Abstract
Objectives
This study compared an opioid-free injectable anaesthetic protocol with or without multimodal analgesia in kittens undergoing ovariohysterectomy.
Methods
In this prospective, randomised, blinded, clinical trial, 29 healthy kittens (mean ± SD weight 1.55 ± 0.46 kg; aged 10 weeks to 6 months) were included. Anaesthesia was performed with an intramuscular injection of ketamine (4 mg/kg), dexmedetomidine (40 μg/kg) and midazolam (0.25 mg/kg). In the multimodal group (MMG), cats (n = 14) received meloxicam (0.1 mg/kg SC) and intraperitoneal bupivacaine 0.25% (2 mg/kg), whereas the same volume of saline was administered in the control group (CG; n = 15). Atipamezole (0.4 mg/kg IM) was given 15 mins after ovariohysterectomy. Postoperative pain was assessed using the UNESP-Botucatu multidimensional feline pain assessment scale – short form. Rescue analgesia (buprenorphine 0.02 mg/kg IM in MMG/CG and meloxicam 0.1 mg/kg SC in CG) was administered if pain scores were ⩾4/12. Soft food intake (after 2 and 60 mins) was evaluated at specific time points postoperatively. Statistical analyses were performed with linear models and post-hoc pairwise comparison with Benjamini–Hochberg corrections (P <0.05).
Results
The prevalence of rescue analgesia was higher in the CG (n = 15/15) than the MMG (n = 1/14; P <0.001). Pain scores at 1 h, 2 h and 4 h postoperatively were higher in the CG (4.1 ± 2.8, 4.8 ± 3.0 and 5.3 ± 1.2, respectively) than in the MMG (1.6 ± 1.0, 1.1 ± 1.0 and 0.9 ± 0.8, respectively; P <0.001). Food intake (%) at 1 h postoperatively was higher in the MMG after 2 and 60 mins (10.4 ± 9 and 71.9 ± 29, respectively) than in the CG (1.4 ± 2 and 13.9 ± 7, respectively; P <0.001).
Conclusions and relevance
This opioid-free protocol using multimodal analgesia produced adequate postoperative pain relief, while almost eliminating the need for rescue analgesia in kittens undergoing ovariohysterectomy. Pain decreased food intake.
Keywords: Analgesia, pain, opioid-free, local anaesthesia, multimodal
Introduction
Animal overpopulation is a societal challenge, particularly in countries with limited resources.1–4 Unwanted litters overpopulate shelters, creating a welfare issue. Particularly in cats, a queen may have up to 18 kittens every year.5–7 This problem grows exponentially, leading to the euthanasia of thousands of cats every year. Furthermore, it is now a concern that the hunting behaviour of owned, stray and feral cats poses a risk to wildlife populations.8–10 On a public health level, some of these cats could be potential vectors of zoonotic diseases.9,10
The One Health initiative by the World Health Organization 11 and the International Society of Feline Medicine (ISFM) support early-age neutering for population control.2,4–6,12 In 2013, prepubertal gonadectomy was promoted by the ISFM’s guidelines on population management and welfare of unowned domestic cats (Felis catus). 2 Early-age neutering provides advantages over traditional-age neutering (ie, ⩾6 months) as anaesthetic recovery is faster in kittens than adults,13–17 there is no increased risk of wound complications and morbidity is low. 18 There is also some evidence that kittens experience less pain than adult cats after ovariohysterectomy (OVH). For example, the prevalence of rescue analgesia using an opioid-free anaesthetic protocol was lower in kittens than in adult cats after OVH. 19
Injectable anaesthesia using ketamine and alpha (α)2-adrenergic receptor agonists is commonly used in high-volume, high-quality spay–neuter programmes.3–6,12,17,19,20 However, these protocols do not always include opioid analgesics, as veterinarians do not always have access to these drugs and volatile anaesthetics due to strict regulatory and bureaucratic constraints in many countries. Additionally, veterinarians do not often use non-opioid analgesic techniques [intraperitoneal (IP) local anaesthesia or non-steroidal anti-inflammatory drugs (NSAIDs)] and surgery is regularly performed with limited or no perioperative analgesia. There is now an interest in the study of opioid-free analgesic protocols,19–21 similar to the human literature, 22 and in investigating whether, for example, multimodal analgesia with NSAIDs and IP administration of local anaesthetics could provide appropriate pain relief and reduce/eliminate the need for opioids after early-age neutering. Moreover, it is not clear how appetite and perioperative food intake are affected by postoperative pain in kittens undergoing OVH when using, or not using, non-opioid multimodal analgesia.
The aims of this study were to compare the analgesic and anaesthetic effects of an opioid-free injectable protocol with or without multimodal analgesia in kittens undergoing OVH, and food intake in groups treated with these two protocols at specific time points. The hypotheses were that the protocol using multimodal analgesia would provide lower postoperative pain scores and prevalence of rescue analgesia when compared with a control group, and that postoperative food intake would be higher in the multimodal analgesia group than in the control group.
Materials and methods
This study is reported in accordance with the CONSORT guidelines (www.consort-statement.org).
