Abstract
Objectives
Feline primary laryngeal or tracheal lymphoma (PLTL) is an uncommon extranodal presentation. Information on long-term survival is scarce, although some small case series describe this being achieved with multiagent protocols; an accurate outcome for cats with PLTL is yet to be determined. The aim of this study was to gather information on the clinical presentation, response to treatment and outcome in a large case series of feline PLTL.
Methods
This retrospective multicentre study included cats with a cytological or histopathological confirmation of PLTL. Histopathology samples, when available, were reassessed for grade and immunophenotype. Clinical (age, signalment, retroviral status, presence of anaemia, clinical signs, location and therapy type) and outcome (response, progression-free survival [PFS] and overall survival [OS]) variables were recorded. Survival analyses to assess the impact of variables on PFS and OS were performed.
Results
Twenty-three cases were included; cats had a median age of 11 years (range 2–16) and the male:female ratio was 3.6:1. Common clinical signs at presentation included increased respiratory effort (74%) and abnormal upper respiratory tract sounds (48%). Immunophenotyping was performed in 48% of cases and all were B cell. Debulking surgery was performed in 26% of cases. All cats received chemotherapy, COP (cyclophosphamide, vincristine and prednisolone; 39%), CHOP (cyclophosphamide, vincristine, doxorubicin and prednisolone; 44%) and other protocols (17%); 35% had a partial response and 65% a complete response. Median PFS and OS were 909 days (range 23–1484) and 909 days (range 23–2423), respectively. Complete response was associated with longer PFS (P <0.001) and OS (P = 0.012). Pretreatment with steroids was associated with longer OS (P = 0.003). No other variable was found to be significant.
Conclusions and relevance
PLTL in cats is mostly of a B-cell phenotype, could be of a low-to-medium grade, and may respond to surgical and medical treatment with a longer survival time than has previously been reported.
Keywords: Feline lymphoma, chemotherapy, prognosis, immunophenotype
Introduction
Lymphoma is the most frequently diagnosed tumour in cats, accounting for 30% of all feline malignancies in most studies,1–3 although squamous cell carcinoma might be over-represented in European Shorthair cats. 4 The incidence of lymphoma affecting the lymphoreticular system has decreased in recent decades owing to the advent of routine vaccination against feline leukaemia virus (FeLV), which has been historically associated with this lymphoma presentation in cats.3,5–11 An extranodal presentation is currently the most frequent form of lymphoma in cats, with abdominal involvement and atypical locations (eg, central nervous system [CNS] or nasopharyngeal region) accounting for 50% and 20% of cases, respectively. 10 Extranodal locations have been shown to have different outcomes, with nasal lymphoma being associated with longer survival times, and CNS involvement with shorter survival. 12
Some studies have reported potential longer survival times for feline primary laryngeal or tracheal lymphoma (PLTL). To our knowledge, just six published papers have described primary feline PLTL lymphoma, and these will be described from the newest to the oldest.12–17 The most recent reports three cats with laryngeal lymphoma treated with partial laryngectomy, where two cats achieved long survival times with and without chemotherapy, respectively. 13 The second most recent paper, and also the most extensive, reported the outcome of 11 cases diagnosed with laryngeal lymphoma, of which eight received a multidrug chemotherapy protocol. All treated cases achieved complete remission, with a median survival time of 112 days. 12 The same study described four tracheal lymphomas; these were included in a miscellaneous group, which meant individual data regarding response and survival were not available. The third paper described four cats with laryngeal lymphoma, with just two receiving chemotherapy and one achieving a survival time of 1440 days before dying of unrelated causes. 14 The fourth paper described two cats with primary disease in the pharyngeal/laryngeal location, and both achieved complete remission, with survival times of 242 and 265 days, respectively, after treatment with a multiagent chemotherapy protocol. 15 The fifth publication described 27 cases of laryngeal or tracheal masses in cats, including nine lymphomas, but no specific survival data on each case were provided; the median survival time for all cases that received miscellaneous therapy was 121 days. 16 The final paper was a case series of tracheal lymphoma in four cats, of which three received chemotherapy ± radiotherapy, with two patients achieving survival times of 17 and 19 months, respectively. 17
The objective of this study was to describe the signalment, clinical presentation, biological behaviour and outcome of cats diagnosed with feline PLTL; specifically, we wanted to investigate immunophenotype, response and survival for each lymphoma location (laryngeal or tracheal) and in a larger number of cats. In addition, potential prognostic factors were also assessed.
