Abstract
To identify genes encoding adhesins that mediate the binding of Candida albicans to endothelial cells, a genomic library from this organism was constructed and used to transform Saccharomyces cerevisiae. These transformed organisms were screened for adherence to endothelial cells, and a highly adherent clone was identified. The adherence of this clone to endothelial cells was over 100-fold greater than that of control S. cerevisiae transformed with the empty plasmid. This clone also exhibited enhanced adherence to epithelial cells. The C. albicans gene contained within this clone was found to be ALS1. These results indicate that ALS1 may encode a candidal adhesin.
The opportunistic pathogen Candida albicans disseminates hematogenously in susceptible hosts. During the process of hematogenous dissemination, it is likely that blood-borne organisms must first adhere to and then penetrate through the endothelial cell lining of the vasculature to invade the target organs. For this reason, the adherence of C. albicans to the vascular endothelium is likely a pivotal step in the initiation of a hematogenously disseminated infection. Characterizing the adhesins that mediate the binding of C. albicans to endothelial cells is important for understanding the mechanism by which this process occurs and developing therapeutic strategies to block it.
Although considerable investigative effort has been devoted to identifying candidal adhesins, it has been difficult to characterize these adhesins at the molecular level. Previously, we used complementation cloning to identify CAD1/AAF1, a gene from C. albicans that, when expressed in Saccharomyces cerevisiae, induces enhanced adherence to endothelial cells and flocculation in vitro (3). However, the sequence of the predicted protein encoded by CAD1/AAF1 and the finding that the CAD1/AAF1 protein does not localize to the plasma membrane or cell wall indicate that the gene does not encode a cell surface adhesin. Furthermore, the endothelial cell adherence of homozygous cad1/AAF1 null mutants of C. albicans is similar to that of the wild-type parent strain (12). These results indicate that the protein encoded by CAD1/AAF1 does not contribute significantly to the adherence of C. albicans to endothelial cells in vitro.
In the present series of investigations, we constructed a genomic library of C. albicans DNA in an S. cerevisiae expression vector. By screening for adherence to human endothelial cells, we identified a clone that was highly adherent to both endothelial and epithelial cells. This clone was found to express ALS1, a gene that has homology to S. cerevisiae AGα1 and is a member of the immunoglobulin gene superfamily. These results suggest that ALS1 may encode a candidal adhesin.
Genomic library construction.
To construct the genomic library, DNA from C. albicans SC5314 was mechanically sheared and the ends were made blunt with T4 DNA polymerase (New England Biolabs, Beverly, Mass.). The DNA fragments were fractionated by agarose gel electrophoresis, after which fragments of 3 to 7 kb were pooled and ligated to XhoI adaptors. The resulting pool of DNA was ligated to XhoI-digested λ-Yes-R (kindly provided by Ronald W. Davis of Stanford University) (1, 11). The ligation mixture was packaged into λ phage with the λ phage packaging system (Stratagene, La Jolla, Calif.). A total of 1 × 107 independent clones were selected and divided into 20 sublibraries of 5 × 105 clones each. To convert the phage library into a pYesR plasmid library, the phages were transfected into a cre-producing strain of Escherichia coli, BNN132 (Ronald W. Davis). Plasmid pYesR is a single-copy vector that contains the S. cerevisiae GAL1 promoter so that expression of the candidal genes within the library can be induced. Analysis of the plasmids contained within representative transformants revealed that 95% contained inserts of candidal DNA with an average size of 4.3 kb.
Selection of adherent clones.
