Abstract
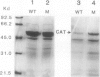
Experiments were conducted with a tobacco (Nicotiana tabacum) mutant with 40 to 50% greater catalase activity than wild type that is associated with a novel form of O2-resistant photosynthesis. The apparent Km for H2O2 was the same in mutant and wild-type leaf extracts. Tobacco RNAs were hybridized with Nicotiana sylvestris catalase cDNA, and a threefold greater steady-state level of catalase mRNA was found in mutant leaves. Steady-state levels of ribulose-1,5-bisphosphate carboxylase small subunit mRNA were similar in mutant and wild type. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of partially purified catalase showed that the protein concentration in the band corresponding to catalase was higher in the mutant than in the wild type. Separation of leaf catalase proteins by isoelectric focusing revealed the presence of five major bands and one minor band of activity. The distribution of the catalase activity among these forms was similar in mutant and wild type, although the total activity was higher in the mutant in all five major bands. The results indicate that the enhanced catalase activity in mutant leaves is caused by an increase in synthesis of catalase protein and that this trait is mediated at the nucleic acid level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clare D. A., Duong M. N., Darr D., Archibald F., Fridovich I. Effects of molecular oxygen on detection of superoxide radical with nitroblue tetrazolium and on activity stains for catalase. Anal Biochem. 1984 Aug 1;140(2):532–537. doi: 10.1016/0003-2697(84)90204-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Havir E. A., McHale N. A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987 Jun;84(2):450–455. doi: 10.1104/pp.84.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havir E. A., McHale N. A. Purification and characterization of an isozyme of catalase with enhanced-peroxidatic activity from leaves of Nicotiana sylvestris. Arch Biochem Biophys. 1990 Dec;283(2):491–495. doi: 10.1016/0003-9861(90)90672-l. [DOI] [PubMed] [Google Scholar]
- Havir E. A., McHale N. A. Regulation of Catalase Activity in Leaves of Nicotiana sylvestris by High CO(2). Plant Physiol. 1989 Mar;89(3):952–957. doi: 10.1104/pp.89.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W., Turley R. B., Trelease R. N. Characterization of a cDNA encoding cottonseed catalase. Biochim Biophys Acta. 1990 Jun 21;1049(2):219–222. doi: 10.1016/0167-4781(90)90044-3. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E. F., Dannelly H. K., Malloy P. J., Reeves H. C. Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal Biochem. 1987 Dec;167(2):290–294. doi: 10.1016/0003-2697(87)90166-7. [DOI] [PubMed] [Google Scholar]
- Zelitch I. Further studies on o(2)-resistant photosynthesis and photorespiration in a tobacco mutant with enhanced catalase activity. Plant Physiol. 1990 Feb;92(2):352–357. doi: 10.1104/pp.92.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Physiological investigations of a tobacco mutant with o(2)-resistant photosynthesis and enhanced catalase activity. Plant Physiol. 1990 Aug;93(4):1521–1524. doi: 10.1104/pp.93.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Selection and characterization of tobacco plants with novel o(2)-resistant photosynthesis. Plant Physiol. 1989 Aug;90(4):1457–1464. doi: 10.1104/pp.90.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]