Materials and methods
This was a retrospective, multi-institutional study; the inclusion criteria were as follows: cytological and/or histopathological diagnosis of lymphoma and presenting clinical signs associated with a non-nasal upper respiratory airway mass (dyspnoea, tachypnoea, dysphagia, dysphonia and/or stridor). The medical records were searched for client-owned cats diagnosed with non-nasal upper respiratory airway (pharyngeal, laryngeal and tracheal) lymphoma from 2004 to 2016 at three veterinary referral hospitals in the UK (Royal Veterinary College; Willows Veterinary Centre and Referral Service; and Animal Health Trust) and one in Spain (AUNA Especialidades Veterinarias). Data regarding signalment, presenting clinical signs, tumour anatomical location (laryngeal or tracheal), retroviral status, diagnostic tests, immunophenotype, histological grade, prior use of corticosteroids, chemotherapy protocol, response to chemotherapy and outcome were reviewed.
Staging procedures were at the discretion of the clinician in charge of the case and consisted of complete blood count (CBC); packed cell volume (PCV); biochemistry (BC); urinalysis (UA); CT of the head/neck, thorax and abdomen; and thoracic radiographs and/or abdominal ultrasound. Cytology samples were collected from abdominal organs, including lymph nodes ± internal organs (eg, liver and spleen).
Regarding lymphoma location, cats were retrospectively assigned to two groups (laryngeal or tracheal) depending on where the main lesion was found, based on direct visualisation or imaging findings. Diagnosis was made based on cytology or histopathology. The histopathology samples, when available, were reviewed by a single board-certified veterinary anatomical pathologist (SLP), in order to confirm the final diagnosis. Lymphoma grading was performed in cases where a histopathology sample was available. 18 Immunohistochemistry or immunocytochemistry were performed using antibodies against CD3 and CD79a in cases in which immunophenotyping had not been performed.
Treatment was recorded for each patient. Surgery was considered part of the treatment modality in cases where a debulking surgery was performed; patients that underwent an incisional biopsy for diagnostic purposes were not considered to have received surgical treatment. Induction chemotherapy protocols were classified into three categories: COP (cyclophosphamide, vincristine and prednisolone)-based protocols, CHOP (cyclophosphamide, vincristine, doxorubicin and prednisolone)-based protocols and other miscellaneous treatments (see Table 1). CHOP protocols were of different durations (19 or 25 weeks), and some patients received L-asparaginase as part of the protocols. Information regarding rescue protocols was also recorded.
Table 1.
Summary of signalment, clinical presentation, pathological findings, therapy, response and survival for all cases included in the study
| Case no. | Signalment (age, sex, breed) | Disease location | Presenting signs | Diagnostic method | Staging method | IHC results | Histological grade | Debulking surgery | Pretreatment with steroids | First chemotherapy treatment | Response to therapy | Main AEs (grade) | Subsequent therapies | Cause of death | PFS (days) | OS (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 11 years 11 months, MN, DSH | Larynx | Lethargy, IRE | C, H | CBC, BC | B cell | Low grade, large cell | No | No | P | CR | None | LP | LTF | 94 | 94 |
| 2 | 11 years, MN, DSH | Larynx | URN, IRE | C, H | CBC, BC, TR | B cell | Low grade, large cell | Yes | Yes | COP | PR | GI (1) | LP | Lymphoma | 23 | 101 |
| 3 | 12 years 9 months, MN, DSH | Trachea | Wheezing and cough | C | CBC, BC, TR | – | – | No | No | COP | CR | Anorexia (1) | LP | Lymphoma | 909 | 909 |