To select clones with enhanced adherence to endothelial cells, S. cerevisiae S150-2B (leu2 his3 trp1 ura3) was transformed with four pYesR sublibraries by the method of Gietz et al. (6). The four pools of yeast, each containing a sublibrary, were grown in minimal medium (yeast nitrogen base broth without amino acids [Difco, Detroit, Mich.] supplemented with 80 μg of l-leucine per ml, 60 μg of l-histidine per ml, and 60 μg of l-tryptophan per ml and containing either galactose, raffinose, or glucose [each 2%, wt/vol]). Expression of the candidal genes was induced by incubating the transformants in minimal medium plus galactose on a rotary shaker overnight at 30°C. The organisms were harvested by centrifugation, washed twice in Dulbecco’s phosphate-buffered saline (PBS), and then suspended in PBS containing Mg2+ and Ca2+ (PBS++). Next, a total of 3 × 108 induced cells was added to confluent monolayers of human umbilical vein endothelial cells in 100-mm-diameter tissue culture dishes. These endothelial cells had been isolated and grown by our modification of the method of Jaffe et al. (2, 9). The S. cerevisiae cells were incubated with the endothelial cells for 30 min at 37°C in 5% CO2, after which the nonadherent clones were removed by rinsing with warm PBS++ in a standardized manner. The endothelial cells and adherent clones were removed from the tissue culture dishes with a cell scraper and then sonicated briefly to lyse the endothelial cells. The S. cerevisiae cells were then transferred to minimal medium plus galactose and incubated overnight at 30°C.
This procedure was repeated a total of five times for each pool of S. cerevisiae. At the end of the selection procedure, we isolated individual clones and determined their adherence to endothelial cells using our previously described assay (5). Briefly, the organisms were grown in minimal medium plus galactose overnight and then 103 organisms in PBS++ were added to confluent endothelial cells in six-well tissue culture plates. This inoculum was confirmed by colony counting. After incubation for 30 min, the nonadherent organisms were removed by rinsing with PBS++. Next, the wells were overlaid with YPD agar (1% [wt/vol] yeast extract [Difco], 2% [wt/vol] peptone [Difco], 2% [wt/vol] glucose) and incubated at 30°C for 36 h. The number of adherent organisms was determined by colony counting, and adherence was expressed as a percentage of the original inoculum.
Testing the adherence induced by pYF-5.
After five rounds of selection, 12 clones were chosen and their adherence to endothelial cells was tested and compared to the adherence of control S. cerevisiae transformed with the empty plasmid. One clone that exhibited significantly greater adherence than did the control organism was identified. The plasmid contained within this clone was designated pYF-5. The adherence of this clone to endothelial cells was then determined. It was grown in minimal medium plus galactose to induce expression of the candidal gene in pYF-5. This clone was also grown in minimal medium plus glucose to suppress the expression of this gene. The adherence of this organism grown under these two conditions was compared to that of control organisms that contained the empty plasmid and were grown under identical conditions. In these experiments, C. albicans SC5314 was included as a positive control.
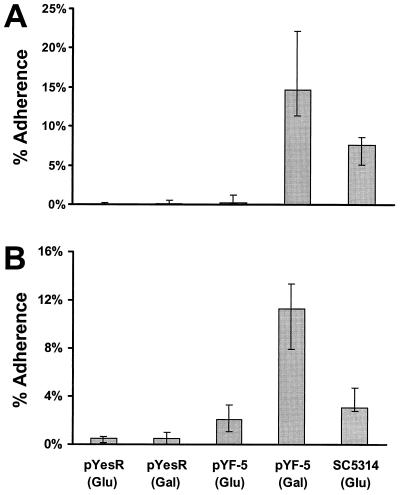
When grown in the presence of galactose, the clone containing pYF-5 exhibited 68-fold greater adherence to endothelial cells than it did when grown in the presence of glucose (P < 0.001 by the Kruskall-Wallace test) (Fig. 1A). Its adherence was also at least 100-fold greater than that of S. cerevisiae that had been transformed with vector alone and grown in either galactose or glucose (P < 0.001 for each comparison). The adherence of this clone was almost twofold greater than that of C. albicans SC5314 (P < 0.001). These findings provide strong evidence that the increased adherence of the clone containing pYF-5 was due to the expression of the candidal gene contained within this clone.
FIG. 1.
