control cats were considered age-matched if the difference in age was ⩽1 year (using the CS cat as reference). Cats were included if they were systemically clinically stable on physical examination with little to no dental disease noted on thorough oral examination. Any previously diagnosed chronic conditions must have been stable with current medical therapy. Cats with hyperthyroidism were excluded, given the effect of this condition on glomerular filtration rate (GFR). Control cats with data collected retrospectively were included if at least all of the following were known: serum creatinine; urea nitrogen (SUN); and urine specific gravity (USG).
Clinicopathologic analyses
Renal function was assessed with serum creatinine, SUN, symmetric dimethylarginine (SDMA) concentration, USG and, when available, UPC. Clinicopathologic analyses were performed by IDEXX Laboratories or the University of Georgia Clinical Pathology Laboratory. Urine electrophoresis was performed by IDEXX Laboratories.
Categorization of renal function
Cats were sorted into categories based on the presence and presumed cause of azotemia, presence and magnitude of proteinuria, and clinicopathologic evidence of renal disease. Regarding azotemia, cats were categorized as non-azotemic (serum creatinine concentration <1.6 mg/dl, SDMA ⩽14 μg/dl and SUN below the laboratory’s upper end of the reference interval [RI]) or having prerenal azotemia (serum creatinine concentration ⩾1.6 mg/dl, SDMA >14 μg/dl, SUN above the upper end of the laboratory’s RI or a combination of these, with a concurrent USG ⩾1.035) or renal azotemia (serum creatinine concentration ⩾1.6 mg/dl, SDMA >14 μg/dl, SUN above the upper end of the laboratory’s RI or a combination of these, with a concurrent USG <1.035). Proteinuria was categorized as non-proteinuria (UPC <0.2), borderline proteinuria (UPC 0.2–0.4) or proteinuria (UPC >0.4), according to the parameters set forth by the International Renal Interest Society (IRIS). 17
Cats were also further sorted into three categories according to the presence or absence of evidence suggesting a diagnosis of kidney disease: ‘no kidney disease detectable’; ‘kidney disease not confirmed or ruled out’; and ‘kidney disease’. Cats were categorized as having ‘no detectable kidney disease’ if they were non-azotemic (as defined above) and all available clinicopathologic parameters reflective of renal function were normal. Cats were classified as ‘kidney disease not confirmed or ruled out’ if they were non-azotemic but had USG <1.035, UPC ⩾0.2 or both. Because a subset of cats with documented kidney disease maintains urinary concentrating ability,18–21 cats with prerenal azotemia were also included in the ‘kidney disease not confirmed or ruled out’ category. Cats were assigned to the ‘kidney disease’ category if they had renal azotemia, as defined above. Given that samples were collected only at a single time point, the chronicity and persistence of azotemia, proteinuria or kidney disease could not be determined.
Histopathologic analysis
Gingival tissues were biopsied at the time of the COHAT procedure for the experimental group and evaluated by each institution’s pathology service to definitively diagnose CS as the cause of gingival inflammation.
Statistics
The distribution of data for continuous variables was assessed for normality by visual examination of histograms and the Shapiro–Wilk test. Normally distributed data are presented as mean ± SD and compared between groups using the two-sample, unequal variance t-test. Non-normally distributed data are presented as median (range) and compared between groups using the Mann–Whitney U-test. Clinicopathologic variables compared between groups included hematocrit, white blood cell count, serum albumin, globulins, creatinine, SUN, potassium, phosphorus and SDMA concentrations, USG and UPC. For statistical analyses, USG values reported as >1.050 or >1.060, and UPC values reported as <0.03 were assigned a value of 1.051 or 1.061, and 0.029, respectively. Categorical data, such as azotemia, proteinuria and evidence of renal disease category, were compared using Fisher’s exact test.
Results
The 44 cats included in the CS group were all confirmed to have CS via histopathologic diagnosis. In all cases, inflammation was characterized as severe lympho-plasmacytic, chronic gingivitis, which is the hallmark of stomatitis in cats. 2 Most cases also showed varying levels of neutrophilic inflammation containing superficial mixed populations of bacteria.
Of the 44 cases with CS, 29 (65.9%) cats were male (all castrated) and 15 (34.1%) were female (all spayed). The median age at presentation was 4 years (mean age 5.5 years; age range 1–16 years). Of the 44 control cats, 28 (63.4%) were male (all castrated) and 16 (36.4%) were female (all spayed). The median age at presentation was 5 years (mean age 6.2 years; age range 1.2–16.5 years).
