Abstract
Practical relevance:
Abdominal ultrasound plays a vital role in the diagnostic work-up of many cats presenting to general and specialist practitioners. Ultrasound examination of the spleen provides important information to aid the investigation of several conditions and is particularly relevant when an enlarged or irregular spleen is identified during abdominal palpation.
Clinical challenges:
Despite ultrasonography being a commonly used modality, many practitioners are not comfortable performing an ultrasound examination or interpreting the resulting images. Even for the experienced ultrasonographer, differentiating between incidental findings and pathological changes can be challenging.
Aim:
This review, part of an occasional series on feline abdominal ultrasonography, discusses the ultrasound examination of the normal and diseased spleen. Aimed at general practitioners who wish to improve their knowledge of and confidence in feline abdominal ultrasound, this review is accompanied by high-resolution images and videos available online as supplementary material.
Equipment:
Ultrasound facilities are readily available to most practitioners, although the use of ultrasonography as a diagnostic tool is highly dependent on operator experience.
Evidence base:
Information provided in this article is drawn from the published literature and the author’s own clinical experience.
Keywords: Ultrasound, splenomegaly, lymphoma, mast cell neoplasia, splenic mass, myelolipoma, accessory spleen, splenunculus
Imaging the spleen
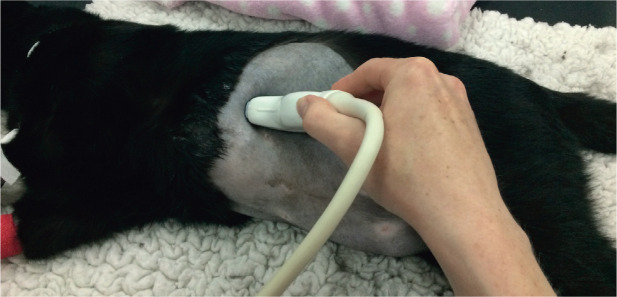
Ultrasound is considered to be the diagnostic imaging modality of choice for assessing the feline spleen, enabling splenic contour, size and parenchymal echogenicity and echotexture to be evaluated. 1 The head of the spleen is located caudolateral to the gastric fundus, to which it is attached by the short gastro-splenic ligament.1–3 Unlike dogs, in which the position of the spleen is highly variable, the body and tail of the feline spleen are fairly consistently positioned along the left abdominal wall, lateral to the left kid-ney. 4 The superficial location of the feline spleen renders it well within the reach of high frequency transducers and, since the spleen is easily accessible, an intercostal approach is not usually required. The author scans the spleen with the cat positioned in right lateral recumbency, using a left flank approach, starting at the head and finishing at the tail of the spleen (Figure 1). As for other abdominal organs, the spleen should be examined in at least two orthogonal planes, usually longitudinal and transverse, to reduce the risk of missing a lesion.
Figure 1.
Approximate transducer positioning for the spleen
Radiographically, the feline spleen is most easily appreciated on a ventrodorsal projection of the abdomen and is small compared with the canine spleen (Figure 2). 56 The head of the spleen may be visible on one or both lateral views in around 50% of cats. In contrast, according to some reports, the tail is rarely visible on a lateral abdominal radiograph and, if seen, is potentially suggestive of splenomegaly.1,3 In the author’s experience, the tail is occasionally visible in cats where there is no suspicion of splenic disease. This is supported by a recent study that documented the presence of a visible splenic tail on lateral abdominal radiographs in 4/15 (27%) non-sedated healthy adult cats. 7 Thus, it may be helpful to interpret such a finding on a case-by-case basis in the context of the history and clinical examination to determine the likely significance, with a view to further investigation if considered appropriate.
Figure 2.
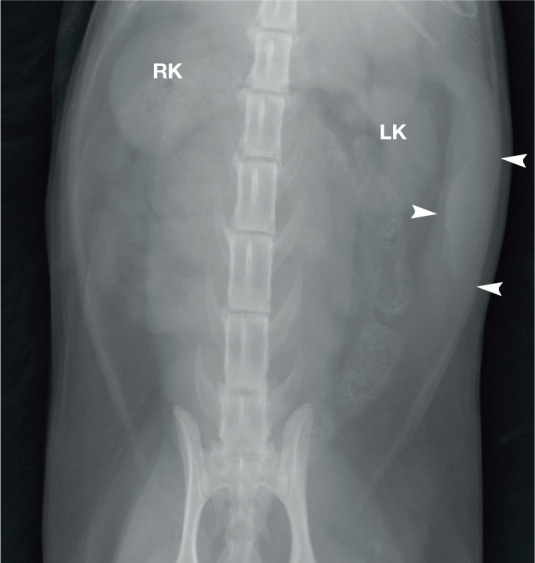
Ventrodorsal radiographic projection of the abdomen of a cat. The spleen (arrowheads) is visible within the left side of the abdomen, lateral to the left kidney (LK). RK = right kidney. Courtesy of Paul Mahoney, IDEXX, UK
Normal appearance of the spleen
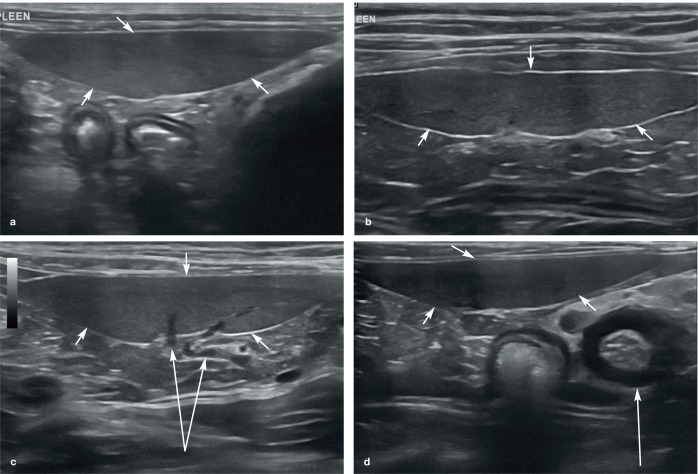
On ultrasound, the feline spleen appears as a small, elongated organ in the long axis and is triangular in cross-section. 5 It is well defined, smoothly marginated and possesses a thin, echogenic capsule, which becomes most apparent when orientated perpendicular to the ultrasound pulses due to specular reflection (Figure 3).4,5,8 Mild undulations may be noted along the mesenteric border where individual splenic vein radicles exit the spleen. 5 Splenic parenchymal echotexture is typically homogeneous and finer than that of the liver, interrupted only by the presence of linear, anechoic intraparenchymal veins coursing longitudinally through the body of the spleen.4,9 Splenic veins are usually less conspicuous in cats than in dogs. 2 Splenic arteries may not be appreciated on B-mode images alone, but can often be seen using colour Doppler. 5

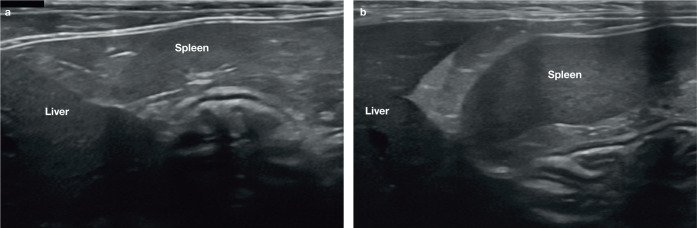
The echogenicity of the spleen relative to the liver and kidney is variable, but is generally greater than that of the liver and less than the kidney (Figure 4).4,5 Some studies have reported the echogenicity of the spleen to be either isoechoic or mildly hyperechoic when compared with the left renal cortex.9,10 The authors of one of these studies noted a difference in relative splenic echogenicity between male and female cats. 9 They found that in 77% (23/30) of castrated male cats the spleen was isoechoic to the renal cortex, whereas in 80% (16/20) of spayed female cats the spleen was mildly hyperechoic relative to the kidney. 9 It was postulated that this difference is likely to be due to the increased propensity of older, castrated male cats to accumulate lipid within the tubules of the renal cortex, thus causing an increase in cortical echogenicity. 11 Readers should note that only the mid-portion of the left renal cortex, which is perpendicular to the ultrasound beam, should be used to compare renal with splenic echogenicity, since artefactual echogenic dropout at the poles of the kidney can give rise to misleading results.5,10 Minimal pressure should be applied to the transducer at the time of comparison and the focal zone should be adjusted to the level of interest. 5 An increase in hepatic echogenicity can occur in cats with vacuolar hepatopathy, thus altering the relationship between the echogenicity of the spleen and liver. 12
Figure 3.
