Abstract
Objectives
Hypothermia is a common complication of anesthesia, particularly in cats. Some veterinarians insulate the extremities of cats as a preventive measure, and there is evidence that heating the extremities of dogs decreases the rate of heat loss from the core. This study investigated whether active warming or passive insulation of the extremities of cats resulted in a slower decrease in rectal temperature during anesthesia.
Methods
Female cats were assigned via block randomization to passive (cotton toddler socks), active (heated toddler socks) or control group (uncovered extremities). Rectal temperature was monitored every 5 mins from induction through return to trap/carrier (final temperature). Multivariable linear regression models were used to compare temperature (rate change and final) between groups.
Results
There were 164 cats with 1757 temperature readings. Mean total duration of anesthesia was 53 ± 13 mins. The temperature of all groups decreased linearly over time (all P <0.0001), with the rates of temperature decrease being −0.039°F/min (95% confidence interval [CI] −0.043 to −0.035)/−0.022°C (95% CI −0.024 to −0.019), −0.039°F/min (95% CI −0.042 to −0.035)/−0.022°C (95% CI −0.023 to −0.019) and −0.029°F/min (95% CI −0.032 to −0.025)/−0.016°C (95% CI −0.018 to −0.014) for the control, passive and active groups, respectively. The control, passive and active groups had median final temperatures of 98.4°F (interquartile range [IQR] 97.6−99.4)/36.9°C (IQR 36.4−37.4), 98.0°F (IQR 97.2–98.7)/36.7°C (IQR 36.2–37.1) and 99.1°F (IQR 97.7–100.0)/37.3°C (IQR 36.5–37.8), respectively. After controlling for weight, postinduction temperature and duration of anesthesia, and as compared with controls, the final temperature of the active group was predicted to be 0.54°F (95% CI 0.03–1.01)/0.3°C (95% CI 0.02–0.56) greater (P = 0.023), while the passive group was not significantly different (P = 0.130).
Conclusions and relevance
The rate of rectal temperature decrease was significantly slower for the active group compared with the other groups. Although the cumulative difference in final temperature reading was modest, superior materials might enhance performance. Cotton toddler socks alone did not slow the rate of temperature decrease.
Keywords: Anesthesia, anaesthesia, surgery, shelter medicine, high-quality high-volume spay–neuter, hypothermia, trap–neuter–vaccinate–return
Introduction
Perioperative hypothermia is a common surgical complication in cats, with moderate-to-severe hypothermia estimated to occur in 71% of cats undergoing anesthesia when moderate hypothermia is defined as body temperature <97.7°F (36.5°C). 1 As body temperature falls below 98.6°F (37.0°C), there are increased risks of prolonged recovery due to decreased metabolism of anesthetic drugs, 2 coagulopathies, myocardial ischemia and – for patients becoming moderately to severely hypothermic – surgical incision site infections.3–8 Smaller animals such as kittens are particularly prone to developing hypothermia due to their large surface-to-volume ratio. 7
Three phases are recognized in the decrease of core temperature induced by anesthesia.6,7 Phase 1 is a rapid decrease in core temperature occurring within the first hour following induction and resulting from the decreased sympathetic tone and opening of arteriovenous shunts, which redistributes blood from the core to the periphery.6,9,10 Minimal heat loss is thought to occur through mechanical means (conduction, convection, etc) during this phase, but a decrease in metabolic heat production of around 22% is reported in humans. 9 Phase 2 begins after the first hour and consists of a linear, progressive decline in core body temperature as heat losses outweigh metabolic heat production. Phase 3 begins after approximately 3 h, during which heat production becomes balanced with losses.6,7
Numerous active and passive warming methods for cats and dogs are supported by research and used clinically, including warm water blankets, reflective blankets, forced warm air blankets and full-body bubble wrap.11–15 In one study, warming dogs’ extremities maintained core body temperature more effectively than truncal warming alone. 16 Passive insulation of the periphery and trunk of cats with bubble wrap and a plastic barrier decreased temperature loss and recovery time. 13
Placing socks over extremities has been recommended as a passive warming method for surgeries performed in animal shelters. 17 During surgery, these socks are typically used in conjunction with truncal warming from circulating warm water blankets, forced air warmers or heating mats made from conductive fabric. To our knowledge, no study has investigated passive or active warming of the extremities of cats in conjunction with truncal warming.