Clinicopathologic data
Serum biochemical analyses, including SUN and creatinine concentrations, were available for all cats. Serum SDMA concentrations were measured for all cats in the CS group and 24/44 control cats.
Two cats in the CS group and one prospectively enrolled cat in the control group did not produce a urine sample during their study visit and, therefore, did not have a urinalysis or UPC performed. In total, 15 CS and 21 control group cats did not have a UPC performed. Of these, seven CS and three control cats had negative protein on the urine dipstick.
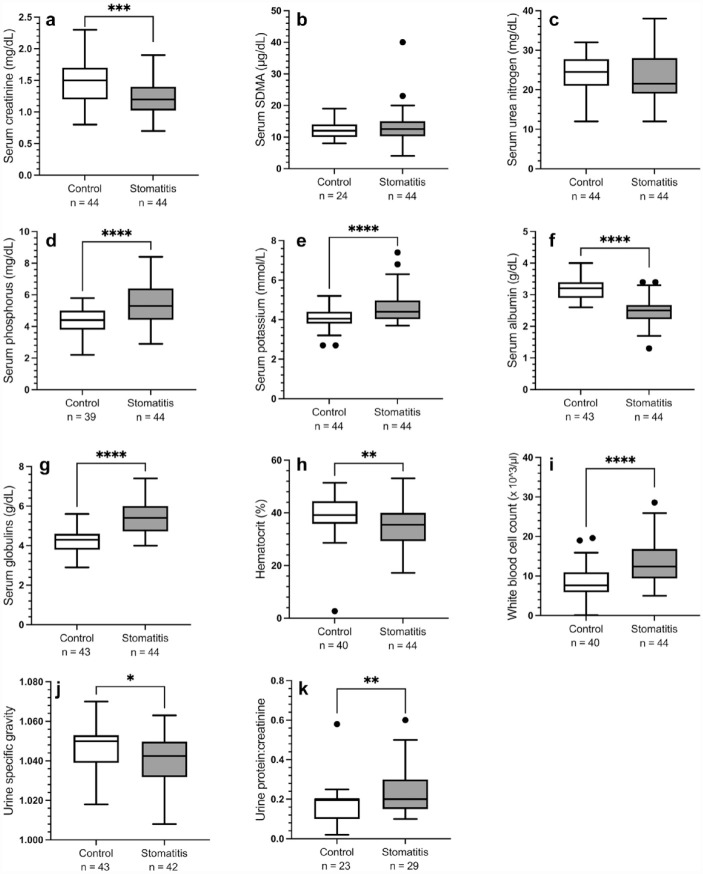
Relative to the control group, cats in the CS group had significantly lower median serum creatinine (P = 0.001) and albumin (P <0.001) concentrations, USG (P = 0.024) and hematocrit (P = 0.003), and higher serum phosphorus (P <0.001), potassium (P <0.001) and globulin (P <0.001) concentrations, white blood cell count (P <0.001) and UPC (P = 0.009) (Figure 1). There were no significant differences in median concentrations of serum SDMA or SUN.
Figure 1.
Box plot of (a) serum creatinine, (b) symmetric dimethylarginine (SDMA), (c) urea nitrogen, (d) phosphorus, (e) potassium, (f) albumin and (g) globulin concentrations, (h) hematocrit, (i) white blood cell count, (j) urine specific gravity and (k) urine protein:creatinine ratio in cats with caudal stomatitis (n = 44) and in age-matched cats without stomatitis (controls; n = 44). The number of cats for which data are available is noted on the x-axis. Boxes represent the interquartile range, and the horizontal bar within each box represents the median. Upper and lower bars and outliers (closed circles) are plotted using Tukey’s method. **P <0.01; ***P <0.001; ****P <0.0001
Urine protein electrophoresis was performed in 30/44 (68.2%) cats in the CS group and none of the control group. Of these 30 cats, 16 had a UPC ⩾0.2. In the cats in which protein electrophoresis was performed, there were no clinically significant findings, and, for financial reasons, routine submission of urine protein electrophoresis was not continued.
Categorization of renal function
There were no significant differences in the frequency of azotemia, proteinuria and evidence of renal disease between cats with CS and age-matched control cats (Table 1). Most cats in both groups were non-azotemic. For the azotemic cats, elevation in a single biomarker of GFR was most common, with only one cat in each group showing concurrent serum creatinine and SDMA concentrations >1.6 mg/dl and 14 μg/dl, respectively (Table 2). A majority of cats in either group were assigned to the ‘kidney disease not confirmed or ruled out’ category, many of which showed prerenal azotemia, borderline proteinuria or both (Table 3).