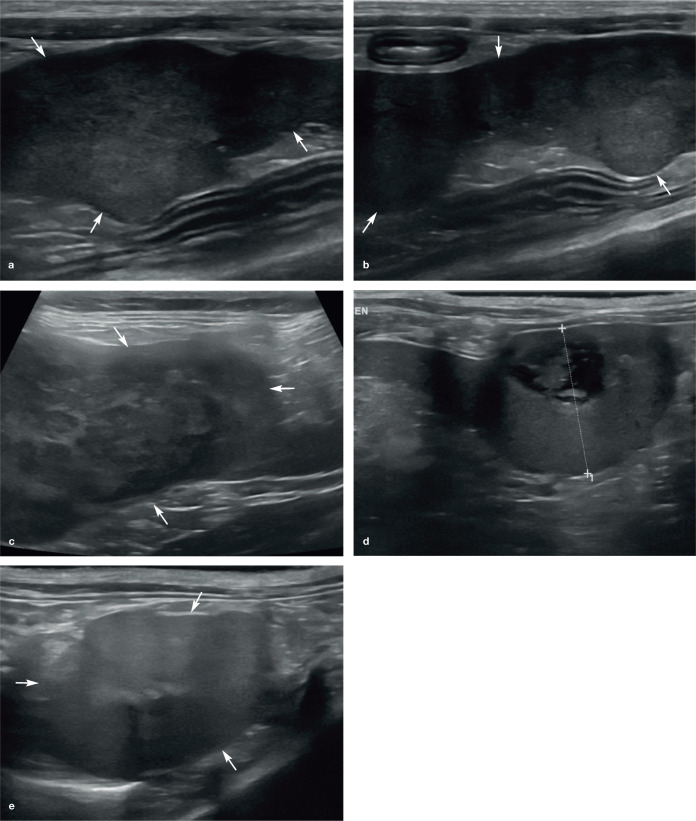
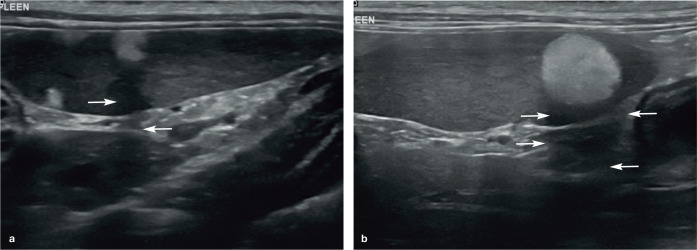
(a–d) Normal appearance of the spleen in four cats. In each example, the spleen (short arrows) appears as an elongated organ with fine echotexture within the near field of the image due to its superficial location within the abdomen. (b) The echogenic capsule surrounding the spleen is particularly conspicuous. (c) Splenic veins (long arrows) are visible within the hilar region of the spleen. (d) Marked thickening of the muscularis layer of the ileum (long arrow) is visible, and is surrounded by hyperechoic reactive fat. Two videos showing the appearance of a healthy spleen in a kitten are available as supplementary material
While in many cats the splenic parenchyma is homogeneous in echogenicity, at higher frequencies, and therefore improved axial resolution, a normal spleen can appear mottled.2,9,13–15 Other terms commonly used to describe this appearance include ‘honeycomb’, ‘Swiss cheese’ and ‘moth-eaten’. In essence, these terms all refer to the presence of numerous small but separate hypoechoic nodules disseminated throughout the splenic parenchyma resulting in a spotted echotex-ture. 13 The cause of this mottling is not known but the ability of high resolution transducers to resolve splenic lymphoid centres has been proposed as a possible explanation. 2 The appearance has also been reported in association with various pathological conditions. The reader is directed to the round cell neo-plasia section below for a further discussion on pathological causes of mottling.
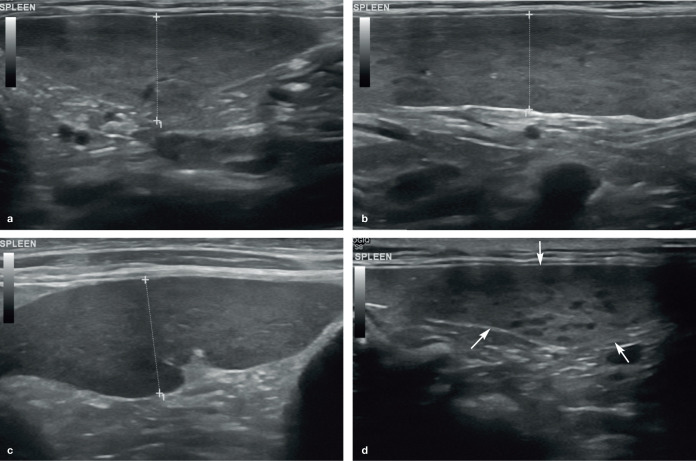
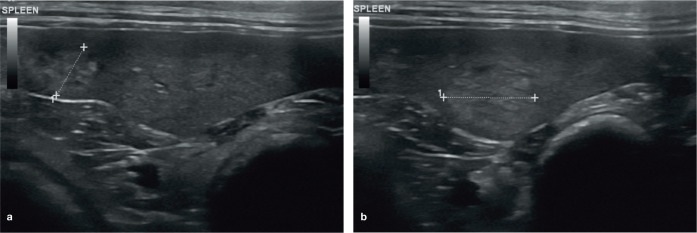
The shape of the spleen is fairly consistent across the species and is characterised by a slender head and body, and a relatively wider tail, resulting in a ‘tongue-like’ appearance. 5 Assessment of splenic size in cats has traditionally been subjective, 4 with one study revealing a poor correlation between radio-graphic and ultrasonographic measurements of splenic thickness (hereafter referred to as ‘height’). 1 However, a study by Reese et al in 2013 reported the mean height of the normal splenic body in 60 healthy cats to be 8.2 mm (SD 1.4 mm), with a range of 5.3–11.1 mm, based on images of the spleen acquired in the transverse plane. 9
A separate study by Sayre and Spaulding in 2014 determined the height of the head, body and tail of the spleen in 31 clinically healthy, non-sedated cats using a 13.5 MHz linear transducer. 5 These authors found that splenic measurements were highly dependent on the segment of the spleen under evaluation, with the tail being most variable in size and having the greatest potential for false-positive results with regards to splenomegaly. To ensure consistent evaluation of the spleen, the authors of the study recommended measuring the height of the proximal third (head) of the spleen in a transverse plane when a splenic radicle is visible at the mesenteric surface. Using this protocol, they reported the mean height of the normal spleen to be 7.1 mm, with a range of 5.1–9.1 mm. They also suggested that a spleen >9.1 mm may be indicative of enlargement, potentially prompting further investigation such as aspiration, depending upon patient presentation and any additional ultrasonographic findings. 5
It should be noted that neither splenic aspiration nor histopathology were performed in either of the above-mentioned 2013/2014 studies and thus definitive confirmation of normality was not possible. Other authors cite an upper limit of 10 mm for the size of the normal spleen in the cat,3,8 based on experience or referencing the study by Reese et al. 9
Abnormalities of the spleen
The majority of the veterinary literature describing the ultrasonographic appearance of splenic disease pertains to dogs. There are, however, a limited number of reports describing the ultrasonographic presentation of splenic lesions in the cat and these are discussed below.