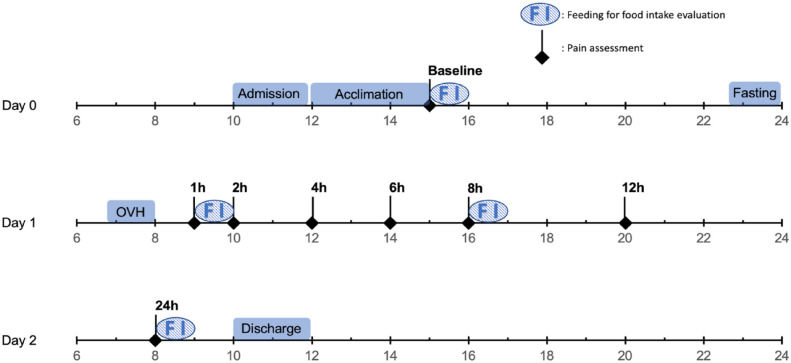
The study was approved by the ethics committee of the Faculty of Veterinary Medicine, Université de Montréal (21-Rech-2132) and a written consent form was signed by an individual representing the shelters responsible for the cats included in the trial. The experiment was conducted between July and October 2021 (Figure 1).
Figure 1.
Example of a timeline showing manipulations and data collection for one kitten during a randomised, prospective, blinded, clinical trial in kittens undergoing ovariohysterectomy (OVH). Pain and food intake were assessed throughout the trial at specific time points
Animals
A total of 34 domestic shorthair (n = 27) and longhair (n = 7) kittens were enrolled in a prospective, blinded, randomised clinical trial. Kittens from local shelters were admitted to the Centre Hospitalier Universitaire Vétérinaire, Faculty of Veterinary Medicine, Université de Montréal, at least 16 h before undergoing OVH. Recruitment was performed by one of the investigators (AJC). Cats were housed in adjacent stainless steel cages in a cat ward. A period of 3 h was provided for acclimation before any manipulation. All handling was done with low-stress, feline-friendly techniques. Cages were enriched with toys, cardboard boxes and blankets. Water was available ad libitum. At the end of the study, kittens were returned to the shelters for adoption.
Inclusion and exclusion criteria
Inclusion criteria included female healthy kittens based on medical history and physical examination, aged between 10 weeks and 6 months, body weight ⩾1 kg, body condition score between 4 and 6 on a scale of 1–9 and of any breed. Exclusion criteria included kittens with hyperthermia (rectal temperature >39.5°C), systemic disease on physical examination, preoperative fear-induced aggressive behaviour or pain at baseline assessment (UNESP-Botucatu multidimensional feline pain assessment scale – short form [UFEPS-SF] 23 scores of ⩾3/12). Kittens were excluded from the food intake part of the trial when they refused to eat any of the soft food offered preoperatively. In this case, another type of soft and/or hard food was offered to that cat.
Randomisation
Kittens were randomly allocated to either the multimodal group (MMG) or the control group (CG). A randomisation plan (www.randomization.com) was generated in May 2021 by an individual (RW) who was not involved with pain assessment, with two allocation blocks (1:1 ratio): 32 animals in the first block and 8 in a second block (see Statistical analyses). Upon hospital admission, each kitten was sequentially assigned a number that was allocated to either MMG or CG.
Anaesthesia, surgery and analgesia
Food, but not water, was withheld for 6–10 h before general anaesthesia. In the morning before surgery, kittens were given 0.5 ml of corn syrup oral transmucosally to prevent hypoglycaemia. The anaesthetic protocol consisted of ketamine (Ketaset; Zoetis; 4 mg/kg), dexmedetomidine (Dexdomitor; Zoetis; 40 μg/kg) and midazolam (Midazolam; Sandoz; 0.25 mg/kg). Drugs were withdrawn and combined in the same syringe by one individual (PVS) in the aforementioned order, and administered into the epaxial lumbar muscle using a 1 ml syringe by two individuals (HLMR, AM) who were blinded to the treatments.
Time to achieve lateral recumbency (onset of action; in seconds) was recorded after drug injection. Patients were then transferred to the operating room, where intubation was performed with a supraglottic airway device (V-gel; Docsinnovent). Kittens were placed in dorsal recumbency on a circulating warm water blanket and the abdominal area was clipped and prepared for surgery using aseptic technique. The cats’ eyes were lubricated with ocular gel before and after surgery.
An off-label subcutaneous (SC) injection of meloxicam (Metacam 0.5%; Boehringer Ingelheim; 0.1 mg/kg) was administered between the shoulder blades in the MMG, whereas kittens in the CG received the same volume of 0.9% saline. These injections were performed during surgical preparation (ie, scrubbing). An IP injection of bupivacaine hydrochloride 0.25% (Bupivacaine injection BP; Sterimax; 2 mg/kg) or 0.9% saline was performed in the MMG and CG, respectively, as previously described before an OVH. 24 Briefly, bupivacaine was withdrawn in a sterile manner using a 3 ml syringe. The solution was equally divided into three parts and instilled over the right and left ovarian pedicles, and the caudal aspect of the uterine body. 24 OVH was performed immediately after IP administration and using the pedicle tie technique by one experienced veterinarian (BPM). The abdominal wall was closed using a simple continuous pattern with poliglecaprone 25 (Monocryl 3-0 or 4-0; Ethicon). The skin and subcutaneous tissue were closed with an intradermal pattern using the same type of suture. At the end of surgery, the skin incision length was measured and a green tattoo line was placed approximately 1 cm parallel to the surgical incision.