Table 1.
Frequency of azotemia, proteinuria and evidence of renal disease in cats with CS (n = 44) and age-matched cats without stomatitis (control group; n = 44)
| Control group | CS group | P value | ||
|---|---|---|---|---|
| Azotemia | Non-azotemic | 25 (56.8) | 25 (56.8) | 0.76 |
| Prerenal | 14 (31.8) | 11 (25.0) | ||
| Renal | 5 (11.4) | 7 (15.9) | ||
| Azotemic but no USG performed | 0 (0) | 1 (2.3) | ||
| Proteinuria | Non-proteinuric | 11 (25.0) | 7 (15.9) | 0.17 |
| Borderline proteinuric | 11 (25.0) | 18 (40.9) | ||
| Proteinuric | 1 (2.3) | 4 (9.1) | ||
| UPC not performed | 21 (47.7) | 15 (34.1) | ||
| Kidney disease | No kidney disease detectable | 16 (36.4) | 9 (20.5) | 0.26 |
| Kidney disease not confirmed or ruled out | 23 (52.3) | 28 (63.6) | ||
| Kidney disease | 5 (11.4) | 7 (15.9) |
Data are n (%) unless otherwise indicated
CS = caudal stomatitis; UPC = urine protein:creatinine ratio; USG = urine specific gravity
Table 2.
Information used to assign cats with CS and age-matched cats without CS to azotemia categories
| Group | Biomarker elevated | Azotemia category | ||
|---|---|---|---|---|
| Prerenal (USG ⩾1.035) | Renal (USG <1.035) | Azotemic but no USG performed | ||
| Control | Creatinine alone (n) | 13 | 3 | 0 |
| Creatinine and SDMA (n) | 1 | 0 | 0 | |
| SDMA alone (n) | 0 | 2 | 0 | |
| Stomatitis | Creatinine alone (n) | 4 | 1 | 0 |
| Creatinine and SDMA (n) | 0 | 1 | 0 | |
| SDMA alone (n) | 6 | 5 | 1 | |
| SUN alone (n) | 1 | 0 | 0 | |
CS = caudal stomatitis; SDMA = symmetric dimethylarginine; SUN = serum urea nitrogen; USG = urine specific gravity
Table 3.
Information used to assign cats with CS and age-matched cats without CS to the ‘kidney disease not confirmed or ruled out’ category
| Group | Clinicopathologic data | n |
|---|---|---|
| Control | Prerenal creatinine elevation | 11 |
| Prerenal creatinine and SDMA elevation | 1 | |
| Prerenal creatinine elevation and borderline proteinuria | 1 | |
| Prerenal creatinine elevation and proteinuria | 1 | |
| Borderline proteinuria | 7 | |
| Borderline proteinuria and USG <1.035 | 1 | |
| USG <1.035 | 1 | |
| Total | 23 | |
| Stomatitis | Prerenal creatinine elevation | 3 |
| Prerenal SDMA elevation | 3 | |
| Prerenal SUN elevation | 1 | |
| SDMA elevation (no USG performed) | 1 | |
| Prerenal creatinine elevation and borderline proteinuria | 1 | |
| Prerenal SDMA elevation and borderline proteinuria | 3 | |
| Borderline proteinuria | 11 | |
| Borderline proteinuria and USG <1.035 | 1 | |
| Proteinuria | 1 | |
| USG <1.035 | 3 | |
| Total | 28 |
CS = caudal stomatitis; SDMA = symmetric dimethylarginine; SUN = serum urea nitrogen; UPC = urine protein:creatinine ratio; USG = urine specific gravity
Discussion
The aim of the present study was to evaluate whether the frequency of detectable renal disease was higher in cats with CS than in those without. The hypothesis stated that cats with CS would have elevated renal biomarker concentrations and a higher frequency of evidence of renal insufficiency compared with the control group. The authors were unable to document a difference in evidence of renal disease between these two groups, and therefore failed to accept this hypothesis.