The central hypothesis of this study was that active warming of the extremities would decrease the rate of core heat loss as measured by rectal body temperature during phase 1 of hypothermia as compared with controls. It was further hypothesized that passive insulation of the extremities with socks would reduce the rate of temperature decrease, compared with controls, and that the final temperature reading of both treatment groups would be greater than controls.
Materials and methods
Survey of common clinical practices for passive insulation
Before experimentation, an anonymous, 11 question survey (see Appendix 1 in the supplementary material) of common practices related to perioperative insulation of extremities in high-quality high-volume spay–neuter environments was distributed via a commercial electronic survey service (Google Forms). Data were collected between 18 September 2020 and 17 October 2020. The survey was advertised on the Association of Shelter Veterinarians, on high-quality, high-volume spay–neuter mailing lists and via social media posts. Cross-posting was encouraged. Responses informed the protocol for passive insulation in the multi-arm, parallel-group randomized controlled trial (RCT).
Multi-arm, parallel-group RCT
Female cats presented to the Midwestern University Shelter and Community Medicine program for ovariohysterectomy (OVH) as part of a trap–neuter–return (TNR) or foster program between 13 March 2021 and 11 September 2021 were enrolled in the study. Inclusion criteria included any intact female cat of any breed older than 6 weeks of age, body condition score (BCS) >2 (scale 1–9) and deemed healthy based on visual examination. Cats requiring any surgical procedure in addition to OVH were excluded. Cats were allocated to one of the three experimental groups (n = 55) via block randomization (www.randomization.com). Experimental groups included a control group (no socks), passive insulation (cotton toddler socks alone) and active warming (heated toddler socks). Blinding was not possible owing to visible differences between treatments. All animal experiments were performed as per the guidelines and regulations set forth by the Midwestern University Institutional Animal Care and Use Committee (approved study protocol #2976).
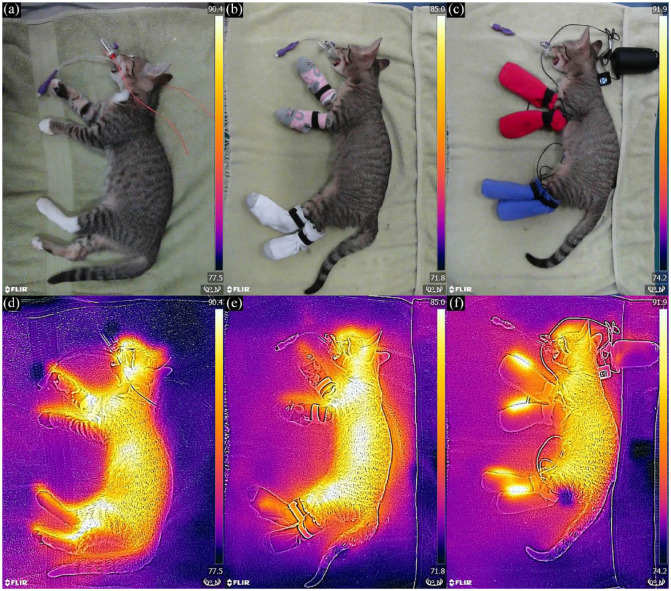
For passive and active groups, socks were placed on all four paws and secured with small Velcro strips (Figure 1). For biosecurity, the cut end of a plastic umbrella bag was placed over each paw before the sock. Commercially available infant- or toddler-sized cotton socks were used for the passive group. Heated socks were created by sewing commercially available, 17.5 × 10 cm, battery-powered, low-voltage, cloth heating pads (Nuonove) into the same type of socks used for the passive group. Heated socks were set to medium temperature (approximately 108°F/42°C). To ensure safety, the first 30 cats enrolled in the heated sock group had their paw temperatures continuously monitored (TP25; ThermoPro) to ensure that the temperature did not exceed 109°F (42.8°C), as previous research suggests cats will react at temperatures above 109°F. 18 Representative images of radiant heat for the three conditions were documented using a thermal imaging camera (Flir ONE Gen 3; Flir) (Figure 1).
Figure 1.
Sock placement and radiant heat imaging for (a,d) control, (b,e) passive and (c,f) active groups
Rectal temperatures were measured every 5 mins from loss of consciousness following induction of anesthesia until return to the trap/carrier. Temperature logging devices (RC-5+ [Elitech] and Vet30 [SunTech]) and digital rectal thermometers (VetOne; MWI Veterinary supply) were used but not systematically assigned to different groups. The socks and monitoring thermometers were placed immediately after determining or confirming the sex of the cat when the heart rate and respiratory rate were recorded per Arizona administrative code R 311-502. Thermometer probes were secured to the patients’ tails, using elastic wrap to prevent dislodging.