S. cerevisiae transformed with pYF-5 exhibits increase adherence to endothelial and epithelial cells. S. cerevisiae cells were transformed with either pYF-5 or the empty plasmid (pYesR). They were grown in either glucose (Glu) to suppress expression of the candidal gene or galactose (Gal) to induce expression of the candidal gene. Next, the adherence of these organisms to human umbilical vein endothelial cells (A) or FaDu epithelial cells (B) was determined. Results are the medians and interquartile ranges from at least four separate experiments, each performed in triplicate.
To confirm that the increased adherence of this clone was the result of pYF-5, the plasmid was rescued by transformation in E. coli and then retransformed into S. cerevisiae. These secondary transformants also exhibited enhanced adherence to endothelial cells when grown in the presence of galactose (data not shown).
The effect of pYF-5 on the adherence of S. cerevisiae to epithelial cells was also tested. In these experiments, the FaDu oropharyngeal epithelial cell line (American Type Culture Collection, Rockville, Md.) was used, and the adherence of the organisms to these cells was measured in a manner similar to that in the endothelial cell experiments. We found that when grown in the presence of galactose, the clone containing pYF-5 exhibited significantly higher adherence to epithelial cells than did all other organisms tested (P < 0.001 for all comparisons) (Fig. 1B).
pYF-5 contained ALS1.
We next analyzed the candidal DNA insert contained within pYF-5. Digestion of pYF-5 with XhoI released a single 6-kb insert. This insert was used as a probe for Southern blotting of DNA from C. albicans SC5314 to confirm that the insert was part of the C. albicans genome (data not shown). Next, the 1.1 kb of DNA adjacent to the GAL1 promoter within pYF-5 was sequenced. The beginning of an open reading frame was identified 82 bp downstream from this promoter. Its sequence was identical to that of ALS1, which has previously been isolated by Hoyer et al. (8).
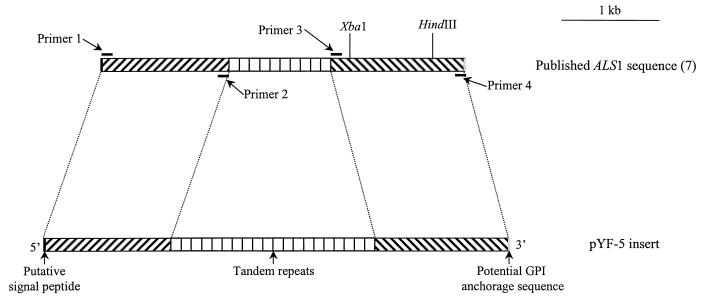
ALS1 is part of the ALS gene family, the members of which are characterized by the presence of conserved tandem repeats (8). Certain members of this gene family have been sequenced, and the 5′ ends of some of these genes are reported to have significant homology with ALS1 (7). Therefore, we performed further analyses to confirm that the insert contained within pYF-5 was ALS1 and not another member of the ALS gene family. We first constructed primers based on nucleotides 1 to 22 (primer 1; sense) and 1296 to 1280 (primer 2; antisense) of the 5′ end of the published ALS1 sequence (Fig. 2). When used in PCR with pYF-5 as the template, the expected 1.3-kb fragment was amplified. Similarly, PCR amplification using primers from positions 2399 to 2420 (primer 3; sense) and 3786 to 3763 (primer 4; antisense) of the 3′ end of the published ALS1 sequence yielded a product that was 1.4 kb, as expected. However, when primers 1 and 4 were used to PCR amplify a fragment from pYF-5, the resulting 4.9-kb product was 1.1 kb longer than was predicted by the published sequence of ALS1 (8). Although the published sequence of ALS1 is 3.8 kb, it has been reported that the size of this gene exhibits strain-to-strain differences in the number of repeats (8). Our PCR results suggest that the additional 1.1 kb within ALS1 obtained from C. albicans SC5314 is due to the presence of additional tandem repeats. Each tandem repeat unit in ALS1 is 108 bp in length; therefore, we estimate that the allele of ALS1 in pYF-5 contains 10 additional tandem repeats (Fig. 2).