Anaesthesia was always performed by the same individual (RW). During the procedure, animals were spontaneously breathing room air. Monitoring was performed using a multiparametric monitor (LifeWindow 6000V veterinary multiparameter monitor; Digicare Animal Health) and included heart rate (HR; in beats/min [bpm]), respiration rate (fR; in respirations/min [rpm]), capnography (end-tidal CO2 concentrations [PETCO2]), pulse oximetry (SpO2), electrocardiography, and indirect systolic, mean and diastolic arterial blood pressure (SAP, MAP and DAP, respectively) using an oscillometry device. Rectal body temperature (T) was measured before and immediately after surgery. Isoflurane in oxygen was administered using a Mapleson D system connected to the supraglottic airway device, if anaesthetic depth was deemed superficial (purposeful movement, presence of palpebral reflexes with indication of high sympathetic stimulation).
Anaesthetic complications
Tachycardia (HR >180 bpm), bradycardia (HR <100 bpm), tachypnoea (fR >30 rpm), bradypnoea (fR <4 rpm), hypercapnia (PETCO2 >50 mmHg), desaturation (SpO2 <90%), hypotension (MAP <50 mmHg) and hypothermia (T <36.0°C) recorded for more than 5 mins of anaesthesia were considered to be anaesthetic complications. Kittens with desaturation or hypercapnia for more than 5 mins received assisted ventilation with intermittent positive pressure using a manual resuscitator connected to the supraglottic airway device. If desaturation persisted, 100% oxygen was administered via the Mapleson D circuit.
Anaesthetic recovery
Kittens received a 5 ml/kg bolus of SC lactated Ringer’s (Lactated Ringer’s Inj. Bag/500 ml; McCarthy & Sons Service) during anaesthetic recovery. Fluids were administered between the shoulder blades. An injection of atipamezole (Antisedan 0.4 mg/kg; Zoetis) was given into the epaxial lumbar muscles 15 mins postoperatively. Duration of anaesthesia (time between the administration of ketamine–dexmedetomidine–midazolam and atipamezole), duration of surgery (time between first skin incision and last suture), time to head lift (between the end of surgery and the first head lift) and time to sternal recumbency (between end of surgery and first sternal recumbency) were recorded. Kittens were transferred back to the cat ward for postoperative pain assessment once they were standing after surgery.
Pain assessment and rescue analgesia
Pain assessment was performed using the UFEPS-SF before surgery (baseline) and at 1, 2, 4, 6, 8, 12 and 24 h postoperatively by a veterinarian (AM), who was blinded to the treatment groups. This observer had 7 years of experience in clinical practice, including feline medicine and surgery, and had completed training using the UFEPS (www.animalpain.org). Additionally, before the study had begun, a total of seven female kittens were neutered using the same methodology so this observer could practise acute pain assessment with two other experienced investigators (PVS and HLMR).
Rescue analgesia was provided when UFEPS-SF scores were ⩾4/12. Intramuscular (IM) buprenorphine (Vetergesic 0.02 mg/kg; Champion Alstoe) was administered to cats in the MMG and CG. Cats in the CG also received a SC injection of meloxicam (0.1 mg/kg). To avoid bias, data collected after the administration of rescue analgesia were not included in the statistical analysis; however, pain assessment continued until patient discharge. An oral dose of meloxicam 0.05 mg/kg (Metacam 0.5 mg/ml oral suspension; Boehringer Ingelheim) was administered 24 h postoperatively to kittens weighing ⩾2 kg and/or older than 16 weeks for additional postoperative analgesia.
Food intake
Food intake was recorded before surgery (baseline) and at 1 h, 8 h and 24 h postoperatively. Each kitten was given a precise amount of canned food (Royal Canin Gastrointestinal Kitten; Mars) calculated according to the following formula: 2.5 × 70 × body weight (kg)0.75. 25 At each of these time points, food was offered in a dish and weighed (in grams) before administration and after periods of 2 mins and 60 mins. For baseline measurements, half of the daily intake was administered on the day of admission, whereas a third of the daily intake was given for each of the three postoperative meals. On the day of surgery, kittens received the remaining third at around 10 pm (Figure 1).
Statistical analyses
Sample size calculations were based on previous studies in which the prevalence of rescue analgesia was used as the primary outcome.19,24 In cats undergoing OVH using multimodal analgesia, the prevalence of rescue analgesia was 6%. 24 In a similar study using an opioid-free injectable protocol, the prevalence of rescue analgesia was 57%. 19 Consequently, a total of 24 kittens (n = 12 in each group) would be needed using two independent groups with a dichotomised primary endpoint, an α value of 5% and a power of 80%. The sample size was increased to 32 (n = 16 per group) to reduce the possibility of type II error. The randomisation included a second block of cats to compensate for any patient exclusion.
Data normality and homoscedasticity were assessed using the Shapiro–Wilk and Levene’s tests, respectively (R version 4.0.3). Demographic data and data related to surgery and anaesthesia were analysed with the Student’s t-test, Wilcoxon or Mann–Whitney U-test, when appropriate. The prevalence of rescue analgesia was compared between groups using a binomial generalised linear model. Temporal changes in pain scores were analysed with a generalised linear model with group and time points as fixed factors. A pairwise post-hoc test with a Benjamini–Hochberg correction was used when an interaction between the two fixed factors was observed (P <0.05). Anaesthetic complications were analysed with generalised linear models for binomial values. Food intake was calculated as a percentage (total soft food consumed/offered in grams × 100%). Two data sets were created, one for 2 mins and another one for 60 mins. The 2 min, but not the 60 min, data set was log10 transformed to obtain normality of the model’s residuals. These data were then evaluated using two linear mixed models with time point and group as fixed factors. Another pairwise post-hoc test with Benjamini–Hochberg correction was used for these results (P <0.05).