Contrary to our hypothesis, the median serum creatinine concentration was significantly higher in the control group relative to the CS group. Although the exact reasons for this difference cannot be known, the authors offer several possible explanations for this finding. Although the frequency of prerenal azotemia was not different between groups, it is possible that subclinical dehydration contributed to the increase in serum creatinine concentration in the control group, as median hematocrit and serum albumin concentration were also found to be higher in this group. Given that cats with CS are more likely to be eating moist/canned cat food or broths due to oral pain, it stands to reason that they would have an increased water intake and, therefore, less pronounced pre-renal factors affecting GFR. Cats in the control group also likely had higher muscle mass due to a more consistent diet and lack of chronic illnesses affecting their ability to eat, resulting in a higher creatinine concentration. 22 Along these same lines, cats with CS may have been consuming fewer calories due to oral pain, causing muscle wasting secondary to this disease. Although SDMA was not consistently evaluated in all control cats, there was no difference in median SDMA concentration between the groups, suggesting that muscle mass might have indeed been a factor in the serum creatinine results observed.23,24 Nonetheless, the cats’ muscle condition scores and hydration status were not consistently recorded during their examinations, and clinical evaluation of dehydration is subjective and insensitive, so neither explanation can be further assessed.
In addition to potential changes in hydration status, the lower albumin in the CS group may also reflect changes related to inflammation as a negative acute phase protein. 25 Cats in the CS group had a significantly higher mean concentration of serum globulins. Elevated globulin concentration is a hallmark of inflammation in nearly all veterinary species and has been previously associated with chronic inflammatory conditions, including CS. 26 The CS group also was noted to have a higher white blood cell count than the control group, further suggesting a systemic inflammatory immune response. Similarly, cats with CS might have a lower hematocrit due to chronic inflammatory disease. 27
Serum phosphorus and potassium concentrations in the CS cats were significantly higher when compared with control cats, although it should be noted that most of these values were still within the normal reference range. There were two cats in the CS group with elevated phosphorus levels (8.4 and 7.6 mg/dl; IDEXX RI 2.9–6.3 mg/dl) and three cats in the CS group with elevated potassium levels (7.4, 5.9 and 6.8 mmol/l; IDEXX RI 3.7–5.2 mmol/l), which were not associated with azotemia in these cats.
Overall, there was no difference in the frequency of proteinuria between the CS and control groups. However, borderline proteinuria was a frequent reason for cats to be classified as ‘kidney disease not confirmed or ruled out’. Despite the lack of difference regarding proteinuria categories between groups, the median UPC was significantly higher in the CS group. Although systemic inflammation and, specifically, periodontitis may cause glomerulonephritis, 28 urine protein electrophoresis was normal in all cats for which it was evaluated, 14 of which were non-proteinuric.
Cats in the present study were assigned to categories to describe their overall level of renal dysfunction, and the frequency of renal disease was similar between cats with and without CS. Prior research has shown an increased prevalence of kidney dysfunction in other species with dental disease overall. 13 The evaluation of chronicity, and therefore a diagnosis of CKD, requires documentation of renal azotemia across multiple time points or renal imaging or histopathology documenting chronic changes. Because the present study compared only one time point between the two groups, and imaging studies were not performed, persistence and chronicity could not be evaluated, which is a main limitation of the present study. Although not statistically significant, a numerically higher number of cats in the CS group were assigned a diagnosis of ‘kidney disease’ or ‘kidney disease not confirmed or ruled out’ or were ‘borderline proteinuric’. Because of the cross-sectional design of the present study and the limited information available for some cats, it cannot be determined how many cats in the ‘kidney disease not confirmed or ruled out’ category might have had normal kidney function had they undergone repeated and more complete clinicopathologic testing. For example, USG <1.035 might be a normal finding in a euhydrated cat, borderline proteinuria might be transient, and mild elevation in serum markers of GFR with concurrent concentrated urine is often due to true prerenal azotemia versus renal disease with retained concentrating ability. On the other hand, it is possible that if tracked over time, cats with ‘kidney disease not confirmed or ruled out’ or those that were ‘borderline proteinuric’ would have been shown to have or develop CKD. Although the present study did not show a significantly higher frequency of renal insufficiency and CS, it may lay the groundwork for future research on this and broader subjects.