Splenic disease in cats is at least as, if not more likely to be neoplastic in nature than in dogs;16,17 the majority of splenic lesions in dogs being benign in nature according to several studies.18–21 Spangler and Culbertson conducted a study in 1992 with the aim of determining the prevalence and type of splenic disease in a cohort of 455 cats. 17 Primary or metastatic neoplasia accounted for 37% of splenic lesions, whereas accessory splenic tissue from the omentum or pancreas was identified in 4% of cats, and nodular hyperplasia and haematomas accounted for a further 4%. Mast cell neoplasia, lymphoma, myeloproliferative disease and haeman-giosarcoma were the most common tumours of the feline spleen (in that order), although these cannot be differentiated based on ultrasound appearance alone. 17 Splenitis was detected in 2% of cases and thromboembolic disease resulting in regional splenic infarction was documented in just 1% of cases. Interestingly, in a subsequent study by Hanson et al in 2001, 4 a much higher percentage (73%) of the splenic changes identified in 101 cats were due to neoplasia.
Diffuse splenomegaly
In contrast to the canine spleen, the spleen of a cat is non-sinusoidal and, as such, does not act as a large blood reservoir. Consequently, physiological variations in size are likely to be minimal and any splenomegaly is more likely to be pathological. Mild enlargement can occur following sedation or anaesthesia, although any increase in size is likely to be less pronounced than that observed in the dog. 2 Sevoflurane, for example, has been found to cause a statistically significant increase in the height, width and cross-sectional area of the feline spleen. However, the magnitude of such change is minimal in real terms and deemed unlikely to be clinically significant. 9 Furthermore, sevoflurane is not associated with significant alterations in splenic echotexture or echogenicity. 9
In a recent study, acepromazine, butor-phanol, dexmedetomidine or a combination of midazolam and butorphanol or dex-medetomidine, butorphanol and ketamine were administered to 15 healthy adult research cats to determine the effect, if any, the five sedative drugs/drug combinations had on ultrasonographic splenic size. 7 All drugs were administered intravenously, with the exception of the final drug combination, which was given by intramuscular injection. Acepromazine was the only drug to result in a statistically significant increase in splenic size, with the effect lasting at least 2–3 h post-sedation. The mean magnitude of this change was 0.9–1.8 mm. Butorphanol produced no significant change in splenic size, while the remaining drugs resulted in a trend towards increased splenic size 15–30 mins following administration, although the change was not statistically significant. In this study, haematocrit decreased significantly following sedation in cats receiving each drug or combination of drugs, again with the exception of butorphanol. However, the greatest increase in splenic size did not correlate with the most significant reduction in haematocrit, leading the authors to hypothesise that while red blood cell sequestration in the spleen may explain the increase in splenic size following sedation, sequestration of red blood cells at other sites is also likely.
In the cat, a spleen measuring greater than 10 mm in height, as mentioned earlier, or one that is folded over upon itself is usually suggestive of splenomegaly (Figure 5).4,8,9
Figure 4.
(a,b) Relative echogenicity of the spleen and liver in two cats. The spleen is mildly hyperechoic compared with the liver
Round cell neoplasia
The spleen is a common site for primary mast cell neoplasia in the cat and mast cell tumours are the most common haematopoietic tumour of the spleen in this species, particularly in older cats (>10 years) (Figure 6).6,22,23 Splenic mast cell neoplasia can result in diffuse splenic enlargement, an irregular contour, diffuse hypoechogenicity, mottling and the presence of hypoechoic nodules.4,24 Some of these changes are discussed in more detail below. A reduction in size of the spleen, hyperechoic nodules and diffuse hyperechogenicity have also, less commonly, been reported in association with mast cell infiltration. 4 Furthermore, an ultrasonographically normal spleen is not sufficient to exclude a diagnosis of mast cell infiltration. 24
Diffuse splenomegaly is also particularly common in cats with lymphoma (Figure 7). In one study, 83% of cats with splenic lymphoma had an enlarged spleen and, in 32% of cases, enlargement was the only splenic change observed. 4 Despite this, and as is the case with mast cell neoplasia, the absence of splenomegaly is insufficient to exclude a diagnosis of lymphoma. 4
Figure 5.
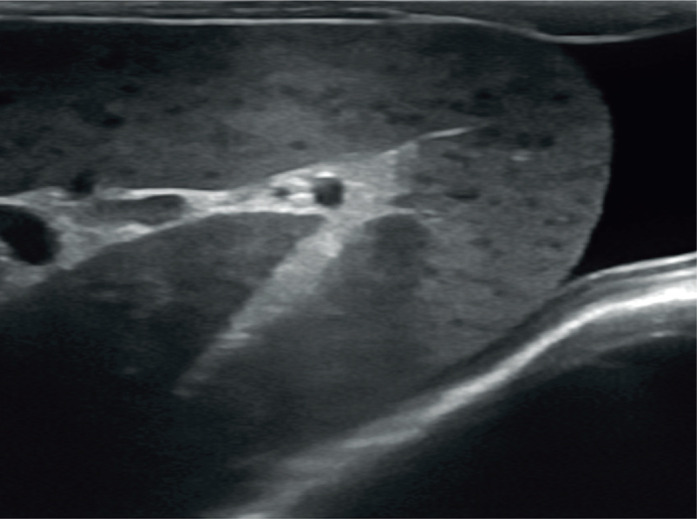
Splenomegaly in a 2-year-old neutered male domestic shorthair cat. The spleen is folded over upon itself, indicating enlargement, and has a mottled appearance due to the presence of numerous hypoechoic foci throughout the parenchyma. A moderate volume of free anechoic peritoneal fluid is visible caudal to the body of the spleen. The final diagnosis was lymphoid hyperplasia. Three videos showing lymphoid hyperplasia ± haematopoiesis of the spleen, and folding over of the spleen, are available as supplementary material
In dogs, a ‘honeycomb’-like or ‘Swiss cheese’ echotexture, indicating the presence of multiple small hypoechoic foci, has been described in association with splenic lymphoma.8,25 Similarly, in one feline study, the most common ultrasonographic presentation of splenic lymphoma, observed in 16/30 cats, was a large, smoothly marginated, hypoechoic or nodular spleen. 4 Lamb et al also described various similar changes occurring in dogs and cats with splenic lymphoma including diffuse hypo-echogenicity, the presence of multiple hypo-echoic foci, an irregular border due to the presence of multiple protuberances, and a cavitary mass, although the study did not specify which changes occurred in each species. 26
Despite the above findings, it is important to exercise caution in the interpretation of a moth-eaten spleen in the cat, since it may also be present in normal cats, as mentioned earlier, and in conjunction with various other splenic pathologies including lymphoid hyperplasia, EMH, passive congestion, carcinoma, histiocytic sarcoma, mast cell infiltration and granu-lomatous splenitis.4,13,14,24,27 This was confirmed by the authors of a recent study who investigated the correlation between a moth-eaten appearance of the spleen and cytological diagnosis. 13 Of the 25/170 cats they identified as having a moth-eaten spleen, only five had malignant neoplasia diagnosed on cytology. Of these five, there were three cases of lymphoma, one of metastatic carcinoma and one of histiocytosis. The remaining 20 cats had a diagnosis of benign or non-neoplastic disease, yielding a positive predictive value of only 20% for screening for malignant neoplasia, thus leading the authors to conclude that a moth-eaten spleen in the cat is not necessarily indicative of lymphoma or other malignancy on cytology.