Results
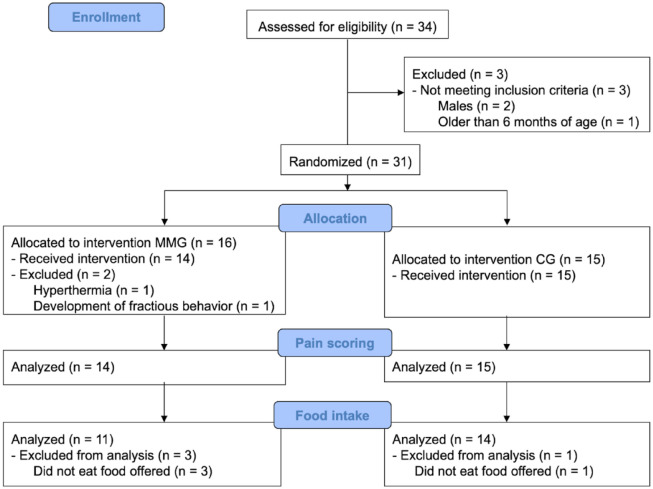
Included and excluded patients are shown in the CONSORT flow diagram (Figure 2). A total of 29 and 25 kittens were included for pain assessment and food intake analysis, respectively. There were no differences between groups for age, body weight or body condition scores, or for variables associated with surgery or anaesthesia (Table 1).
Figure 2.
CONSORT flow diagram of a randomised, prospective, blinded, clinical trial in kittens undergoing ovariohysterectomy after an intramuscular injection of ketamine–dexmedetomidine–midazolam. Kittens were randomly allocated to either the multimodal analgesia group (MMG) or the control group (CG). Pain and food intake were assessed throughout the trial at specific time points
Table 1.
Demographic data and parameters related to surgery and anaesthesia in kittens undergoing ovariohysterectomy after an intramuscular injection of ketamine–dexmedetomidine–midazolam and randomly allocated to the multimodal analgesia group (MMG) or the control group (CG)
| CG | MMG | P value | |
|---|---|---|---|
| Body condition score (1–9) | 4.5 (4.5–5) | 5 (4.5–5) | 0.591 |
| Age (weeks) | 16.2 ± 4.2 | 16.6 ± 5.1 | 0.801 |
| Weight (kg) | 1.6 ± 0.5 | 1.5 ± 0.4 | 0.688 |
| Duration of surgery (mins) | 14 (13–18) | 14 (13–15) | 0.354 |
| Incision length (cm) | 1.4 (1.2–1.6) | 1.3 (1.1–1.5) | 0.467 |
| Onset of action of anaesthesia (s) | 105 (86–116) | 101 (72–114) | 0.214 |
| Duration of anaesthesia (mins) | 41.0 (38.5–45.5) | 40.5 (38.25–41.75) | 0.481 |
| Time to head lift (mins) | 17 (16–18) | 19 (18–20) | 0.062 |
| Time to sternal recumbency (mins) | 18 (17–19) | 20 (19–20) | 0.226 |
Data are presented as mean ± SD or median (interquartile range)
The prevalence of rescue analgesia was higher in cats in the CG (n = 15/15) than in cats in the MMG (n = 1/14; P <0.001) (Table 2). At 6 h postoperatively, all kittens in the CG had received rescue analgesia; therefore, statistical comparisons for pain scores and food intake were not performed after this time point.
Table 2.
Cumulative number of kittens that received rescue analgesia during postoperative pain assessment of kittens undergoing ovariohysterectomy after an intramuscular injection of ketamine–dexmedetomidine–midazolam and randomly allocated to the multimodal analgesia group (MMG) or the control group (CG)
| Time point (h) | CG (n = 15) | MMG (n = 14) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 7 | 0 |
| 4 | 12 | 0 |
| 6 | 15 | 1 |
| 8 | 15 | 1 |
| 12 | 15 | 1 |
| 24 | 15 | 1 |
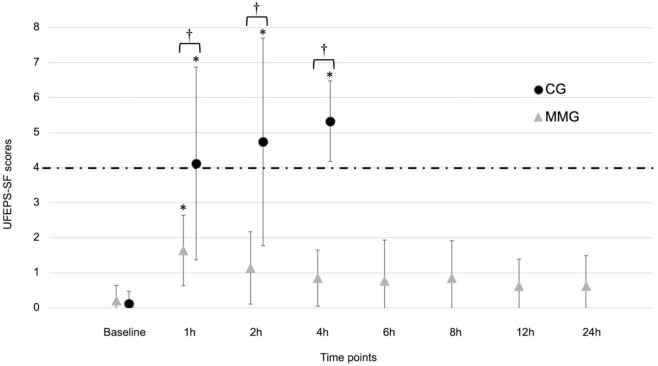
Pain scores were higher (P <0.001) in cats in the CG at 1, 2 and 4 h postoperatively (4.1 ± 2.8, 4.8 ± 3.0 and 5.3 ± 1.2, respectively) than in cats in the MMG (1.6 ± 1.0, 1.1 ± 1.0 and 0.9 ± 0.8, respectively; Figure 3). When compared with baseline (0.1 ± 0.4 for the CG and 0.2 ± 0.4 for the MMG), pain scores were increased at 1 h, 2 h and 4 h in the CG (4.1 ± 2.8, 4.8 ± 3.0 and 5.3 ± 1.2, respectively; P <0.001) and at 1 h in the MMG (1.6 ± 1.0; P = 0.027).