There are several limitations to this study. As mentioned above, this was a cross-sectional study, and the diagnosis of CKD could not be established. In addition, the kidney disease and azotemia categories were largely based on criteria for the staging of CKD set forth by IRIS, 17 which are intended to be applied to cats for which a diagnosis of CKD has been established. Based on the IRIS staging scheme, a serum creatinine concentration of 1.6 mg/dl was used to define azotemia. This value is within the RI of several laboratories, including those used in the present study, and might have promoted a larger number of cats to be assigned to an azotemic category. CS is a disease affecting cats of all ages, 1 although our particular experimental group is skewed toward younger cats, with 34 of the 44 cats aged <10 years. As such, the prevalence of renal disease may have been low at that life stage and might increase more rapidly over time in that group. Also, as cases were recruited at different institutions and a subset of controls was enrolled retrospectively, a few clinicopathologic data points were occasionally missing. For example, a CBC and SDMA was not performed in all control cats. Further, a sample size of 44 cats in each group may not provide enough power to detect significant differences in the frequency of azotemia, renal disease or proteinuria categories, when those are overall modest.
CKD remains the most commonly diagnosed metabolic disease of domesticated cats, with estimates of overall prevalence in the range of 1–3% 29 to 50%. 30 There are many studies completed and ongoing evaluating possible risk factors for the development of CKD in the domestic cat population. Several associations have been identified for the development of CKD in domestic cats (lower body weight, lower frequency of veterinary visits, history of moderate to severe periodontal disease, previous diagnosis of hypertension, cystitis, living anywhere in the contiguous USA except the northeast, higher frequency of vaccination), but very few of these parameters have demonstrated causality over time, and exploration into the pathophysiological mechanism of renal injury for these parameters is ongoing.29,31,32 Therefore, it would be of interest in future research to re-evaluate renal biomarkers in cats with CS and other types of dental disease in a longitudinal manner. The goal of this type of study would be to determine whether cats with untreated periodontal disease are more prone to the development of CKD later in life, or whether they develop this condition earlier than cats with either no dental disease or with dental disease which is well managed/routinely addressed. Within this study model, the question of whether certain types of dental disease (ie, periodontitis, tooth resorption, CS) are more likely to lead to renal damage than others could be investigated. This research would not only provide more information as to the possible causation of CKD but would also arm veterinarians with evidence to keep their clients more informed and feline patients healthier for longer periods of their life.
Conclusions
Although several significant differences were noted between the groups for the aforementioned parameters, the present study does not demonstrate a significant difference in the frequency of kidney disease between cats with and without CS. Given the limitations discussed above, prospective, longitudinal research is needed to determine whether cats with CS and other dental diseases are at higher risk of developing renal damage later in life.
Footnotes
Accepted: 17 May 2023
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by a grant from the University of Georgia Small Animal Medicine and Surgery Department (grant number CR-522).
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognized high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: C Autumn M Vetter  https://orcid.org/0000-0002-8543-0258
https://orcid.org/0000-0002-8543-0258
Bianca N Lourenço  https://orcid.org/0000-0001-5249-4645
https://orcid.org/0000-0001-5249-4645
References
- 1. Healey KA, Dawson S, Burrow R, et al. Prevalence of feline chronic gingivo-stomatitis in first opinion veterinary practice. J Feline Med Surg 2007; 9: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greenfield B. Chronic feline gingivostomatitis: proven therapeutic approaches & new treatment options. Today’s Veterinary Practice. https://todaysveterinarypractice.com/dentistry/chronic-feline-gingivostamatitis-proven-therapeutic-approaches-new-treatment-optionsce-article/ (2016, accessed 1 June 2021).