| 4 | 14 years 6 months, MN, DSH | Trachea | Inspiratory dyspnoea, stertor, IRE | C, H | PCV | B cell | Low grade, medium cell | No | Yes | L-COP | CR | Grade 4 neutropenia | LP | Other | NA | 534 |
| 5 | 9 years 7 months, MN, DSH | Larynx | Dysphonia, IRE | H | CT | B cell | Medium grade, large cell | Yes | Yes | COP | CR | None | LP | Lymphoma | NA | 734 |
| 6 | 10 years, MN, DSH | Larynx | Lethargy, dyspnoea | C, H | CBC, BC, UA, TR, AUS | B cell | Low grade, medium cell | No | No | L-asparaginase, P | PR | None | – | Lymphoma | 23 | 23 |
| 7 | 2 years 6 months, ME, DSH | Larynx | URN | C, H | CBC, BC, TR | B cell | Low grade, medium cell | No | Yes | CHOP | PR | GI (1), urinary (1) | Cyclophosphamide | Lymphoma | 93 | 2423 |
| 8 | 5 years 1 month, MN, DSH | Trachea | Dyspnoea, URN | C, H | CBC, BC, TR | T-cell rich, B cell | Low grade, large cell | Yes | Yes | CHOP | CR | Neutropenia (2) | Other | 973 | 973 | |
| 9 | 8 years, FN, DSH | Larynx | Stertor, dysphagia | C, H | PCV | B cell | Low grade, medium cell | No | No | COP | CR | Anorexia (1), neutropenia (2) | Vinblastine 3, LP | NA – alive | NA | 457 |
| 10 | 16 years 4 months, FN, DSH | Larynx | IRE, stertor, cough | C, H | None | – | – | Yes | No | COP | CR | Neutropenia (2) | LP | Lymphoma | NA | 558 |
| 11 | 10 years 9 months, MN, Siamese | Larynx | Chronic cough, wheezing, dysphonia | H | CBC, BC, TR, AUS | – | – | No | Yes | LP | CR | None | Vincristine | Other | 78 | 803 |
| 12 | 13 years, MN, DSH | Larynx | Inspiratory dyspnoea, dysphonia, shaking head | C | None | – | – | No | Yes | COP | CR | Lethargy (1), neutropenia (4) | LP | LTF | NA | 549 |
| 13 | 8 years 11 months, MN, DSH | Larynx | Stridor, dysphonia | C | CBC, BC, TR, AUS | – | – | No | Yes | CHOP | CR | Acute dyspnoea after doxorubicin | Second CHOP, third L-CP | Lymphoma | 957 | 1086 |
| 14 | 15 years 1 month, MN, Tonkinese | Trachea | Inspiratory dyspnoea | Necropsy | CBC, BC, TR, AUS, UA | – | – | No | No | L-CHOP (mitoxantrone) | PR | None | – | Lymphoma | 144 | 168 |
| 15 | 16 years 3 months, MN, Siamese | Trachea | Inspiratory dyspnoea | C | TR | – | – | No | No | L-CHOP | CR | None | – | Lymphoma | 336 | 360 |
| 16 | 12 years 6 months, FN, DSH | Trachea | Inspiratory dyspnoea | Necropsy | CBC, BC, TR, AUS, UA | – | – | No | No | L-asparaginase (×5) | PR | None | – | Lymphoma | 168 | 184 |
| 17 | 9 years 2 months, MN, DSH | Larynx | Stridor and inspiratory dyspnoea | C | CBC, BC, TR, AUS | – | – | No | No | L-CHOP | CR | Alopecia (1) | – | NA – alive | NA | 1484 |
| 18 | 12 years 3 months, ME, DSH | Larynx | Lethargy, dyspnoea | C, H | CBC, BC, TR, UA | – | – | No | No | L-COP | PR | Anorexia (2) | – | Lymphoma | 98 | 160 |
| 19 | 10 years 2 months, MN, Siamese | Larynx/tonsils | Anorexia, inspiratory dyspnoea | C | CBC, BC, TR, AUS | – | – | No | Yes | L-CHOP | CR | GI (2), neutropenia (2) | – | NA – alive | NA | 597 |
| 20 | 7 years 2 months, MN, DSH | Pharynx | Anorexia, inspiratory dyspnoea | C, H | CBC, BC, TR, AUS | – | – | No | No | L-CHOP | PR | Anorexia (2) | Lomustine | Lymphoma | 86 | 543 |
| 21 | 13 years 7 months, FN, DSH | Larynx | Stridor, wheezing, dyspnoea | C, H | CBC, BC | B cell | Medium grade, medium cell | Yes | Yes | CHOP (mitoxantrone) | CR | None | – | Lymphoma | 1003 | 1012 |
| 22 | 15 years 8 months, FN, DSH | Larynx | Cough, retching, dysphagia | C, H | CBC, BC, CT | B cell | Low grade, large cell | No | No | CHOP | PR | GI (1) | – | Lymphoma | 89 | 89 |
| 23 | 9 years 3 months, MN, DSH | Larynx | IRE, retching, dysphonia | H | CBC, BC, TR | B cell | Low grade, medium cell | Yes | No | COP | CR | Diarrhoea (1), neutropenia (1) | Second lomustine, third DMAC | Lymphoma | 242 | 313 |