Veterinary students monitored and maintained anesthesia and performed the OVH under veterinary supervision in the Midwestern University College of Veterinary Medicine student surgery suite. A single technician induced anesthesia with an intramuscular (IM) injection of tiletamine/zolazepam (3 mg/kg), dexmedetomidine (7.5 μg/kg) and butorphanol (0.15 mg/kg). Ambient room temperature was recorded at induction of anesthesia as part of each patient’s record. Recumbent cats unresponsive to stimuli were placed on standard terrycloth towels for transport throughout the clinic. Physical examinations were performed by veterinary students or technicians and included rectal temperature (postinduction temperature, via VetOne), heart rate, respiratory rate, age estimate based on dentition and BCS on a scale of 1–9. Physical examination of unsocial cats was performed after loss of consciousness, consistent with Arizona administrative code R 311-502. Cats were shaved from xiphoid to pubis, eye lubrication was applied, bladders were emptied and a supraglottic airway control device (V-gel; Docsinnovent) was inserted.
Cats were positioned in dorsal recumbency on the surgical table and connected to non-rebreathing anesthetic circuits (T-Piece or Bain). Anesthesia was maintained with 1.5% isoflurane and 2 l/min oxygen unless otherwise clinically indicated. An electrically resistant conductive fabric blanket (HotDog; Augustine Surgical) set at 109.4°F (43°C) and positioned underneath the patient provided truncal heat support. A towel was interposed between the cat and the warming pad.
All surgical and perioperative protocols followed the Association of Shelter Veterinarians’ 2016 Veterinary Medical Care Guidelines for Spay–Neuter Programs. 19 The OVH technique was as follows: a 1 cm incision was made on the ventral midline midway between the umbilicus and pubis; the uterus was exteriorized with a spay hook; ovarian pedicles were autoligated; a single miller’s knot was placed on the uterine body; and the body wall and skin were closed with a cruciate and a purse string pattern, respectively, using absorbable monofilament sutures. This technique was modified for pregnant cats by extending the incision; applying uterine artery stick ties distal to the miller’s knot on the uterine body; closing the body wall with a simple continuous pattern; and closing the subcutaneous tissue and skin with a modified Colorado pattern. 20 The duration of surgery was from the start of the incision to the placement of the final suture, while the duration of anesthesia was from the induction of anesthesia through extubation. All cats were tattooed on their abdomen, and cats admitted through the TNR program were ear-tipped by removing the distal 1 cm of ear, to identify them as sterilized. 21
Postoperatively, the cats were moved to the recovery area for vaccination, injection of pain medication (robenacoxib 2 mg/kg SC for cats >4 months of age or buprenorphine 0.02 mg/kg IM for cats aged ⩽4 months) and monitoring. Supraglottic devices were removed once cats could swallow. Patients with recovery temperatures below 97°F (36.1°C) received rescue heat support from a resistant conductive fabric blanket. Atipamezole (0.04 mg/kg IM) to antagonize the dexmedetomidine (reversal) was administered to patients with decreasing temperatures and those failing to regain palpebral reflex after 10 mins. Postoperative pain was subjectively assessed by looking for signs of discomfort, as there are no pain scales validated for unsocial/feral cats. Patients showing signs attributable to pain or dysphoria, such as vocalization and restlessness, were administered low-dose dexmedetomidine (0.001 mg/kg IM). If these signs persisted beyond 15 mins, they were attributed to pain and the cat was administered buprenorphine (0.02 mg/kg IM). 22
Statistical analysis
A sample size calculation was performed before the study, using a clinically meaningful difference of 1°F (0.6°C) and a SD of 1.8 (1.0), alpha of 0.05 and power of 0.8 to determine a sample size of 50 for each group. The results were summarized using descriptive statistics, with mean ± SD reported for normally distributed variables and median and interquartile range (IQR; reported as quartile 1 and quartile 3), for non-normally distributed variables. Normality was assessed using tests of skewness and kurtosis. Point estimates were reported with 95% confidence intervals (CIs). To ensure that groups did not differ, values for age, incision size, ambient temperature and surgical duration were compared between groups using Kruskal–Wallis tests, while anesthetic induction temperature and weight were compared between groups using ANOVA. The need for reversal and for heat support were compared between groups using Fisher’s exact test. The linear relationship of continuous, independent variables to dependent variables was confirmed via visualization, and the shape of the best fit line for the change in temperature over time was confirmed with Lowess curves. Lowess lines were constrained to 60 mins to minimize the effect of decreasing sample size on the distal portion of the curve.