FIG. 2.
Comparison of the published sequence of ALS1 (top) with the sequence of ALS1 contained in plasmid pYF-5 (bottom). GPI, glycosylphosphatidylinositol.
To confirm that the gene within pYF-5 was ALS1, we sequenced the 3′ end of this insert corresponding to positions 2610 to 3485 of the published sequence of ALS1. This region was identical to the published sequence. Using the published sequence of ALS1 as a guide, we also performed restriction mapping of the insert contained within pYF-5. These results confirmed our findings with PCR. Enzymes that cut in either the 5′ or 3′ region yielded fragments of the expected size, based on the published sequence of ALS1. However, when the DNA was digested with enzymes that cut in both the 5′ and 3′ regions, the resultant fragments were 1.1 kb longer than expected. Based on these combined results, we conclude that the allele of ALS1 contained within pYF-5 is 4.9 kb in length and contains approximately 20 tandem repeats (Fig. 2).
The ALS1 gene product has motifs characteristic of a cell surface protein.
The predicted ALS1 protein has several motifs which are characteristic of a protein expressed on the cell surface (8). First, there appears to be a glycosylphosphatidylinositol attachment site in its C terminus. Second, the N terminus contains a region resembling a signal peptide. Third, the ALS1 protein is predicted to be highly glycosylated. Fourth, the N and C termini of ALS1 have extensive homology with the S. cerevisiae AGα1 gene product, a glycoprotein that mediates cell-to-cell adhesion during mating (10). Interestingly, the N termini of both the ALS1 and AGα1 gene products have homology with the immunoglobulin superfamily (13). This superfamily contains adhesins such as intercellular adhesion molecules 1, 2, and 3 which mediate the cell-to-cell attachment of mammalian cells. This similarity to S. cerevisiae and mammalian adhesins is consistent with the hypothesis that ALS1 encodes a candidal adhesin. Our finding that expressing this gene in S. cerevisiae causes a very large increase in adherence to endothelial and epithelial cells provides additional support for this hypothesis.
Previously, we have found that another gene of C. albicans, CAD1/AAF1 causes increased endothelial cell adherence when expressed in S. cerevisiae. However, CAD1/AAF1 is significantly different from ALS1/AAF1. For example, the sequence of CAD1/AAF1 is more consistent with that of a transcription factor than that of a surface protein in that it contains multiple nuclear localization sequences but lacks a discernible signal peptide or transmembrane sequence (3). Also, S. cerevisiae expressing CAD1/AAF1 strongly flocculates, whereas S. cerevisiae expressing ALS1 was only weakly flocculent (data not shown). In addition, the adherence of S. cerevisiae transformed with CAD1/AAF1 was only fivefold greater than that of organisms transformed with the empty plasmid. However, although this increase in adherence is much less than that induced by the expression of ALS1, these two values are difficult to compare because CAD1/AAF1 was contained in a different vector than was ALS1.
Recently, Gaur and Klotz (4) described another member of the ALS gene family, ALA1. This gene was identified by its ability to cause S. cerevisiae to bind to extracellular matrix proteins. S. cerevisiae expressing ALA1 also exhibited enhanced adherence to human buccal epithelial cells. The N terminus of the predicted ALA1 gene product has significant homology with that of the ALS1 gene product. Also, the tandem repeats of the ALA1 protein are similar but not identical to those of the ALS1 protein. However, the C termini of the two predicted proteins are quite dissimilar. The functional similarities between ALS1 and ALA1 suggest that at least two members of the ALS gene family may encode candidal adhesins.
Why ALA1 or other members of the ALS gene family were not identified by the screening process used in the present investigations is unclear. One possibility is that other ALS-type genes would have been identified if we had screened more sublibraries. Alternatively, it is possible that proteins encoded by other members of the ALS gene family are not expressed in as functional a manner in S. cerevisiae as is ALS1. Finally, the ALS1 gene product may be the dominant candidal adhesin for endothelial cells. Further studies to define the role of the ALS1 gene product in the adherence of C. albicans to human cells are currently in progress.