IHC = immunohistochemistry; AEs = adverse events; PFS = progression-free survival; OS = overall survival (days); MN = male neutered; DSH = domestic shorthair; IRE = increased respiratory effort; C = cytology; H = histopathology; CBC = complete blood count; BC = biochemistry; P = prednisolone; CR = complete remission; LP = chlorambucil and prednisolone; LTF = lost to follow-up; URN = upper respiratory noises; TR = thoracic radiographs; COP = cyclophosphamide, vincristine and prednisolone; PR = partial remission; GI = gastrointestinal; PCV = packed cell volume; L-COP = L-asparaginase, cyclophosphamide, vincristine and prednisolone; NA = not applicable; UA = urinalysis; AUS = abdominal ultrasound; ME = male entire; CHOP = cyclophosphamide, vincristine, doxorubicin and prednisolone; FN = female neutered; L-CP = L-asparaginase, cyclophosphamide and procarbazine; L-CHOP = L-asparaginase, cyclophosphamide, vincristine, doxorubicin and prednisolone; DMAC = dexametasone, melphalan, actinomycin D and cytarabine
Remission status was assessed using the Veterinary Cooperative Oncology Group (VCOG) Response Evaluation Criteria in Solid Tumours (RECIST) criteria for nodal lymphoma extrapolated to these extranodal forms of lymphoma and was also based on clinical presentation owing to the unique location. 19 Complete remission (CR) was defined as the resolution of all clinical signs and/or disappearance of all evidence of disease on the basis of direct observation or diagnostic imaging available. Partial remission (PR) was defined as a marked improvement of the clinical signs without complete resolution or ⩾30% but <100% reduction in size of the measurable masses. Stable disease (SD) was defined as no change in clinical signs or a <30% reduction in size of measurable masses. Progressive disease (PD) was defined by a deterioration of the clinical signs, an increase in the size of measurable masses of at least 20% or the appearance of new lesions in other locations.
Statistical analysis
CR and PR rates were defined as the number of cats achieving CR or PR, expressed as a percentage of the total number of cats treated and for which response information was available from the medical records. Median survival time (MST) was defined as the time between the start of treatment (chemotherapy or surgery) and the date of death, including causes unrelated to the primary tumour. Patients that were lost to follow-up or were alive by the end of the study period were censored for the purpose of survival analysis. Progression-free survival (PFS) was defined as the time from treatment initiation to the date of relapse or disease progression. PFS was censored for those patients where disease relapse was never documented.
The Kaplan–Meier product limit analysis and the log-rank test were used for survival analysis. The χ2 or Fisher’s exact test was used for comparative analysis of groups. The log-rank test was used to evaluate the following variables for influence on survival times: age, breed, sex, anatomical location (laryngeal or tracheal), retroviral status, anaemia, previous treatment with corticosteroids and response to chemotherapy. A P value <0.05 was considered to be statistically significant. All statistical analyses were performed using commercial software (SPSS Statistics, version 18.0).
Results
Twenty-three cats met the inclusion criteria for the study. One cat was excluded due to an equivocal diagnosis and lack of follow-up. The clinical characteristics of all cases are summarised in Table 1. Median age at diagnosis was 11 years (range 2–16). Breeds included 19 domestic shorthairs, three Siamese and one Tonkinese cat. There were five neutered females, 16 neutered males and two entire males. The male:female ratio was 3.6:1.