In a separate study, lymphoma was identified in only 8/33 cats with a honeycomb pattern, all of which also had evidence of splenomegaly, with the remaining cats having diagnoses of EMH, lymphoid hyperplasia, splenitis and, in one case, histiocytic sarcoma. 14 Similarly, a further study involving 25 cats with a honeycomb splenic pattern identified neoplasia in only 16% of cases (of which 3/4 were lymphoma), with the remainder having lymphoid hyperplasia (64%), EMH (12%) or splenitis (8%). 15
Additional findings may be present on ultrasound in cats with round cell neoplasia. For example, cats with lymphoma are much more likely to have evidence of concurrent abdominal lymphadenopathy and/or an abdominal effusion. 4 Changes such as these may also be observed in cats with mast cell disease, although the incidence is perhaps lower in comparison with lymphoma.4,24
Hepatic abnormalities, such as hepatomegaly, a diffuse increase or decrease in hepatic echogenicity and the presence of hepatic nodules, are common findings in cats with lymphoma and concurrent hepatic involvement. 4 Similarly, hepatic abnormalities and abdominal effusion occur in approximately one-third of cats with mast cell disease. Mesenteric lymph node involvement and, occasionally, the presence of renal and small intestinal masses have also been reported in cats with mast cell disease.4,24
Extramedullary haematopoiesis and lymphoid hyperplasia
EMH is relatively common in cats and usually the result of an underlying process such as anaemia, rather than representing a primary splenic disease.4,6 Lymphoid hyperplasia and EMH often occur simultaneously, and in one study around 40% of cats with one or both conditions had splenomegaly associated with normal echogenicity. 4 Multiple non-shadowing hyperechoic foci and focal hypo-echoic nodules or masses up to 3 cm in diameter have also been described in cats with EMH and/or lymphoid hyperplasia (Figure 8). 4 As for round cell neoplasia, splenic echotexture and echogenicity may appear normal, hypoechoic and/or mottled and such observations are not considered to be pathognomonic for a particular condition. 4
Figure 6.
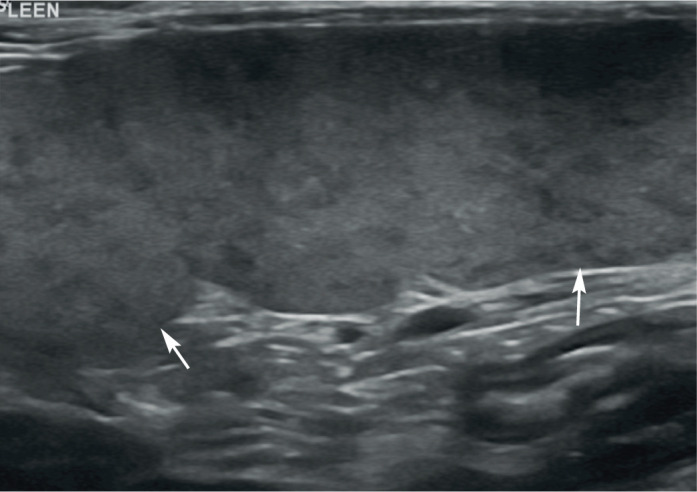
Diffuse splenomegaly owing to mast cell infiltration in a 15-year-old neutered female domestic shorthair cat. The spleen (arrows) is enlarged and contains multiple, small, poorly defined hypoechoic foci throughout the parenchyma. Additional ultrasonographic findings included hepatomegaly, abdominal lymphadenopathy and a small peritoneal effusion. Mast cell infiltration of the liver and spleen was confirmed following ultrasound-guided fine-needle aspiration of both organs. A video showing multiple splenic masses due to mast cell infiltration is available as supplementary material
Feline infectious peritonitis
Feline coronavirus, and also acromegaly, have been reported to cause enlargement in an otherwise unremarkable or diffusely hypoechoic spleen with or without an irregular contour (Figure 9).4,28 Conversely, in one study of 16 cats with feline infectious peritonitis, in the vast majority of cases (n = 14, 88%), the spleen appeared normal on ultrasound.28 However, it should be noted that this was a retrospective study performed over 10 years ago and ultrasound equipment, and therefore ultrasound images, have improved considerably over this time period. Potential concurrent findings in feline infectious peritonitis include renomegaly, an irregular renal contour, a hypoechoic subcapsular rim, peritoneal and/or retroperitoneal effusion, abdominal lymphadenopathy and diffuse intestinal thickening. 28
Figure 7.