Acknowledgments
We thank the Perinatal nurses at Harbor-UCLA Medical Center for collecting umbilical cords; Alison Orozco, Toshiko Lamkin, and Michael Mador for helping with tissue culture; and Toyota USA for donating the Olympus phase-contrast microscope used in these studies.
This work was supported in part by Public Health Service grants R01 AI-19990, P01 AI-37194, R29 AI040636, and MO1 RR00425 from the National Institutes of Health.
REFERENCES
- 1.Elledge S J, Mulligan J T, Ramer S W, Spottswood M, Davis R W. Lambda YES: a multifunctional cDNA expression vector for the isolation of genes by complementation of yeast and Escherichia coli mutations. Proc Natl Acad Sci USA. 1991;88:1731–1735. doi: 10.1073/pnas.88.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filler S G, Pfunder A S, Spellberg B J, Spellberg J P, Edwards J E., Jr Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun. 1996;64:2609–2617. doi: 10.1128/iai.64.7.2609-2617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu, Y., S. G. Filler, B. J. Spellberg, W. A. Fonzi, A. S. Ibrahim, T. Kanbe, M. A. Ghannoum, and J. E. Edwards, Jr. Cloning and characterization of CAD1/AAF1, a gene from Candida albicans that induces adherence to endothelium when expressed in Saccharomyces cerevisiae. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 4.Gaur N K, Klotz S A. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect Immun. 1997;65:5289–5294. doi: 10.1128/iai.65.12.5289-5294.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghannoum M A, Filler S G, Ibrahim A S, Fu Y, Edwards J E., Jr Modulation of the interactions of Candida albicans and endothelial cells by fluconazole and amphotericin B. Antimicrob Agents Chemother. 1992;36:2239–2244. doi: 10.1128/aac.36.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gietz D, Schiestl R H, Williams A R, Woods R A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 7.Hoyer L L, Payne T L, Bell M, Meyers A M, Scherer S. Abstracts of the 13th Congress of the International Society for Human and Animal Mycology, 1997. Leeds, United Kingdom: International Society for Human and Animal Mycology; 1997. The ALS gene family of Candida albicans, abstr. P216. [Google Scholar]
- 8.Hoyer L L, Scherer S, Shatzman A R, Livi G P. Candida albicans ALS1: domains related to a Saccharomyces cerevisiae sexual agglutinin separated by a repeating motif. Mol Microbiol. 1995;15:39–54. doi: 10.1111/j.1365-2958.1995.tb02219.x. [DOI] [PubMed] [Google Scholar]
- 9.Jaffe E A, Nachman R L, Becker C G, Ninick C R. Culture of human endothelial cells derived from umbilical veins: identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipke P N, Wojciechowicz D, Kurjan J. AGα1 is the structural gene for the Saccharomyces cerevisiae α-agglutinin, a cell surface glycoprotein involved in cell-cell interactions during mating. Mol Cell Biol. 1989;9:3155–3165. doi: 10.1128/mcb.9.8.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramer S W, Elledge S J, Davis R W. Dominant genetics using a yeast genomic library under the control of a strong inducible promoter. Proc Natl Acad Sci USA. 1992;89:11589–11593. doi: 10.1073/pnas.89.23.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rieg, G., Y. Fu, A. S. Ibrahim, X. Zhou, W. A. Fonzi, S. G. Filler, and J. E. Edwards, Jr. Heterogeneity among single/double knock-out mutants of CAD1/AAF1 in Candida albicans. Submitted for publication.
- 13.Wojciechowicz D, Lu C-F, Kurjan J, Lipke P N. Cell surface anchorage and ligand-binding domains of the Saccharomyces cerevisiae cell adhesion protein α-agglutinin, a member of the immunoglobulin superfamily. Mol Cell Biol. 1993;13:2554–2563. doi: 10.1128/mcb.13.4.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]