Information on presenting clinical signs was available for all cases and included increased respiratory effort (n = 17), abnormal upper respiratory tract sounds (n = 11), dysphonia (n = 5), inappetence/lethargy (n = 5), cough (n = 4), retching (n = 2) and other signs (Table 1). The mean duration of clinical signs before presentation was recorded in 21/23 cases and was 57.5 days (range 2–515). The diagnosis of lymphoma was based on cytology and histopathology in 11 cats, cytology alone in seven cats and histopathology alone in three cats. Lymphoma grade was reviewed in 11 cases, of which nine were classified as low grade and two as intermediate grade. 18 Paraffin blocks were not available for review in 12 cases. Information on cell size is summarised in Table 1. Immunohistochemistry was available in 11 cases (48%); all were consistent with B-cell lymphoma (CD79a+ and CD3−). One of these 11 cases was defined as T-cell rich, B-cell lymphoma and had histological characteristics of Hodgkin-like lymphoma.
Regarding anatomical location, 17 cases were classified as laryngeal and six as tracheal. Staging methods included CBC in 17 cases, PCV in two cases, BC in 17 cases, UA in four cases, thoracic radiographs in 15 cases, abdominal ultrasound in eight cases and CT in two cases; for the remaining cases, this information was not available (Table 1). No cases had lymphoma documented in a location other than the primary site. Fourteen cats tested negative for FeLV and feline immunodeficiency virus (FIV) antibodies, two tested positive for FIV antibodies and none tested positive for FeLV antigenaemia; seven cases were not tested or the information was not available. Prior to commencing a multidrug chemotherapy protocol, 10 cats had received corticosteroids for a mean of 42 days (range 6–515).
Complete records for the evaluation of treatment response and survival analysis were available in all cats. Debulking surgery before medical therapy was performed in six cats (26%). Nine cats received a COP protocol (39%), 10 cats received a CHOP protocol (44%) and four (17%) received different treatments (prednisolone only, prednisolone and chlorambucil, and L-asparaginase) as monotherapy. The overall response rate to COP and CHOP protocols combined was 100%: 16 (65%) CR and seven (35%) PR. Of the nine cats receiving a COP protocol, seven (77.8%) achieved CR and two (22.2%) PR. Of the 10 cats receiving the CHOP protocol, seven (70%) achieved CR and three (30%) PR. Of the cats that received different treatments, the ones receiving prednisolone alone (n = 1) or chlorambucil plus prednisolone (n = 1) achieved CR, whereas the ones receiving L-asparaginase with or without prednisolone (n = 2) achieved PR. The cat receiving prednisolone and chlorambucil as the first-line treatment was rescued successfully with a COP protocol. Thirteen cats received rescue therapy with at least one chemotherapy protocol; this information is summarised in Table 1. Of those cats, three achieved much longer PFS with the rescue protocol than with the induction protocol, as shown by a much higher overall survival vs the PFS described for the induction protocol (Table 1). All protocols were well tolerated with self-limiting, mild-to-moderate adverse events that were mainly gastrointestinal or haematopoietic in nature (Table 1). There was no statistically significant difference in response rate and proportion of PR and CR between any of the protocols. Age, breed, sex, retroviral status, tumour location and pretreatment with corticosteroids were not significantly associated with treatment response.
Outcome and follow-up
At the time of writing, 18 treated cats had died or had been euthanased, three cats were still alive and two were lost to follow-up. Based on the available data, the cause of death or euthanasia recorded was progression of lymphoma in 15 cats and causes considered unlikely related to lymphoma in three cats, including renal disease in one case and euthanasia due to general deterioration or decreased quality of life without upper airway respiratory signs in two cases (Table 1). Of these three cats, none had lymphoma properly excluded as the cause of death. The 6-month, 1-year and 2-year survival rates were 74%, 61% and 35%, respectively. Median PFS and OS was 909 days (range 23–1484) and 909 days (range 23–2423), respectively (Figure 1). PFS and OS were not statistically different in cats receiving a COP protocol (610 days and range 78–909 for both) and those receiving the CHOP protocol (957 days [range 86–2470] and 973 days [range 89–2470], respectively). Longer PFS and OS were observed for cats achieving CR (909 days for both) vs cats achieving PR (89 days and 160 days, respectively; P <0.001). Age, breed, sex, retroviral status, anatomical location, anaemia at presentation, debulking surgery and pretreatment with corticosteroids did not affect PFS or OS.