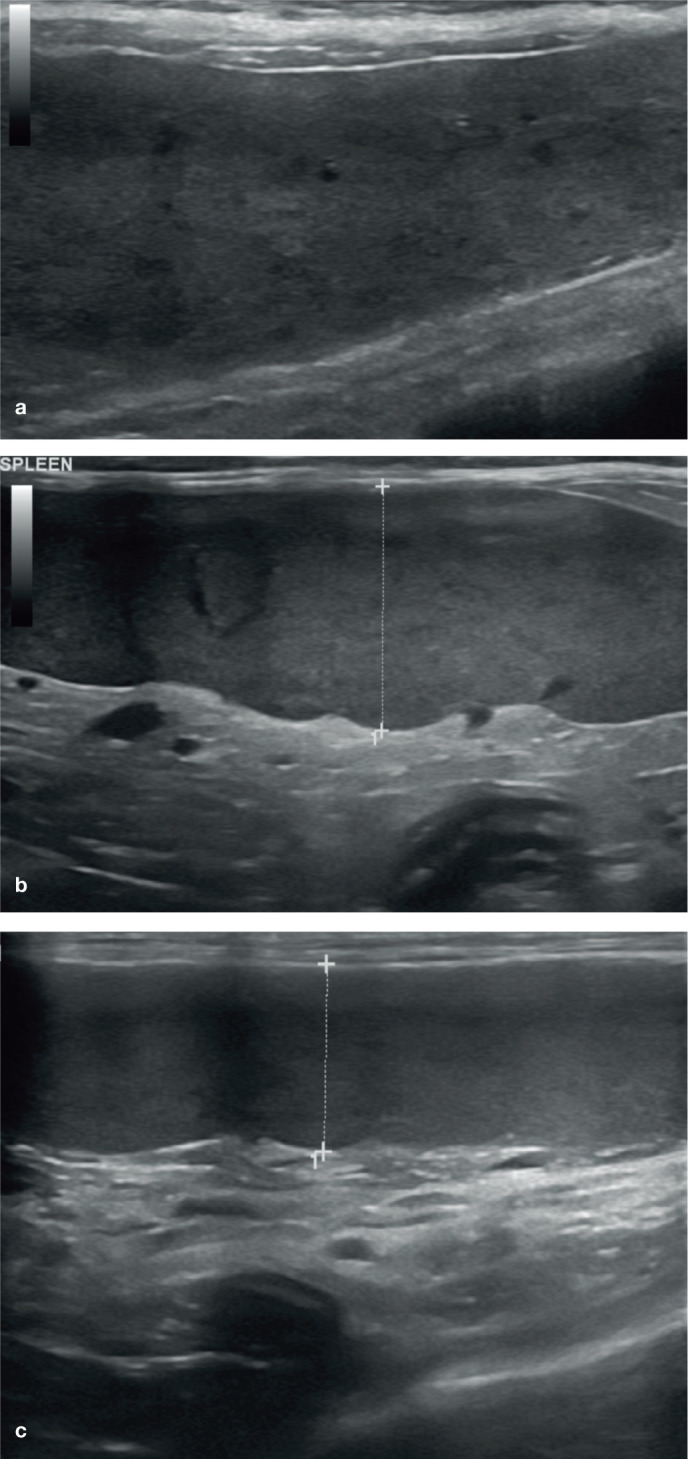
(a–c) Diffuse splenomegaly (indicated between the measuring calipers in [b] and [c]) in three cats owing to lymphoma. In (a) and (b), the splenic parenchyma is heterogeneous due to the presence of small, often coalescing, hypoechoic foci; in (c), the parenchymal echotexture is uniform. In all three cases there was concurrent hepatomegaly and abdominal lymphadenopathy. A video showing splenic lymphoma is available as supplementary material
Miscellaneous
Less common causes of splenomegaly include myeloproliferative disease, histoplasmosis, erythroleukaemia, eosinophilic syndrome, plasma cell tumour, mycobacteriosis, malignant histiocytosis, myelodysplasia and haemophagocytic syndrome.4,29–34
Myeloproliferative disease has been reported to cause a concurrent reduction in echogenicity of the spleen in two cats. 4 Although no hepatic changes or abdominal lymphadenopathy were documented in these cases, one cat had an abdominal effusion. A 3–4 cm diameter hypoechoic splenic mass has also been reported in a cat with myeloproliferative disease. 35
Histoplasmosis is the second-most common fungal infection in the cat in endemic countries (after cryptococcosis) and is caused by systemic infection with the dimorphic fungus Histoplasma capsulatum.36,37 Disseminated histoplasmosis with splenic involvement most commonly results in an enlarged and hypo echoic spleen.36,38 It is thought that the splenic enlargement may be the result of histoplasmosis-induced splenic macrophage proliferation. Chronic histoplasmosis can cause increased splenic echogenicity with focal regions of parenchymal mineralisation. 2 Less common findings include a mottled appearance to the spleen and the presence of discrete nodules. 36 Concurrent hepatomegaly, renomegaly, adrenomegaly and lymphadenopathy may be recognised,39–41 and ultrasoundguided aspiration of the spleen can be useful to confirm the diagnosis.
Malignant histiocytosis has occasionally been reported in cats and ultrasonographic findings including diffuse splenomegaly with irregular margination, hepatomegaly, mesenteric lymphadenopathy and peritoneal effusion have all been observed.34,42,43 Even with multiple organ involvement, abdominal ultrasound findings may in some cases be normal. 34
Splenic torsion is a further differential for splenomegaly in the dog.2,8 To the best of the author’s knowledge, this condition has not been reported in the cat.
Focal nodules and masses
Focal lesions of the spleen are generally much more readily detected on ultrasound than diffuse alterations in echogenicity (Figure 10). As mentioned earlier, focal or multifocal splenic nodules and masses are not as common in cats as they are in dogs and, when they occur, are most commonly isoechoic to hypoechoic relative to normal splenic parenchyma. Similar differential diagnoses apply to both species and include round cell and metastatic neoplasia, lymphoid hyperplasia, EMH and, very rarely, haematoma, abscess and granuloma. 4
Figure 8.
(a,b) Diffuse splenomegaly due to reactive lymphoid hyperplasia and neutrophilic inflammation of unknown aetiology in a 3-year-old neutered male domestic shorthair cat. Multiple poorly defined hypoechoic foci are scattered throughout the splenic parenchyma. (c) Splenomegaly owing to extramedullary haematopoiesis in an 11-year-old neutered male Bengal with bilateral perinephric pseudocysts (not shown). The enlarged spleen is indicated between the measuring calipers in (a–c). (d) A normal size spleen (arrows) containing multiple well-defined hypoechoic foci in an 8-year-old neutered male domestic shorthair cat with reactive lymphoid hyperplasia
In one study, a splenic mass >10 mm diameter was reported to be suggestive of a cytological diagnosis of malignancy. However, this was based on splenic masses in only 15 cats, of which eight were subsequently diagnosed as being malignant (four carcinomas, two lymphomas, one multiple myeloma and one haemangiosarcoma). 13 In contrast, splenic masses were more commonly associated with non-neoplastic disease in a separate study, although the authors used different size criteria to differentiate between nodules and masses. 4 As will become evident below, size alone cannot be used to discriminate between malignant and benign disease.
Malignant neoplasia
One or more hypoechoic splenic nodules or masses have been reported in cats with lymphoma, 4 and histiocytic sarcoma causing two masses in the spleen has also been reported in a cat. 44 Similarly, multiple hypoechoic nodules varying in size up to 13 mm in diameter and 1–2 cm diameter anechoic to hypoechoic nodules have been described in cats with metastatic fibrosarcoma 45 and metastatic carcinoma, 4 respectively.
In contrast to the situation in dogs, feline splenic haemangiosarcoma is relatively rare but, when present, may present as anechoic foci or hyperechoic nodules (Figure 11).4,6 Abdominal effusion has also been reported in cats with splenic haemangiosarcoma, although the nature of the effusion was not described. 4
Figure 9.