Figure 3.
Perioperative UNESP-Botucatu multidimensional feline pain assessment scale – short form (UFEPS-SF) scores (mean ± SD) in kittens undergoing ovariohysterectomy after an intramuscular injection of ketamine–dexmedetomidine–midazolam. Kittens were randomly allocated to either the multimodal analgesia group (MMG) or the control group (CG).
*Significant difference between time point and baseline. †Significant difference between treatment groups
Anaesthetic complications are reported in Table 3. The prevalence of cats presenting with tachycardia was significantly higher in the CG (n = 4) than in the MMG (n = 0; P = 0.015).
Table 3.
Anaesthetic complications in kittens undergoing ovariohysterectomy after an intramuscular injection of ketamine–dexmedetomidine–midazolam and randomly allocated to the multimodal analgesia group (MMG) or the control group (CG)
| CG (n = 15) | MMG (n = 14) | P value | |
|---|---|---|---|
| Isoflurane needed | 2 | 0 | 0.096 |
| Desaturation | 2 | 1 | 0.581 |
| Tachycardia | 4 | 0 | 0.015 |
| Bradycardia | 0 | 1 | 0.222 |
| Tachypnoea | 10 | 5 | 0.093 |
Data are presented as the number of kittens presenting each complication
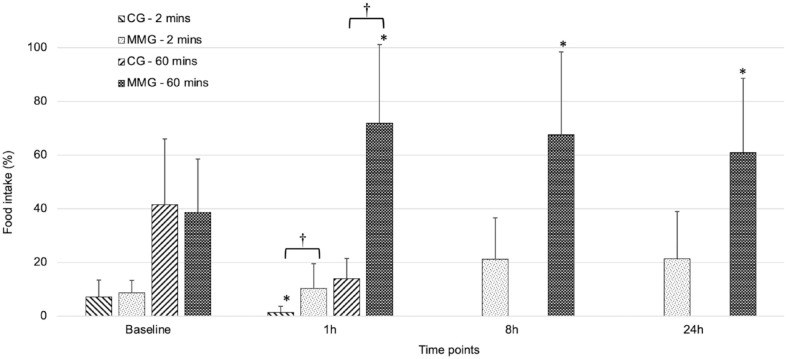
Food intake (%) was higher in cats in the MMG than in the CG for 2 mins (15.2 ± 13 and 4.2 ± 5, respectively; P <0.001) and 60 mins (58.9 ± 30 and 29.9 ± 23, respectively; P = 0.008) when all time points were considered. Baseline values were not statistically different between groups after 2 mins (9.0 ± 5 for the MMG and 7.0 ± 6 for the CG; P = 0.329) and 60 mins (39.6 ± 21 for the MMG and 40.5 ± 24 for the CG; P = 0.716) of food intake (Figure 4). At 1 h postoperatively, food intake (%) after 2 mins and 60 mins was higher in cats in the MMG (10.4 ± 9 and 71.9 ± 29, respectively) than in cats in the CG (1.4 ± 2 and 13.9 ± 7, respectively; P <0.001).
Figure 4.
Food intake (% total soft food consumed/offered [g]; mean ± SD) in kittens undergoing ovariohysterectomy after an intramuscular injection of ketamine–dexmedetomidine–midazolam. Kittens were randomly allocated to either the multimodal analgesia group (MMG) or the control group (CG).
*Significant difference between baseline and time point. †Significant difference between treatment groups
In the CG, food intake (%) was lower at 1 h postoperatively than at baseline for 2 mins (7.0 ± 6 vs 1.4 ± 2; P <0.001) but not for 60 mins. In the MMG, baseline food intake (%) for 60 mins (39.6 ± 21) was lower than at all other time points (1 h: 71.9 ± 29 [P = 0.002]; 8 h: 67.6 ± 31 [P = 0.006]; 24 h: 61.0 ± 28 [P = 0.012]) but not after 2 mins of food intake. Rescue analgesia was positively correlated with food intake after both 2 mins and 60 mins of food intake (r = 0.96 ± 0.19; likelihood ratio test = 24.48; P <0.001).
Discussion
This clinical trial showed that multimodal analgesia using IP bupivacaine and preoperative SC administration of meloxicam almost eliminated the need for opioid therapy in kittens undergoing OVH using injectable anaesthesia. The hypotheses of the study were corroborated, as a lower prevalence of rescue analgesia, lower postoperative pain scores and higher food intake were observed in cats in the MMG vs cats in the CG. Additionally, pain scores were significantly increased up to 1 h in cats in the MMG vs 4 h in cats in the CG when compared with baseline, demonstrating another benefit of using non-opioid, multimodal analgesia in early-age neutering. There is now evidence that food intake is decreased and compromised in painful kittens when multimodal analgesia is not used.