Multivariable linear regression with robust SEs was used to compare the rate of temperature change for each group by including group as an interaction term with time and clustering by animal and controlling for other significant variables. A similar multivariable linear regression model was used to compare the final predicted temperature of each group while controlling for other significant variables. Regression models were built using backwards selection guided by variables significant at an alpha of 0.2 in univariable regression, prior literature and biological plausibility. Competing models were evaluated using Akaike information criterion and Bayesian information criterion, and final models were validated by inspection of the residuals. Variables evaluated using univariable regression included induction temperature, ambient temperature, age (months), weight (kg), BCS (both as categorical and binary), incision length, surgery duration, total anesthetic duration and research arm. All statistical analysis was performed using standard statistical software (STATA version 17), and significance was set at P <0.05 for all tests unless otherwise specified.
Results
Passive insulation practices survey
Of the 52 respondents who completed the survey regarding the perioperative insulation of feline extremities, 22 (42%) reported using passive insulation at least sometimes. Respondents who reported insulating extremities most typically used toddler-sized cotton socks (77%) placed on all four feet (100%). Most respondents (91%) noted that cleaned socks were used for each cat.
RCT
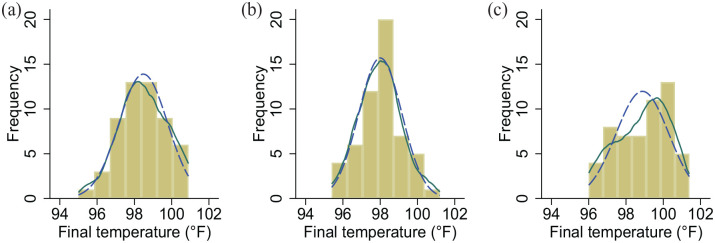
A total of 165 female cats were enrolled in the study (n = 55/group). One cat in the passive group was excluded due to an additional enucleation procedure. Thus, 164 cats completed the study, and 1757 temperature readings were used for analysis. The population had a median age of 12 months (IQR 5–24), mean weight of 2.7 ± 1.0 kg, mean postinduction temperature of 101.2 ± 0.9°F (38.4 ± 0.5°C), median surgical duration of 30 mins (IQR 20–37) and mean total duration of anesthesia of 53 ± 13 mins. These values did not differ between groups (Table 1, Figure 1). Final temperature distributions differed between the groups (Figure 2), with control and passive groups normally distributed and the active group having a left skew. The control, passive and active groups had median final temperatures of 98.4°F (IQR 97.6–99.4, range 95.0–100.9)/36.9°C (IQR 36.4–37.4, range 35.0–38.3), 98.0°F (IQR 97.2–98.7, range 95.4–101.2)/36.7°C (IQR 36.2–37.1, range 35.2–38.4) and 99.1°F (IQR 97.7–100.0, range 96.0–101.4)/37.3°C (IQR 36.5–37.8, range 35.6–38.6), respectively.
Table 1.
Values for the control, passive and active groups
| Variable | Control (n = 54) | Passive (n = 55) | Active (n = 55) | P value |
|---|---|---|---|---|
| Age (months) | 12 (6–30) | 12 (5–24) | 12 (5–24) | 0.689 |
| Weight (kg) | 2.7 ± 1.0 | 2.7 ± 1.1 | 2.5 ± 0.9 | 0.762 |
| BCS (1–9) | 4 (4–4) | 4 (4–4) | 4 (4–4) | 0.471 |
| BCS <4/9 | 1 (2) | 3 (5) | 7 (13) | 0.070 |
| Duration of anesthesia (mins) | 54 ± 13 | 58 ± 14 | 55 ± 13 | 0.428 |
| Duration of surgery (mins) | 25 (20–34) | 35 (20–45) | 30 (20–35) | 0.473 |
| Incision size (cm) | 1 (1–2) | 1 (1–3) | 1 (1–2) | 0.242 |
| Ambient temperature (°F/°C) | 68 (67–69)/20 (19–21) | 68 (67–69)/20 (19–21) | 68 (66–69)/20 (19–21) | 0.440 |
| Postinduction temperature (°F/°C) | 101.4 ± 0.9/38.6 ± 0.5 | 101.0 ± 0.9/38.3 ± 0.5 | 101.1 ± 1.0/38.4 ± 0.6 | 0.729 |
| Final temperature (°F/°C) | 98.4 (97.6–99.4)/36.9 (36.4–37.4) | 98.0 (97.2–98.7)/36.7 (36.2–37.1) | 99.1 (97.7–100.0)/37.3 (36.5–37.8) | 0.004 |
Data are presented as mean ± SD, median (interquartile range [IQR]) or n (%). P values were determined via ANOVA or Kruskal–Wallis test. No P value for the baseline values was significant, while there was a P value <0.05 for the final temperature.