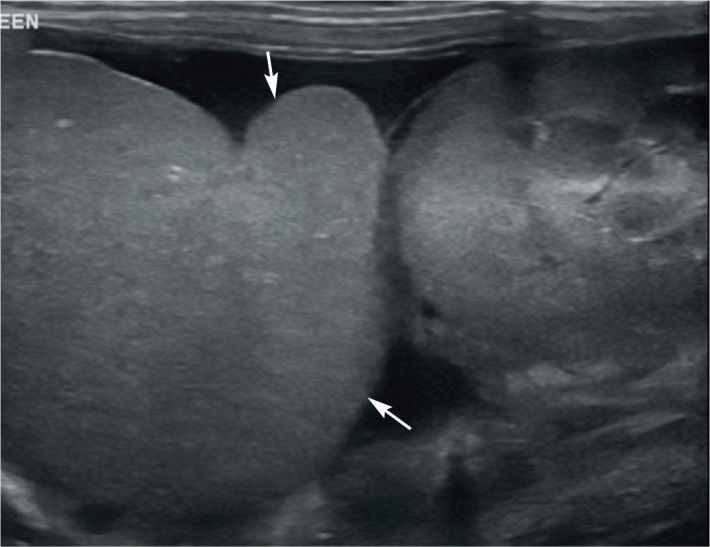
Diffuse splenomegaly (arrows) resulting in rounding of the splenic margins in a 10-month-old neutered male domestic shorthair cat with feline infectious peritonitis. A small volume of free fluid is also present
Target lesions (nodules or masses with a hyperechoic centre and hypoechoic rim) in the spleen and liver have been reported as having a positive predictive value of 74% for malignancy in the dog (Figure 12). 46 While, to the author’s knowledge, the same strong association has not been reported in the feline spleen, there is a description of metastatic bile duct carcinoma resulting in a target-like lesion within the spleen of a cat. 29
Figure 10.
Splenic masses. (a,b) Multiple large masses (arrows) are present within the diffusely hypoechoic spleen of a 10-year-old neutered female domestic shorthair cat due to metastatic mast cell neoplasia. (c) Heterogeneous splenic mass (arrows) in an 11-year-old neutered male domestic shorthair cat with lymphoma. Marked intra-abdominal lymphadenopathy and a small peritoneal effusion were also identified during the ultrasound examination. (d) Large, well-circumscribed mass (between the measuring calipers) at the tail of the spleen in a 12-year-old neutered female domestic longhair cat with carcinomatosis. The mass is isoechoic relative to the remaining splenic parenchyma with an eccentrically located heterogeneously anechoic region. The spleen was normal on ultrasound examination performed 3 months earlier and metastatic or possibly primary neoplastic splenic disease were considered the most likely differentials. (e) A large, approximately 3 cm diameter, well-defined mass (arrows) is visible at the tail of the spleen of an 11-year-old neutered female domestic shorthair cat. There is a centrally located focal hyperechoic region within the mass associated with distal acoustic shadowing. Results from ultrasound-guided fine-needle aspiration were consistent with nodular lymphoid hyperplasia
Benign lesions
Lymphoid hyperplasia and EMH Hypo-echoic nodules and masses measuring up to 3 cm in diameter have been reported in cats with lymphoid hyperplasia and/or EMH.2,4
Haematomas Splenic haematomas usually arise as a result of trauma or coagulopathy or in association with neoplasia and can be intra-parenchymal or subcapsular. 2 Haematomas may have a complex appearance depending on their age and can mimic both benign and neoplastic conditions. 2 However, they typically reduce in size over time, frequently resolving in a few months; this is different to tumours, which generally increase, rather than decrease, in size. 47
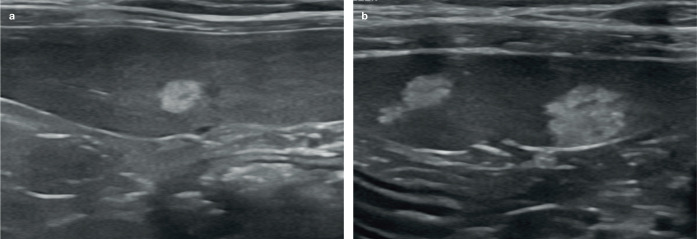
Myelolipomas and other hyperechoic lesions In older cats and other feline species including cheetahs, focal or multifocal, well-defined strongly hyperechoic nodules are sometimes visible surrounding the splenic vessels along the mesenteric border of the spleen or occasionally within the parenchyma.8,9,48,49 These represent benign fatty nodules known as myelolipomas and are considered to be incidental findings.50–52 They may be solitary or multiple and are often accompanied by faint acoustic shadowing (Figure 13).
Figure 11.
(a,b) Multiple poorly defined hyperechoic splenic nodules (between the measuring calipers), varying in size up to 1.7 cm diameter, in a 9-year-old neutered male Maine Coon with haemangiosarcoma
Although the size of myelolipomas is variable, they can become quite large in some cats. Furthermore, while the appearance of myelolipomas is typically fairly characteristic, they must be differentiated from mast cell tumours, which have also been reported to present as small hyperechoic nodules within the spleen or to cause diffuse splenic hyper-echogenicity (Figure 14). 4 In some cases aspiration may be required to differentiate between the two. 4
Figure 12.
Target lesions within the spleen of an elderly Border Collie. Both nodules are well defined and have a hyperechoic centre surrounded by a hypoechoic rim. Aspirates taken under ultrasound guidance were consistent with carcinoma and considered most likely to be metastatic in origin from an undetermined primary lesion
Multiple hyperechoic parenchymal foci resulting in a speckled appearance, and representing mineralisation, have been described in association with endocrinopathies in dogs.2,8 However, to the author’s knowledge, a similar finding has not to date been described in the cat.
Figure 14.
(a,b) Hyperechoic splenic nodules in two cats. Nodules such as these most likely represent myelolipomas. However, consideration should be given to follow-up ultrasound and/or sampling of these to rule out mast cell neoplasia
Miscellaneous conditions
Infarction
Infarction may be focal or it can involve the entire spleen. The ultrasonographic appearance of splenic infarction has not been described to date in the cat. In dogs, the appearance depends on the age of the infarct. 2 Infarcted tissue frequently causes splenic enlargement (focal or diffuse) and has a hypoechoic 53 and possibly lacy appearance. 54 The lacy appearance is due to the presence of hyperechoic vessel walls contrasting with the reduced echogenicity of the abnormal tissue. 54 Crucially, there will be no blood flow within the area of infarcted tissue when interrogated with colour Doppler. If present, immobile echogenic material visible within the splenic vein is suggestive of a thrombus 53 and, in cases of splenic torsion, a hyperechoic perive-nous triangle may be visible at the splenic hilus. 55 The latter is thought to be due to bulging of splenic tissue against the mesentery either side of the hilar vessels resulting in a triangular appearance to the mesenteric fat surrounding the distended veins. 55 Concurrent hypercoagulable conditions are common such as hyperadrenocorticism and neoplasia.53,54
Splenunculus
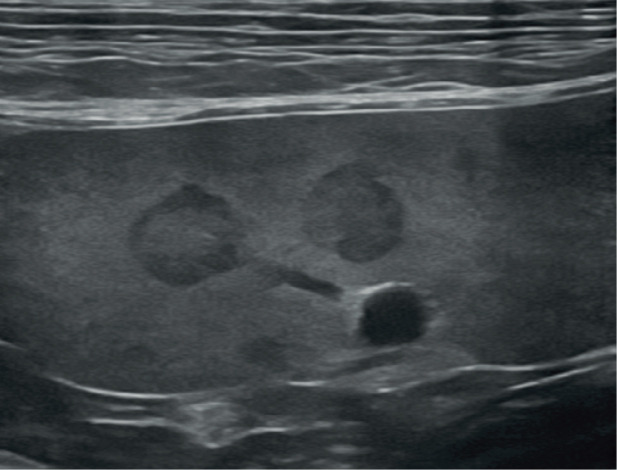
Splenunculi, also known as supernumerary spleens, accessory spleens or splenules, are congenital islands of normal splenic tissue. They are incidental and arise as a result of failure of fusion of the one or more components of the splenic anlage (cluster of embryonic cells from which the spleen originates) during development. 56 Reported locations for accessory splenic tissue include the omentum and pancreas (ie, intra-pancreatic).17,56 On ultrasound, accessory splenic tissue is expected to be isoechoic to the spleen and may present as discrete, smoothly marginated island(s) of tissue within the omentum close to the spleen or within the pancreas (Figure 15).2,56 The primary reason for identification is to differentiate splenunculi from clinically significant pathology such as neoplasia. A single case of mast cell tumour in an intrapancreatic accessory spleen has been reported in the cat. 57
Figure 13.