Figure 1.
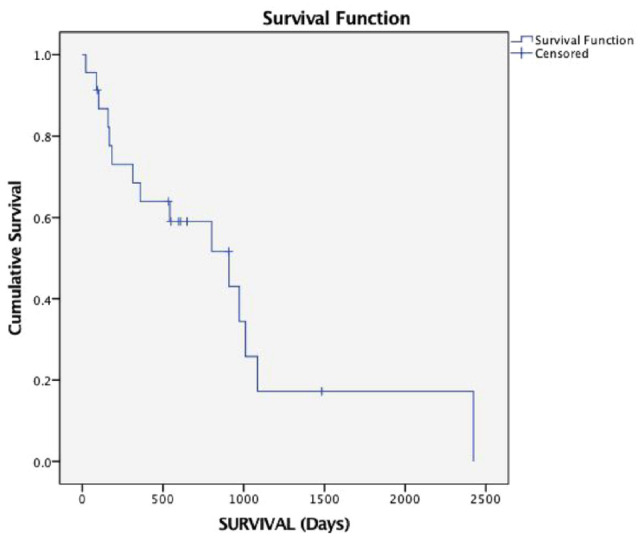
Kaplan–Meier curve depicting survival time of all cats in the study
Discussion
The results of this case series suggest that survival in cats with feline PLTL is considerably longer than previously reported.12,15–17 However, considering the limitations of a retrospective study with a relatively low number of cases, a significant proportion of patients were censored as they were lost to follow-up or still alive at the time of writing. The majority of cats that were alive 1 year after diagnosis were also alive 2 years after, and some of those died of causes presumably unrelated to the tumour or were still alive. Nevertheless, not all cats that died of unrelated causes had full staging at diagnosis or prior to euthanasia; therefore, we cannot exclude lymphoma as the cause of death for the majority of these patients. The group experiencing a longer survival could have had a low-grade tumour or simply a longer remission status. Low-grade lymphoma in cats has been correlated to longer survival not just in intestinal lymphoma, but also in nasal lymphoma cases.12,20–24
Remission rates in this study should be interpreted with caution as they are based on the assessment of clinical signs rather than an objective, measurable response evaluation. Even when no cats were documented to die of systemic lymphoma, full staging was not performed in all cases at the beginning or during the course of the disease, and therefore we cannot guarantee that all patients had primary laryngeal or tracheal lymphoma or that death was not due to lymphoma in those cases that had a different cause of death. Lymphoma should then be considered as possible cause of death in those patients, but further studies with more systematic complete staging in cases of PLTL will be needed to better determine the classic distribution of this disease in cats. Based on our findings, local restaging effort might be more justified in order to detect relapses in its initial phase and plan a rescue treatment as soon as this is confirmed, but systemic restaging should always be recommended as the gold standard in cats with PLTL. Nevertheless, in our cases, local assessment of response or relapse monitoring was rarely carried out due to difficulties in accessing the area and resolution of clinical signs. In this particular disease, owing to its location, the clinical signs as described above might appear early in the course of the disease/relapse and should be interpreted by the clinician as a warning sign as it happens in nasal lymphoma.12,20–22
The difference in days between PFS and MST could be explained by the fact that some patients had a greater response to the first rescue protocol than to the induction protocol, and also by the fact that many of the animals that were still alive at the time of writing were already long-term survivors. Interestingly, those patients that had a fast disease progression after a first-line CHOP-based protocol had a longer remission time after a lomustine single-agent first-rescue protocol, suggesting that some of the feline PLTLs (mainly low or intermediate grade in nature, as discussed later) might have a better response to alkylating agent regimens. This could be supported by the fact that low-grade intestinal lymphoma has been traditionally treated with single-agent chlorambucil and prednisolone with good mid-to-long-term survival described.24–27 Also, several studies have reported the success of single-agent cyclophosphamide as a rescue for low-grade intestinal lymphomas in cats.24–28 Nevertheless, one patient had a quick progression after a first-line chlorambucil-based protocol and also achieved a long survival time with second-line vincristine-based therapy, showing that rescue protocols may work equally well or better in these cases and that it might be difficult to predict which therapy will have the higher efficacy in each case.