BCS = body condition score
Figure 2.
Frequency distribution of final temperature reading for (a) control, (b) passive and (c) active groups overlaid with kernel density estimate (solid green line) and normal curve (dashed blue line)
The temperature of all groups decreased linearly (Figure 3) over time (all P <0.0001), with the rates of temperature decrease being −0.039°F/min (95% CI −0.043 to −0.035)/−0.022°C/min (95% CI −0.024 to −0.019), −0.039°F/min (95% CI −0.042 to −0.035)/−0.022°C/min (95% CI −0.023 to −0.019) and −0.029°F/min (95% CI −0.032 to −0.025)/−0.016 °C/min (95% CI −0.018 to −0.014) for control, passive and active groups, respectively, after controlling for postinduction temperature (b = 0.39, P <0.0001, 95% CI 0.32–0.45) and weight (b = 0.30, P <0.0001, 95% CI 0.24–0.37). Comparison of CIs revealed that the rate of temperature drop was significantly less in the active group compared with the passive and control groups, with cats in the active group losing 0.01°F (0.01 °C) less body heat/min compared with the control group. CIs for the control and passive groups overlapped almost perfectly, indicating that the rates of change were not different.
Figure 3.
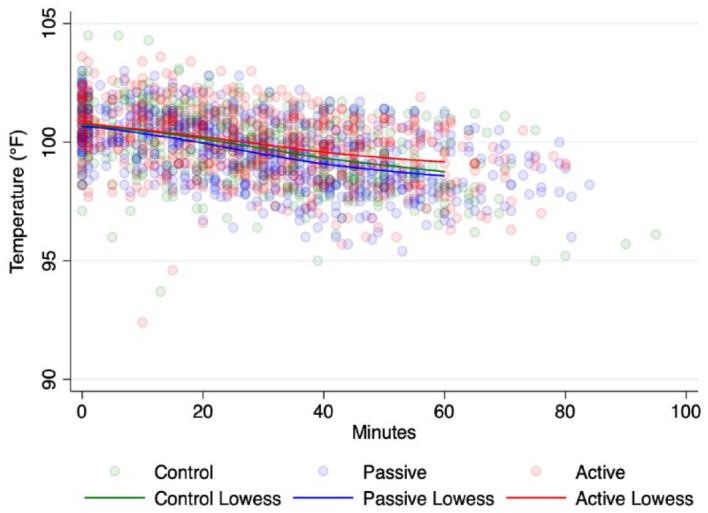
Scatterplot of temperature over time for each of the groups overlaid with Lowess lines. Lowess was constrained to 60 mins due to comparatively few data points after 60 mins (n = 101/1757 temperature readings)
A similar multivariable model (Table 2) was used to predict the final temperature for the treatment groups as compared with controls, with the active group predicted to have a final temperature significantly (P = 0.023) warmer 0.54°F (95% CI 0.07–1.00)/0.3°C (95% CI 0.04–0.56), while the passive group was not significantly different (b = −0.33, P = 0.130, 95% CI −0.76 to 0.10), controlling for postinduction temperature (b = 0.29, P = 0.005, 95% CI 0.09–0.50), weight (b = 0.42, P <0.0001, 95% CI 0.23–0.62) and duration of anesthesia (b = −0.02, P = 0.025, 95% CI −0.03 to −0.00). In recovery (Table 3), the proportion of cats reversed (overall 21%) or requiring heat support (overall 22%) did not differ between groups (P = 0.970 and P = 0.202, respectively).
Table 2.