Splenic myelolipomas. (a) Three well-defined hyperechoic nodules are visible within the spleen, the largest of which is associated with distal acoustic shadowing (arrows). (b) A large (approximately 1 cm diameter), well-defined hyperechoic nodule is present within the body of the spleen. Moderate distal acoustic shadowing (arrows) is evident. A video showing a splenic myelolipoma is available as supplementary material
Figure 15.
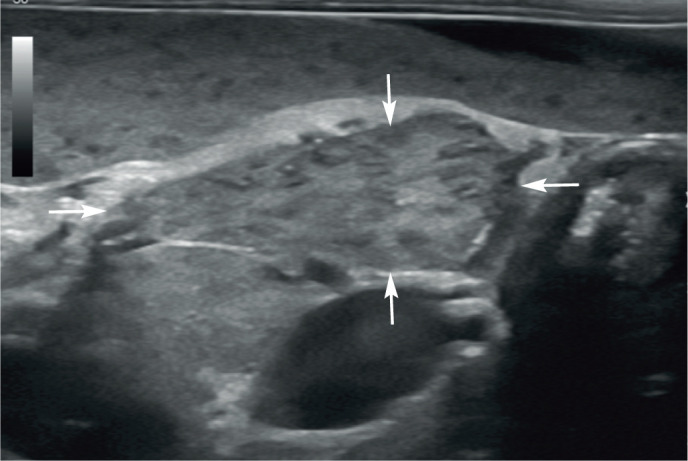
Island of accessory splenic tissue (arrows), measuring 13 mm x 24 mm, located in the omentum adjacent to the spleen in a 2-year-old neutered male domestic shorthair cat. The tissue has the same echogenicity and mottled appearance as the adjacent spleen. Cytology from fine-needle aspiration under ultrasound guidance confirmed the presence of lymphoid hyperplasia

Key Points
The body and tail of the feline spleen are commonly located deep to the left abdominal wall and lateral to the left kidney.
If available, a high frequency linear transducer should be used to examine the spleen.
Generalised splenomegaly may be suspected in a spleen that is folded over, or one that measures >10 mm in height.
Mast cell neoplasia and lymphoma are the most common round cell tumours causing splenomegaly in the cat. Additional findings such as peritoneal effusion and lymphadenopathy may be present.
Anaesthesia is likely to have less effect on the size of the spleen in cats than is observed in dogs.
Splenic masses occur less commonly than diffuse splenomegaly in cats.
In most cases, the ultrasonographic appearance of splenic masses is non-specific.
Hyperechoic splenic lesions are frequently myelolipomas, although mast cell neoplasia remains an important differential.
FNA of the spleen under ultrasound guidance is a useful technique for investigating both diffuse and focal lesions.
Footnotes
The author declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author received no financial support for the research, authorship and/or publication of this article.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not specifically required for publication in JFMS.
Informed consent: This work did not involve the use of animals and therefore informed consent was not required. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
References
- 1. Johnson KL, Porter EG, Berry CR. Analysis of feline splenic radiographic measurements and their correlation to ultrasonographic measurements. J Feline Med Surg 2017; 19: 985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nyland TG, Mattoon JS. Spleen. In: Mattoon JS, Nyland TG. (eds). Small animal diagnotic ultrasound. 3rd ed. St Louis, MO: Elsevier, 2015, pp 400–437. [Google Scholar]
- 3. Larson MM. Liver and spleen. In: Thrall DE. (ed). Textbook of veterinary diagnostic radiology. 7th ed. St Louis, MO: Elsevier, 2018, pp 792–822. [Google Scholar]
- 4. Hanson JA, Papageorges M, Girard E, et al. Ultrasonographic appearance of splenic disease in 101 cats. Vet Radiol Ultrasound 2001; 42: 441–445. [DOI] [PubMed] [Google Scholar]
- 5. Sayre RS, Spaulding KA. Formulation of a standardized protocol and determination of the size and appearance of the spleen in healthy cats. J Feline Med Surg 2014; 16: 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Argyle DJ, O’Brien RT. Nonneoplastic diseases of the spleen. In: Ettinger SJ, Feldman EC, Cote E. (eds). Textbook of veterinary internal medicine. 8th ed. St Louis, MO: Elsevier, 2017, pp 877–887. [Google Scholar]
- 7. Auger M, Fazio C, de Swarte M, et al. Administration of certain sedative drugs is associated with variation in sonographic and radiographic splenic size in healthy cats. Vet Radiol Ultrasound 2019; 60: 717–728. [DOI] [PubMed] [Google Scholar]
- 8. Hecht S, Mai W. Spleen. In: Penninck D, d’Anjou MA. (eds). Atlas of small animal ultrasonography. 2nd ed. Iowa: John Wiley & Sons, 2015, pp 239–258. [Google Scholar]
- 9. Reese SL, Zekas LJ, Lazbik MC, et al. Effect of sevoflurane anesthesia and blood donation on the sonographic appearance of the spleen in 60 healthy cats. Vet Radiol Ultrasound 2013; 54: 168–175. [DOI] [PubMed] [Google Scholar]
- 10. Yabuki A, Endo Y, Sakamoto H, et al. Quantitative assessment of renal cortical echogenicity in clinically normal cats. Anat Histol Embryol 2008; 37: 383–386. [DOI] [PubMed] [Google Scholar]
- 11. Yeager AE, Anderson WI. Study of association between histologic features and echogenicity of architecturally normal cat kidneys. Am J Vet Res 1989; 50: 860–863. [PubMed] [Google Scholar]
- 12. Yeager AE, Mohammed H. Accuracy of ultrasonography in the detection of severe hepatic lipidosis in cats. Am J Vet Res 1992; 53: 597–599. [PubMed] [Google Scholar]
- 13. Bertal M, Norman Carmel E, Diana A, et al. Association between ultrasonographic appearance of splenic parenchyma and cytology in cats. J Feline Med Surg 2018; 20: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quinci M, Sabattini S, Agnoli C, et al. Ultrasonographic honeycomb pattern of the spleen in cats: correlation with pathological diagnosis in 33 cases. J Feline Med Surg 2020; 22: 800–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harel M, Touzet C, Barthelemy A, et al. Prevalence and diagnostic value of the ultrasonographic honeycomb appearance of the spleen in cats. J Feline Med Surg 2020; 22: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spangler WL, Culbertson MR. Prevalence, type, and importance of splenic diseases in dogs: 1,480 cases (1985–1989). J Am Vet Med Assoc 1992; 200: 829–834. [PubMed] [Google Scholar]
- 17. Spangler WL, Culbertson MR. Prevalence and type of splenic diseases in cats: 455 cases (1985–1991). J Am Vet Med Assoc 1992; 201: 773–776. [PubMed] [Google Scholar]
- 18. Spangler WL, Kass PH. Pathologic factors affecting postsplenectomy survival in dogs. J Vet Intern Med 1997; 11: 166–171. [DOI] [PubMed] [Google Scholar]