This study provides additional information on opioid-free analgesic techniques in cats, particularly in kittens; it highlights age differences in response to surgery and analgesia in this species. For example, a recent clinical trial that included adult cats demonstrated that the same opioid-free, multimodal analgesic protocol used herein was appropriate for some, but not all, cats. A single preoperative dose of IM buprenorphine was required to eliminate the need for rescue analgesia in adult cats. 21 Contrarily, the present study showed that the opioid-free multimodal analgesia protocol provided adequate analgesia in kittens and that opioids may not always be required in cats younger than 6 months of age for adequate pain management in early-age neutering. Indeed, another previous clinical trial showed that the same opioid-free anaesthetic protocol with ketamine–dexmedetomidine–midazolam produced a lower prevalence of rescue analgesia in kittens than in adult cats. 19 It is unknown if kittens demonstrate fewer behavioural signs of pain than adult cats or express pain behaviours differently, or if veterinarians are not properly trained to recognise the subtle presence of pain in these young patients.4,19 Furthermore, feline acute pain assessment tools have not been uniquely validated in kittens and it is difficult to know how their specific behaviours may have affected pain scoring with the UFEPS-SF. In order to overcome this limitation in future studies, video recordings were performed in this study for the ongoing validation of the Feline Grimace Scale 26 in kittens.
Anaesthetic complications were similar between the two treatment groups. However, the prevalence of tachycardia during surgery was higher in cats in the CG than in cats in the MMG. Moreover, there was a trend for a higher prevalence of tachypnoea and the need for isoflurane in cats in the CG vs kittens in the MMG that was not statistically significant but could be of clinical relevance. These parameters could indicate high intraoperative sympathetic nervous stimulation caused by poor antinociception in cats in the CG but not in those in the MMG. These results emphasise the importance of the administration of NSAIDs and IP analgesia with bupivacaine in perioperative pain management in kittens, especially using opioid-free techniques. Overall, this study corroborates previous findings that the injectable protocol using ketamine–dexmedetomidine–midazolam provides a short onset of action with rapid immobilisation and unconsciousness, smooth anaesthetic induction, muscle relaxation, adequate depth and duration of anaesthesia for OVH, and minimal anaesthetic complications, particularly when multimodal analgesia is used and atipamezole administered.19,21 However, oxygen was required in one cat due to desaturation, and tachypnoea was observed in five cats in the MMG. It is unclear if the lack of oxygen could have compromised this particular patient outcome during field anaesthesia considering hypoxaemia. The observed tachypnoea in the other cats could be related not only to surgical stimulation, but also to an adequate and preserved response to maintaining normal CO2 levels during anaesthesia. Overall, it is important to note that this injectable protocol did not require IV administration of ketamine during surgery, which could be an advantage during busy early-age spay–neuter programmes, when IV catheters are often not placed in kittens. However, fluid therapy was provided via the administration of SC fluids postoperatively to avoid dehydration and minimise any potential NSAID-induced deleterious effects on kidney function.
Our results showed that food intake was decreased in animals in the CG when multimodal analgesia had not been used. The study design involving food intake assessment after 2 mins and 60 mins allowed us to show that kittens in the CG ate more slowly within a short period of time (2 mins) and, overall, less (60 mins) than those in the MMG. Of interest, food intake was improved with the administration of rescue analgesia. Additionally, adequate analgesia in the MMG ensured appropriate food intake postoperatively. It is unknown if food intake in kittens in the MMG was improved because they were hungry postoperatively due to preoperative fasting, if they felt more acclimated to the hospital setting after surgery and/or if any of the anaesthetics used increased postoperative appetite in the absence of pain. For example, benzodiazepines (eg, midazolam) are known to increase appetite. 27 As much as these results could have been influenced by the type of food, time points and anaesthetic/analgesic protocol used in the study, including opioid analgesia, these findings are aligned with previous literature showing that food intake is affected by acute pain. 28 For example, both dry and soft food intake after 3 mins and dry food intake after 2 h were significantly lower in painful cats undergoing multiple dental extractions compared with cats undergoing dental cleaning. 29 The present study demonstrates that nutrition is affected by acute pain, including after OVH, but it is difficult to predict how it could compromise kittens on a long-term basis, especially if rescue analgesia had not been administered. However, it is clear that appetite is affected by analgesia. Indeed, the original UNESP-Botucatu multidimensional feline pain assessment scale included appetite, 23 even if it can be a subjective assessment in most cases as it may be influenced by fear, anxiety, food type and choice, or simply if the cat is not hungry.
Many high-volume spay–neuter programmes involving early-age neutering do not include anaesthetic plans that provide significant analgesia. Anaesthetic protocols commonly include a combination of dissociative anaesthetics, benzodiazepines and α2-adrenergic receptor agonists.30,31 As much as some of these drugs have antinociceptive properties, this study showed that ketamine–dexmedetomidine–midazolam alone did not provide clinically relevant analgesia. Previous studies have mostly investigated the anaesthetic and physiological effects of these protocols, deemed adequate in terms of low mortality rate, complications, injection volumes, feasibility and financial costs.31–33 However, these studies have rarely investigated if postoperative analgesia is adequate, especially considering that patients are discharged shortly after the procedure and pain assessment cannot always be easily performed due to the feral nature of the population and also personnel availability. Even when an opioid analgesic is added to the protocol, analgesia is commonly overlooked in the study design. 34 As much as the lack of perioperative analgesia in several spay–neuter programmes is alarming, our study design mimics these practices after ethics approval. Analgesia was ensured with continuous postoperative pain assessment at predefined time points and the immediate administration of rescue analgesia once a cutoff was reached. The protocol for rescue analgesia could have included other opioids, but buprenorphine was chosen as the drug is known as an analgesic in the cat. The onset of action of IM buprenorphine has not been determined in kittens, but our clinical observations revealed that kittens expressed normal behaviours (eg, playing) no later than 15 mins after drug administration.35,36 Additionally, the observer responsible for pain recognition was trained in acute feline pain assessment before the study began. Our results showed that cat spay–neuter programmes using similar injectable protocols must include multimodal analgesia to ensure pain relief; analgesia-free protocols used in these campaigns and as reported in the literature are unacceptable because cats will be painful postoperatively. In the case of early-age neutering, our results showed that the addition of a dose of SC meloxicam and the administration of IP analgesia provided sufficient postoperative pain management. As previously reported, most cats older than 6 months would also require opioid analgesia as part of the aforementioned multimodal protocol. 21 As much as opioids require strict dispensing, storage and prescribing practices, NSAIDs and local anaesthetics are widely available and could be easily incorporated into spay–neuter programmes.35,36
Conclusions
This opioid-free protocol using multimodal analgesia produced anaesthesia with minimal anaesthetic complications, and it is a promising alternative to high-volume, high-quality, early-age spay–neuter programmes. It also provided adequate postoperative pain relief, while almost eliminating the need for rescue analgesia in kittens undergoing OVH. This study demonstrated that pain decreases food intake postoperatively when analgesia is not administered.