Variables contained within the univariable and multivariable model to predict final temperature
| Variable | Univariable coefficient | Univariable 95% CI | Univariable P value | Multivariable coefficient | Multivariable 95% CI | Multivariable P value |
|---|---|---|---|---|---|---|
| Research arm | ||||||
| Control | Reference | |||||
| Passive | −0.47 | −0.94 to 0.00 | 0.048 | −0.33 | −0.76 to −0.10 | 0.130 |
| Active | 0.40 | −0.11 to 0.92 | 0.123 | 0.54 | 0.07 to 1.00 | 0.023 |
| Postinduction temperature | 0.40 | 0.19 to 0.61 | <0.0001 | 0.29 | 0.09 to 0.50 | 0.005 |
| Weight | 0.41 | 0.21 to 0.61 | <0.0001 | 0.42 | 0.23 to 0.62 | <0.0001 |
| BCS | ||||||
| 5 | Reference | |||||
| 4 | −0.29 | −0.80 to 0.21 | 0.255 | |||
| 3 | −1.59 | −2.62 to −0.57 | 0.003 | |||
| BCS <4/9 | −0.69 | −1.68 to 0.30 | 0.169 | |||
| Duration of surgery | 0.01 | −0.01 to 0.02 | 0.364 | |||
| Duration of anesthesia | −0.01 | −0.02 to 0.01 | 0.331 | −0.015 | −0.028 to −0.002 | 0.025 |
| Incision size | 0.07 | −0.08 to 0.21 | 0.364 | |||
| Ambient temperature | −0.16 | −0.33 to 0.02 | 0.075 | |||
CI = confidence interval; BCS = body condition score
Table 3.
Rescue interventions in recovery for the treatment groups
| Control | Passive | Active | P value | |
|---|---|---|---|---|
| Heat support | 9/43 (21) | 13/46 (28) | 6/43 (14) | 0.272 |
| Reversed | 11/54 (20) | 11/55 (20) | 12/55 (22) | 1.000 |
Data are presented as n (%). Fisher’s exact test was used to calculate the P values. Denominators for heat support differ owing to missing data
Discussion
The cats in this study had a mean total duration of anesthesia of 53 mins, indicating that temperature decreases mainly occurred during phase 1 hypothermia, in which redistribution and metabolism are the primary mechanisms of heat loss. A significantly slower rate of temperature drop and higher final temperature resulted from active warming compared with the control. The rate of temperature drop and final temperature were no different for the passive group compared with the control cats. As the predicted final temperature resulting from active warming differed from the control by barely more than 0.5°F (0.3°C) and a similar proportion of cats required reversal or heat support in recovery regardless of group, the benefit from active warming – at least with this experimental setup – was considered modest.
Consistent with previous study findings,11,15,23 the data revealed four predictors of final temperature: group, weight, duration of anesthesia and postinduction temperature. Although a BCS <4 has been reported to be predictive of postoperative temperature, 23 this association was not observed in this study, possibly due to residual confounding from differences in the number of cats within this lower BCS range in the different study groups: 13%, 2% and 4% in the active, passive and control groups, respectively.
In contrast to our study, two studies have found a benefit to passive insulation.13,24 In one, cocooning cats in cotton hand towels or reflective blankets was superior to no insulation for decreasing temperature loss during anesthesia, although passive insulation was found to be inferior to all active measures studied, including heat tent, heat lamp, water blanket and water blanket cocoon. 24 In the second study showing a benefit to passive insulation, bubble wrap applied immediately after the induction of anesthesia to the limbs and thorax of cats led to higher temperatures at the start and end of surgery than in uninsulated controls. 13 Both groups of cats in this study became transiently moderately hypothermic (core temperature <96.8°F/36.0°C) 25 between anesthesia induction and the start of surgery, with a mean of 96.8°F/36.0°C for wrapped cats and 94.1°F/34.5°C for control cats. The mean duration of this interval was approximately 1 h, which has previously been associated with a 50% incidence of perioperative hypothermia. 25 These temperatures rebounded by the end of surgery, associated with forced air warming on the surgical table, and were not markedly different from the final temperatures reported herein. However, we observed no pattern of transient temperature decrease with a median duration of total anesthesia of approximately 1 h. These results suggest that investing in personnel education to shorten the time interval between anesthesia induction and the start of surgery might help to reduce the incidence of hypothermia.