All cases where the immunophenotype was available were B cell, as previously reported for other extranodal lymphomas in cats, namely nasal lymphoma, which has been reported to be mostly of B-cell origin. 29 As the immunophenotype of feline PLTLs has not been described previously, further studies will be needed to confirm this finding. Regarding grading, no patients were classified as having high-grade lymphoma and the majority were classified as low grade. This may suggest that PLTL could have a similar behaviour to nasal lymphomas, where the disease is mainly localised and low grade in nature, with local recurrence of the disease being the limiting factor, rather than systemic involvement.12,20–22 Also, this finding is in concordance with the long survival times described in this study.
Conclusions
Although this was a retrospective case series, our results suggest that laryngeal and tracheal lymphoma in cats is mostly of a B-cell phenotype, could be of low-to-medium grade and may respond to surgical and medical treatment with longer survival times than previously reported. Clinicians should be aware that this location of lymphoma in cats might carry a better prognosis than previously reported and discussion with owners should take this information in consideration.
Footnotes
Accepted: 16 November 2022
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognised high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore, additional informed consent for publication was not required.
ORCID iD: Ignasi Rodriguez-Piza  https://orcid.org/0000-0002-1339-274X
https://orcid.org/0000-0002-1339-274X
Elisabetta Treggiari  https://orcid.org/0000-0002-5081-2440
https://orcid.org/0000-0002-5081-2440
References
- 1. Dorn CR, Taylor DO, Hibbard HH. Epizootiologic characteristics of canine and feline leukemia and lymphoma. Am J Vet Res 1967; 28: 993–1001. [PubMed] [Google Scholar]
- 2. Mooney SC, Hayes AA, MacEwen EG, et al. Treatment and prognostic factors in lymphoma in cats: 103 cases (1977–1981). J Am Vet Med Assoc 1989; 194: 696–702. [PubMed] [Google Scholar]
- 3. Vail DM, Moore AS, Ogilvie GK, et al. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med 1998; 12: 349–354. [DOI] [PubMed] [Google Scholar]
- 4. Manuali E, Forte C, Vichi G, et al. Tumours in European Shorthair cats: a retrospective study of 680 cases. J Feline Med Surg 2020; 22: 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Endo Y, Cho KW, Nishigaki K, et al. Molecular characteristics of malignant lymphomas in cats naturally infected with feline immunodeficiency virus. Vet Immunol Immunopathol 1997; 57: 153–167. [DOI] [PubMed] [Google Scholar]
- 6. Gabor LJ, Jackson ML, Trask B, et al. Feline leukaemia virus status of Australian cats with lymphosarcoma. Aust Vet J 2001; 79: 476–481. [DOI] [PubMed] [Google Scholar]
- 7. Rezanka LJ, Rojko JL, Neil JC. Feline leukemia virus: pathogenesis of neoplastic disease. Cancer Invest 1992; 10: 371–389. [DOI] [PubMed] [Google Scholar]
- 8. Rojko JL, Kociba GJ, Abkowitz JL, et al. Feline lymphomas: immunological and cytochemical characterization. Cancer Res 1989; 49: 345–351. [PubMed] [Google Scholar]
- 9. Takahashi R, Goto N, Ishii H, et al. Pathological observations of natural cases of feline lymphosarcomatosis. Nihon Juigaku Zasshi 1974; 36: 163–173. [DOI] [PubMed] [Google Scholar]
- 10. Louwerens M, London CA, Pedersen NC, et al. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med 2005; 19: 329–335. [DOI] [PubMed] [Google Scholar]
- 11. Cristo TG, Biezus G, Noronha LF, et al. Feline lymphoma and a high correlation with feline leukaemia virus infection in Brazil. J Comp Pathol 2019; 166: 20–28. [DOI] [PubMed] [Google Scholar]