Acknowledgments
The authors thank Tristan Juette for statistical analysis. Nathanael Lutevele was the recipient of the World Small Animal Veterinary Association Global Pain Council Teach the Teachers programme, which allowed him to participate in the study.
Footnotes
Accepted: 2 February 2023
Author note: An abstract of this study was presented at the Association of Veterinary Anaesthetists Spring Meeting in Nafplio, Greece on 20 May 2022. This manuscript represents a portion of an MSc degree thesis of the first author (Université de Montréal).
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was awarded the Zoetis Investment in Innovation Fund, which provided partial funding for the study. Zoetis did not have any participation in the study design or manuscript review and approval. Paulo V Steagall’s laboratory is funded by a Discovery Grant of the Natural Sciences and Engineering Research Council of Canada (RGPIN-2018-03831).
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognised high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). For any animals or people individually identifiable within this publication, informed consent (verbal or written) for their use in the publication was obtained from the people involved.
ORCID iD: Annie Malo  https://orcid.org/0000-0001-7692-9527
https://orcid.org/0000-0001-7692-9527
Beatriz P Monteiro  https://orcid.org/0000-0002-5722-5687
https://orcid.org/0000-0002-5722-5687
Nathanael Lutevele  https://orcid.org/0000-0003-0647-1068
https://orcid.org/0000-0003-0647-1068
Sabrine Marangoni  https://orcid.org/0000-0001-9196-585X
https://orcid.org/0000-0001-9196-585X
Marta Garbin  https://orcid.org/0000-0002-8156-3950
https://orcid.org/0000-0002-8156-3950
Ryota Watanabe  https://orcid.org/0000-0001-8361-8794
https://orcid.org/0000-0001-8361-8794
Paulo V Steagall  https://orcid.org/0000-0003-4150-6043
https://orcid.org/0000-0003-4150-6043
References
- 1. Griffin B, Bushby PA, McCobb E, et al. The Association of Shelter Veterinarians’ 2016 veterinary medical care guidelines for spay-neuter programs. J Am Vet Med Assoc 2016; 249: 165–188. [DOI] [PubMed] [Google Scholar]
- 2. Sparkes A, Bessant C, Cope K, et al. ISFM guidelines on population management and welfare of unowned domestic cats (Felis catus). J Feline Med Surg 2013; 15: 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Posner LP, Applegate J, Cannedy A, et al. Total injectable anesthesia of dogs and cats for remote location veterinary sterilization clinic. BMC Vet Res 2020; 16: 304. DOI: 10.1186/s12917-020-02525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polson S, Taylor PM, Yates D. Effects of age and reproductive status on postoperative pain after routine ovariohysterectomy in cats. J Feline Med Surg 2014; 16: 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joyce A, Yates D. Help stop teenage pregnancy! Early-age neutering in cats. J Feline Med Surg 2011; 13: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olson P, Kustritz M, Johnston S. Early-age neutering of dogs and cats in the United States (a review). J Reprod Fertil Suppl 2001; 57: 223–232. [PubMed] [Google Scholar]
- 7. Nutter F, Levine J, Stoskopf M. Reproductive capacity of free-roaming domestic cats and kitten survival rate. J Am Vet Med Assoc 2004; 225: 1399–1402. [DOI] [PubMed] [Google Scholar]
- 8. Dickman CR, Newsome TM. Individual hunting behaviour and prey specialisation in the house cat Felis catus: implications for conservation and management. Appl Anim Behav Sci 2015; 173: 76–87. [Google Scholar]
- 9. Duffy DC, Capece P. Biology and impacts of Pacific island invasive species. 7. The domestic cat (Felis catus). Pac Sci 2012; 66: 173–212. [Google Scholar]
- 10. Deak BP, Ostendorf B, Taggart DA, et al. The significance of social perceptions in implementing successful feral cat management strategies: a global review. Animals 2019; 9: 617. DOI: 10.3390/ani9090617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. One Health Initiative. www.who.int/teams/one-health-initiative (2022, accessed 11 October 2022).