Several limitations must be considered in this study. First, owing to the short duration of anesthesia, the results reported here are limited to the physiology of heat loss associated with phase 1 hypothermia. It is unclear whether the modest advantage of warming the extremities would continue beyond the redistribution phase, particularly as it has been observed that the temperature of dogs undergoing active peripheral warming plateaus similarly to dogs experiencing only truncal warming, albeit at a higher temperature. 16 Second, the material and fit of the socks were pragmatically informed by responses to the initial survey and not optimized for reducing heat loss. A better insulating material might enhance the active warming device’s performance, such as a study that used bubble wrap and a down cloth blanket to insulate patients warmed by a hot water bottle. 26 The heating element is visible in the active group’s thermal image (see Figure 1), indicating poor insulation. While better materials might improve the performance of passive insulation, superior warming has consistently been observed with a well-insulated heat source as compared with high-quality insulation alone.24,26 Third, drugs used in anesthetic protocols have different effects on vasoconstriction and may be associated with different responses to heat loss prevention, particularly during phase 1 hypothermia. Specifically, acepromazine, thiopentone sodium and halothane promoted vasodilation in dogs treated with warm water mattresses applied to their extremities, potentially increasing heat transfer to the extremities. 16 Dexmedetomidine was administered in the induction protocol of this study and – based on its half-life – its vasoconstrictive effects were potentially present in the first hour of anesthesia. 27 The resulting vasoconstriction may have limited the ability of the active warming device to transfer heat to the extremities or further mitigate heat loss. Finally, different brands of thermometers were used, which were not calibrated. While this could affect the temperature values, there was no systematic bias to the assignment of thermometer brand to treatment group.
Conclusions
The rate of core temperature decrease was significantly slower for the actively warmed group compared with the control or passive groups. Although the cumulative difference in final temperature reading was only 0.54°F/0.3°C), materials with better insulating properties may enhance performance of active warming devices. Cotton toddler socks alone did not slow the rate of temperature decrease, and the passive group was indistinguishable from controls.
Supplemental Material
‘Kitten mitten’ pilot survey
Footnotes
Author note: Study findings were presented as a research abstract at the 2022 American Board of Veterinary Practitioners conference.
Supplementary material: The following file is available online:
Appendix 1: ‘Kitten mitten’ pilot survey.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Article processing charges were provided via Midwestern University departmental funds.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals and procedures that differed from established internationally recognised high standards (‘best practice’) of veterinary clinical care for the individual patient. The study therefore had prior ethical approval from an established (or ad hoc) committee as stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Rachael E Kreisler  https://orcid.org/0000-0002-5562-5521
https://orcid.org/0000-0002-5562-5521
References
- 1. Redondo JI, Suesta P, Gil L, et al. Retrospective study of the prevalence of postanaesthetic hypothermia in cats. Vet Rec 2012; 170: 206. DOI: 10.1136/vr.100184. [DOI] [PubMed] [Google Scholar]
- 2. Pottie RG, Dart CM, Perkins NR, et al. Effect of hypothermia on recovery from general anaesthesia in the dog. Aust Vet J 2007; 85: 158–162. [DOI] [PubMed] [Google Scholar]
- 3. Sessler DI. Mild perioperative hypothermia. N Engl J Med 1997; 336: 1730–1737. [DOI] [PubMed] [Google Scholar]
- 4. Reynolds L, Beckmann J, Kurz A. Perioperative complications of hypothermia. Best Pract Res Clin Anaesthesiol 2008; 22: 645–657. [DOI] [PubMed] [Google Scholar]
- 5. Öner Cengiz H, Uçar S, Yilmaz M. The role of perioperative hypothermia in the development of surgical site infection: a systematic review. AORN J 2021; 113: 265–275. [DOI] [PubMed] [Google Scholar]
- 6. Armstrong SR, Roberts BK, Aronsohn M. Perioperative hypothermia. J Vet Emerg Crit Care 2005; 15: 32–37. [Google Scholar]