- 3. Brown CA, Elliott J, Schmiedt CW, et al. Chronic kidney disease in aged cats: clinical features, morphology, and proposed pathogeneses. Vet Pathol 2016; 53: 309–326. [DOI] [PubMed] [Google Scholar]
- 4. O’Reilly P, Claffey NM. A history of oral sepsis as a cause of disease. Periodontol 2000; 23: 13–18. [DOI] [PubMed] [Google Scholar]
- 5. Joshipura K, Rimm EB, Douglass CW, et al. Poor oral health and coronary heart disease. J Dent Res 1996; 75: 1631–1636. [DOI] [PubMed] [Google Scholar]
- 6. Debowes L, Mosier D, Logan E, et al. Association of periodontal disease and histologic lesions in multiple organs from 45 dogs. J Vet Dent 1996; 12: 57–60. [PubMed] [Google Scholar]
- 7. Lund E, Armstrong PJ, Kirk CA, et al. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc 1999; 214: 1336–1341. [PubMed] [Google Scholar]
- 8. Mehta J, Saldeen T, Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll Cardiol 1998; 31: 1217–1225. [DOI] [PubMed] [Google Scholar]
- 9. Haraszthy V, Zambon JJ, Trevisan M, et al. Identification of periodontal pathogens in atheromatous plaques. J Periodontol 2000; 71: 1554–1560. [DOI] [PubMed] [Google Scholar]
- 10. DeStefano F, Anda RF, Kahn HS, et al. Dental disease and risk of coronary heart disease and mortality. BMJ 1993; 306: 688–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beck D, Slade GD, Offenbacher S. Oral disease, cardiovascular disease and systemic inflammation. Periodontol 2000; 23: 110–120. [DOI] [PubMed] [Google Scholar]
- 12. Renvert S, Wirkstrom M, Mugrabi M, et al. Histological and microbiological aspects of ligature-induced periodontitis in beagle dogs. J Clin Periodontol 1996; 23: 310–319. [DOI] [PubMed] [Google Scholar]
- 13. Pavlica Z, Petelin M, Juntes P, et al. Periodontal disease burden and pathological changes in organs of dogs. J Vet Dent 2008; 25: 97–105. [DOI] [PubMed] [Google Scholar]
- 14. Hoffmann T, Gaengler P. Clinical and pathomorphological investigation of spontaneously occurring periodontal disease in dogs. J Small Anim Pract 1996; 37: 471–479. [DOI] [PubMed] [Google Scholar]
- 15. Cave N, Bridges JP, Thomas DG. Systemic effects of periodontal disease in cats. Vet Q 2012; 32: 131–144. [DOI] [PubMed] [Google Scholar]
- 16. Lee D, Verstraete FJM, Arzi B. An update on feline chronic gingivostomatitis. Vet Clin North Am Small Anim Pract 2020; 50: 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. International Renal Interest Society. Staging of chronic kidney disease guidelines. http://www.iris-kidney.com/guidelines/staging.html (2019, accessed March 1, 2022).
- 18. Williams T, Archer J. Evaluation of urinary biomarkers for azotaemic chronic kidney disease in cats. J Small Anim Pract 2016; 57: 122–129. [DOI] [PubMed] [Google Scholar]
- 19. Jepson R, Brodbelt D, Vallance C, et al. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med 2009; 23: 806–813. [DOI] [PubMed] [Google Scholar]
- 20. Schmiedt CW, Brainard BM, Hinson W, et al. Unilateral renal ischemia as a model of acute kidney injury and renal fibrosis in cats. Vet Pathol 2016; 53: 87–101. [DOI] [PubMed] [Google Scholar]
- 21. Ross L, Finco D. Relationship of selected clinical renal function tests to glomerular filtration rate and renal blood flow in cats. Am J Vet Res 1981; 42: 1704–1710. [PubMed] [Google Scholar]
- 22. Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol 2008; 3: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miyagawa Y, Takemura N, Hirose H. Assessments of factors that affect glomerular filtration rate and indirect markers of renal function in dogs and cats. J Vet Med Sci 2010; 72: 1129–1136. [DOI] [PubMed] [Google Scholar]
- 24. Hall JA, Yerramilli M, Obare E, et al. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med 2014; 28: 1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ceron J, Eckersall P, Martvnez-Subiela S. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet Clin Pathol 2005; 34: 85–99. [DOI] [PubMed] [Google Scholar]
- 26. Werner L, Turnwald G, Willard MD. Immunologic and plasma protein disorders. In: Willard MD, Tvedten H. (eds). Small animal clinical diagnosis by laboratory methods. Philadelphia, PA: WB Saunders, 2004, pp 290–305. [Google Scholar]
- 27. Hutter J, Van der Velden U, Varoufaki A, et al. Lower numbers of erythrocytes and lower levels of hemoglobin in periodontitis patients compared to control subjects. J Clin Periodontol 2001; 28: 930–936. [DOI] [PubMed] [Google Scholar]
- 28. Ardalan M, Ghabili K, Pourabbas R, et al. A causative link between periodontal disease and glomerulonephritis: a preliminary study. Ther Clin Risk Manag 2011; 7: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reynolds B, Lefebvre HP. Feline CKD: pathophysiology and risk factors – what do we know? J Feline Med Surg 2013; 15: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marino C, Lascelles BD, Vaden SL. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2014; 16: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Greene J, Lefebvre SL, Wang M, et al. Risk factors associated with the development of chronic kidney disease in cats evaluated at primary care veterinary hospitals. J Am Vet Med Assoc 2014; 244: 320–327. [DOI] [PubMed] [Google Scholar]
- 32. Finch NC, Syme HM, Elliott J. Risk factors for development of chronic kidney disease in cats. J Vet Intern Med 2016; 30: 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]