- 12. Porters N, de Rooster H, Moons CPH, et al. Prepubertal gonadectomy in cats: different injectable anaesthetic combinations and comparison with gonadectomy at traditional age. J Feline Med Surg 2015; 17: 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spain CV, Scarlett JM, Houpt KA. Long term risks and benefits of early-age gonadectomy in cats. J Am Vet Med Assoc 2004; 224: 372–380. [DOI] [PubMed] [Google Scholar]
- 14. Moons CPH, Valcke A, Verschueren K, et al. Effect of early-age gonadectomy on behavior in adopted shelter kittens – the sequel. J Vet Behav 2018; 26: 43–47. [Google Scholar]
- 15. Howe LM. Short-term results and complications of prepubertal gonadectomy in cats and dogs. J Am Vet Med Assoc 1997; 211: 57–62. [PubMed] [Google Scholar]
- 16. Porters N, de Rooster H, Verschueren K, et al. Development of behavior in adopted shelter kittens after gonadectomy performed at an early age or at a traditional age. J Vet Behav 2014; 9: 196–206. [Google Scholar]
- 17. Bruniges N, Taylor PM, Yates D. Injectable anaesthesia for adult cat and kitten castration: effects of medetomidine, dexmedetomidine and atipamezole on recovery. J Feline Med Surg 2016; 18: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roberts M, Beatty J, Dhand N, et al. Effect of age and surgical approach on perioperative wound complication following ovariohysterectomy in shelter-housed cats in Australia. JFMS Open Rep 2015; 1. DOI: 10.1177/2055116915613358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diep TN, Monteiro BP, Evangelista MC, et al. Anesthetic and analgesic effects of an opioid-free, injectable protocol in cats undergoing ovariohysterectomy: a prospective, blinded, randomized clinical trial. Can Vet J 2020; 61: 621–628. [PMC free article] [PubMed] [Google Scholar]
- 20. Polson S, Taylor PM, Yates D. Analgesia after feline ovariohysterectomy under midazolam-medetomidine-ketamine anaesthesia with buprenorphine or butorphanol, and carprofen or meloxicam: a prospective, randomised clinical trial. J Feline Med Surg 2012; 14: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rufiange M, Ruel H, Monteiro B, et al. A randomized, prospective, masked clinical trial comparing an opioid-free vs. opioid-sparing anesthetic technique in adult cats undergoing ovariohysterectomy. Front Vet Sci 2022; 9. DOI: 10.3389/fvets.2022.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hollingsworth H, Herndon C. The parenteral opioid shortage: causes and solutions. J Opioid Manag 2018; 14: 81–82. [DOI] [PubMed] [Google Scholar]
- 23. Belli M, de Oliveira AR, de Lima MT, et al. Clinical validation of the short and long UNESP-Botucatu scales for feline pain assessment. PeerJ 2021; 9. DOI: 10.7717/peerj.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benito J, Monteiro B, Beaudry F, et al. Efficacy and pharmacokinetics of bupivacaine with epinephrine or dexmedetomidine after intraperitoneal administration in cats undergoing ovariohysterectomy. Can J Vet Res 2018; 82: 124–130. [PMC free article] [PubMed] [Google Scholar]
- 25. Chandler ML, Takashima G. Nutritional concepts for the veterinary practitioner. Vet Clin North Am Small Anim Pract 2014; 44: 645–666. [DOI] [PubMed] [Google Scholar]
- 26. Cheng AJ, Malo A, Garbin M, et al. Construct validity, responsiveness, and reliability of the Feline Grimace Scale in kittens [abstract]. Vet Anaesth Analg 2023; 50: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Macy DW, Gasper PW. Diazepam-induced eating in anorexic cats. J Am Anim Hosp Assoc 1985; 21: 17–20. [Google Scholar]
- 28. Steagall P, Monteiro B. Acute pain in cats: recent advances in clinical assessment. J Feline Med Surg 2019; 21: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watanabe R, Doodnaught G, Proulx C, et al. A multidisciplinary study of pain in cats undergoing dental extractions: a prospective, blinded, clinical trial. PLoS One 2019; 14. DOI: 10.1371/journal.pone.0213195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pestean C, Oana L, Bel L, et al. A survey of canine anaesthesia in veterinary practice in Cluj-Napoca. Bull UASVM Vet Med 2016; 73: 325–328. [Google Scholar]
- 31. Williams LS, Levy JK, Robertson SA. Use of the anesthetic combination of tiletamine, zolazepam, ketamine, and xylazine for neutering feral cats. J Am Vet Med Assoc 2002; 220: 1491–1495. [DOI] [PubMed] [Google Scholar]
- 32. Khenissi L, Nikolayenkova-Topie O, Broussaud S, et al. Comparison of intramuscular alfaxalone and ketamine combined with dexmedetomidine and butorphanol for castration in cats. J Feline Med Surg 2017; 19: 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cistola AM, Golder FJ, Centonze LA, et al. Anesthetic and physiologic effects of tiletamine, zolazepam, ketamine, and xylazine combination (TKX) in feral cats undergoing surgical sterilization. J Feline Med Surg 2004; 6: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ko JC, Abbo LA, Weil AB, et al. A comparison of anesthetic and cardiorespiratory effects of tiletamine-zolazepam-butorphanol and tiletamine-zolazepam-butorphanol-medetomidine in cats. Vet Ther 2007; 8: 164–176. [PubMed] [Google Scholar]
- 35. Steagall PV, Robertson S, Simon B, et al. 2022 ISFM consensus guidelines on the management of acute pain in cats. J Feline Med Surg 2022; 24: 4–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monteiro BP, Lascelles BDX, Murrell J, et al. 2022 WSAVA guidelines for the recognition, assessment and treatment of pain. J Small Anim Pract. Epub ahead of print 27 October 2022. DOI: 10.1111/jsap.13566. [DOI] [Google Scholar]