- 7. Clark-Price S. Inadvertent perianesthetic hypothermia in small animal patients. Vet Clin North Am Small Anim Pract 2015; 45: 983–994. [DOI] [PubMed] [Google Scholar]
- 8. Madrid E, Urrútia G, Roqué i, Figuls M, et al. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev 2016; 4. DOI: 10.1002/14651858.CD009016.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsukawa T, Sessler DI, Sessler AM, et al. Heat flow and distribution during induction of general anesthesia. Anesthesiology 1995; 82: 662–673. [DOI] [PubMed] [Google Scholar]
- 10. Van Duren A. Perioperative prewarming: heat transfer and physiology. AORN J 2022; 115: 407–422. [DOI] [PubMed] [Google Scholar]
- 11. Clark-Price SC, Dossin O, Jones KR, et al. Comparison of three different methods to prevent heat loss in healthy dogs undergoing 90 minutes of general anesthesia. Vet Anaesth Analg 2013; 40: 280–284. [DOI] [PubMed] [Google Scholar]
- 12. Franklin MA, Rochat MC, Payton ME, et al. Comparison of three intraoperative patient warming systems. J Am Anim Hosp Assoc 2012; 48: 18–24. [DOI] [PubMed] [Google Scholar]
- 13. Sakata H, Walsh V, Chambers JP, et al. Effect of insulation with bubble wrap and an absorbent pad on heat loss in anaesthetised cats. N Z Vet J 2020; 68: 324–330. [DOI] [PubMed] [Google Scholar]
- 14. Tan C, Govendir M, Zaki S, et al. Evaluation of four warming procedures to minimise heat loss induced by anaesthesia and surgery in dogs. Aust Vet J 2004; 82: 65–68. [DOI] [PubMed] [Google Scholar]
- 15. Tünsmeyer J, Bojarski I, Nolte I, et al. Intraoperative use of a reflective blanket (Sirius rescue sheet) for temperature management in dogs less than 10 kg. J Small Anim Pract 2009; 50: 350–355. [DOI] [PubMed] [Google Scholar]
- 16. Cabell LW, Perkowski SZ, Gregor T, et al. The effects of active peripheral skin warming on perioperative hypothermia in dogs. Vet Surg 1997; 26: 79–85. [DOI] [PubMed] [Google Scholar]
- 17. Robertson S. Principles of anesthesia, analgesia, safety, and monitoring. In: White S. (ed). High-quality, high-volume spay and neuter and other shelter surgeries. Chichester: John Wiley and Sons, 2020, pp 125–152. [Google Scholar]
- 18. Robertson SA, Taylor PM, Lascelles BDX, et al. Changes in thermal threshold response in eight cats after administration of buprenorphine, butorphanol and morphine. Vet Rec 2003; 153: 462–465. [DOI] [PubMed] [Google Scholar]
- 19. Griffin B, Bushby PA, McCobb E, et al. The Association of Shelter Veterinarians’ 2016 veterinary medical care guidelines for spay–neuter programs. J Am Vet Med Assoc 2016; 249: 165–188. [DOI] [PubMed] [Google Scholar]
- 20. ASPCA. Spay/neuter surgery: closure – female dogs. www.youtube.com/watch?v=7Q_p68xpqg0 (2017, accessed 26 October 2022).
- 21. Dalrymple AM, MacDonald LJ, Kreisler RE. Ear-tipping practices for identification of cats sterilized in trap–neuter–return programs in the USA. J Feline Med Surg 2022; 24: e302–e309. DOI: 10.1177/1098612X221105843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steagall PV, Robertson S, Simon B, et al. 2022 ISFM consensus guidelines on the management of acute pain in cats. J Feline Med Surg 2022; 24: 4–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kreisler RE, Douglas ML, Harder KN. Comparison of the effect of isopropyl alcohol and chlorhexidine solution rinses on body temperature of female cats undergoing sterilization surgery. J Feline Med Surg 2021; 23: 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haskins SC. Hypothermia and its prevention during general anesthesia in cats. Am J Vet Res 1981; 42: 856–861. [PubMed] [Google Scholar]
- 25. Rodriguez-Diaz JM, Hayes GM, Boesch J, et al. Decreased incidence of perioperative inadvertent hypothermia and faster anesthesia recovery with increased environmental temperature: a nonrandomized controlled study. Vet Surg 2020; 49: 256–264. [DOI] [PubMed] [Google Scholar]
- 26. Onozawa E, Azakami D, Seki S, et al. Effect of an insulation device in preventing hypothermia during magnetic resonance imaging examinations for dogs and cats under general anesthesia. Animals 2021; 11: 2378. DOI: 10.3390/ani11082378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pypendop BH, Honkavaara J, Ilkiw JE. Pharmacokinetics of dexmedetomidine, MK-467 and their combination following intramuscular administration in male cats. Vet Anaesth Analg 2017; 44: 823–831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
‘Kitten mitten’ pilot survey