- 12. Taylor SS, Goodfellow MR, Browne WJ, et al. Feline extranodal lymphoma: response to chemotherapy and survival in 110 cats. J Small Anim Pract 2009; 50: 584–592. [DOI] [PubMed] [Google Scholar]
- 13. Moser J, Haimel G, Tichy A, et al. Partial laryngectomy for the management of laryngeal masses in six cats. J Feline Med Surg 2022; 24: 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor SS, Harvey AM, Barr FJ, et al. Laryngeal disease in cats: a retrospective study of 35 cases. J Feline Med Surg 2009; 11: 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simon D, Eberle N, Laacke-Singer L, et al. Combination chemotherapy in feline lymphoma: treatment outcome, tolerability, and duration in 23 cats. J Vet Intern Med 2008; 22: 394–400. [DOI] [PubMed] [Google Scholar]
- 16. Jakubiak MJ, Siedlecki CT, Zenger E, et al. Laryngeal, laryngotracheal, and tracheal masses in cats: 27 cases (1998–2003). J Am Anim Hosp Assoc 2005; 41: 310–316. [DOI] [PubMed] [Google Scholar]
- 17. Brown MR, Rogers KS, Mansell KJ, et al. Primary intratracheal lymphosarcoma in four cats. J Am Anim Hosp Assoc 2003; 39: 468–472. [DOI] [PubMed] [Google Scholar]
- 18. Valli VE, San Myint M, Barthel A, et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol 2011; 48: 198–211. [DOI] [PubMed] [Google Scholar]
- 19. Vail DM, Michels GM, Khanna C, et al. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0) – a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol 2010; 8: 28–37. [DOI] [PubMed] [Google Scholar]
- 20. Haney SM, Beaver L, Turrel J, et al. Survival analysis of 97 cats with nasal lymphoma: a multi-institutional retrospective study (1986–2006). J Vet Intern Med 2009; 23: 287–294. [DOI] [PubMed] [Google Scholar]
- 21. Fujiwara-Igarashi A, Fujimori T, Oka M, et al. Evaluation of outcomes and radiation complications in 65 cats with nasal tumours treated with palliative hypofractionated radiotherapy. Vet J 2014; 202: 455–461. [DOI] [PubMed] [Google Scholar]
- 22. Sfiligoi G, Théon AP, Kent MS. Response of nineteen cats with nasal lymphoma to radiation therapy and chemotherapy. Vet Radiol Ultrasound 2007; 48: 388–393. [DOI] [PubMed] [Google Scholar]
- 23. Gabor LJ, Malik R, Canfield PJ. Clinical and anatomical features of lymphosarcoma in 118 cats. Aust Vet J 1998; 76: 725–732. [DOI] [PubMed] [Google Scholar]
- 24. Fondacaro JV, Richter KP, Carpenter JL, et al. Feline gastrointestinal lymphoma: 67 cases (1988–1996). Eur J Comp Gastroenterol 1999; 4: 69–74. [Google Scholar]
- 25. Kiselow MA, Rassnick KM, McDonough SP, et al. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995–2005). J Am Vet Med Assoc 2008; 232: 405–410. [DOI] [PubMed] [Google Scholar]
- 26. Stein TJ, Pellin M, Steinberg H, et al. Treatment of feline gastrointestinal small-cell lymphoma with chlorambucil and glucocorticoids. J Am Anim Hosp Assoc 2010; 46: 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pope KV, Tun AE, McNeill CJ, et al. Outcome and toxicity assessment of feline small cell lymphoma: 56 cases (2000–2010). Vet Med Sci 2015; 1: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim C, Wouda RM, Borrego J, et al. Cyclophosphamide rescue therapy for relapsed low-grade alimentary lymphoma after chlorambucil treatment in cats. J Feline Med Surg 2021; 23: 976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santagostino SF, Mortellaro CM, Boracchi P, et al. Feline upper respiratory tract lymphoma: site, cyto-histology, phenotype, FeLV expression, and prognosis. Vet Pathol 2015; 52: 250–259. [DOI] [PubMed] [Google Scholar]