- 19. Cleveland MJ, Casale S. Incidence of malignancy and outcomes for dogs undergoing splenectomy for incidentally detected nonruptured splenic nodules or masses: 105 cases (2009–2013). J Am Vet Med Assoc 2016; 248: 1267–1273. [DOI] [PubMed] [Google Scholar]
- 20. Yankin I, Nemanic S, Funes S, et al. Clinical relevance of splenic nodules or heterogeneous splenic parenchyma assessed by cytologic evaluation of fine-needle samples in 125 dogs (2011–2015). J Vet Intern Med 2020; 34: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee M, Park J, Choi H, et al. Presurgical assessment of splenic tumors in dogs: a retrospective study of 57 cases (2012–2017). J Vet Sci 2018; 19: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blackwood L, Murphy S, Buracco P, et al. European consensus document on the management of canine and feline mast cell disease. Vet Comp Oncol 2012; 10: e1–e29. [DOI] [PubMed] [Google Scholar]
- 23. Litster AL, Sorenmo KU. Characterisation of the signalment, clinical and survival characteristics of 41 cats with mast cell neoplasia. J Feline Med Surg 2006; 8: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sato AF, Solano M. Ultrasonographic findings in abdominal mast cell disease: a retrospective study of 19 patients. Vet Radiol Ultrasound 2004; 45: 51–57. [DOI] [PubMed] [Google Scholar]
- 25. Crabtree AC, Spangler E, Beard D, et al. Diagnostic accuracy of gray-scale ultrasonography for the detection of hepatic and splenic lymphoma in dogs. Vet Radiol Ultrasound 2010; 51: 661–664. [DOI] [PubMed] [Google Scholar]
- 26. Lamb CR, Hartzband LE, Tidwell AS, et al. Ultrasonographic findings in hepatic and splenic lymphosarcoma in dogs and cats. Vet Radiol 1991; 32: 117–120. [Google Scholar]
- 27. Allan R, Halsey TR, Thompson KG. Splenic mast cell tumour and mastocytaemia in a cat: case study and literature review. N Z Vet J 2000; 48: 117–121. [DOI] [PubMed] [Google Scholar]
- 28. Lewis KM, O’Brien RT. Abdominal ultrasonographic findings associated with feline infectious peritonitis: a retrospective review of 16 cases. J Am Anim Hosp Assoc 2010; 46: 152–160. [DOI] [PubMed] [Google Scholar]
- 29. Ballegeer EA, Forrest LJ, Dickinson RM, et al. Correlation of ultrasonographic appearance of lesions and cytologic and histologic diagnoses in splenic aspirates from dogs and cats: 32 cases (2002–2005). J Am Vet Med Assoc 2007; 230: 690–696. [DOI] [PubMed] [Google Scholar]
- 30. Barry M, Taylor J, Woods JP. Disseminated Mycobacterium avium infection in a cat. Can Vet J 2002; 43: 369–371. [PMC free article] [PubMed] [Google Scholar]
- 31. Gunn-Moore DA. Feline mycobacterial infections. Vet J 2014; 201: 230–238. [DOI] [PubMed] [Google Scholar]
- 32. Hisasue M, Okayama H, Okayama T, et al. Hematologic abnormalities and outcome of 16 cats with myelodysplastic syndromes. J Vet Intern Med 2001; 15: 471–77. [DOI] [PubMed] [Google Scholar]
- 33. Wilkinson AR, Carr SV, Klahn SL, et al. Hemophagocytic syndrome in a cat. JFMS Open Rep 2018; 4. DOI: 10.1177/2055116918795023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kraje AC, Patton CS, Edwards DF. Malignant histiocytosis in 3 cats. J Vet Intern Med 2001; 15: 252–256. [DOI] [PubMed] [Google Scholar]
- 35. Feeney DA, Johnston GR, Hardy RM. Two-dimensional, gray-scale ultrasonography for assessment of hepatic and splenic neoplasia in the dog and cat. J Am Vet Med Assoc 1984; 184: 68–81. [PubMed] [Google Scholar]
- 36. Atiee G, Kvitko-White H, Spaulding K, et al. Ultrasonographic appearance of histoplasmosis identified in the spleen in 15 cats. Vet Radiol Ultrasound 2014; 55: 310–314. [DOI] [PubMed] [Google Scholar]
- 37. Lin Blache J, Ryan K, Arceneaux K. Histoplasmosis. Compend Contin Educ Vet 2011; 33: E1–10. [PubMed] [Google Scholar]
- 38. Taylor AR, Barr JW, Hokamp JA, et al. Cytologic diagnosis of disseminated histoplasmosis in the wall of the urinary bladder of a cat. J Am Anim Hosp Assoc 2012; 48: 203–208. [DOI] [PubMed] [Google Scholar]
- 39. Reinhart JM, Kukanich KS, Jackson T, et al. Feline histoplas-mosis: fluconazole therapy and identification of potential sources of Histoplasma species exposure. J Feline Med Surg 2012; 14: 841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aulakh HK, Aulakh KS, Troy GC. Feline histoplasmosis: a retrospective study of 22 cases (1986–2009). J Am Anim Hosp Assoc 2012; 48: 182–187. [DOI] [PubMed] [Google Scholar]
- 41. Kerl ME. Update on canine and feline fungal diseases. Vet Clin North Am Small Anim Pract 2003; 33: 721–747. [DOI] [PubMed] [Google Scholar]
- 42. Cortese L, Paciello O, Papparella S. Morphological characterisation of malignant histiocytosis in a cat. Folia Morphol (Warsz) 2008; 67: 299–303. [PubMed] [Google Scholar]
- 43. Court EA, Earnest-Koons KA, Barr SC, et al. Malignant histiocytosis in a cat. J Am Vet Med Assoc 1993; 203: 1300–1302. [PubMed] [Google Scholar]
- 44. Ide K, Setoguchi-Mukai A, Nakagawa T, et al. Disseminated histiocytic sarcoma with excessive hemophagocytosis in a cat. J Vet Med Sci 2009; 71: 817–820. [DOI] [PubMed] [Google Scholar]
- 45. Haddad JL, Goldschmidt MH, Patel RT. Fibrosarcoma arising at the site of a retained surgical sponge in a cat. Vet Clin Pathol 2010; 39: 241–246. [DOI] [PubMed] [Google Scholar]
- 46. Cuccovillo A, Lamb CR. Cellular features of sonographic target lesions of the liver and spleen in 21 dogs and a cat. Vet Radiol Ultrasound 2002; 43: 275–278. [DOI] [PubMed] [Google Scholar]
- 47. Sofer M, Michowitz M, Mandelbaum Y, et al. Percutaneous drainage of subcapsular splenic hematoma: an experimental model in dogs. Am Surg 1998; 64: 1212–1214. [PubMed] [Google Scholar]
- 48. Carstens A, Kirberger RM, Spotswood T, et al. Ultrasonography of the liver, spleen, and urinary tract of the cheetah (Acinonyx jubatus). Vet Radiol Ultrasound 2006; 47: 376–383. [DOI] [PubMed] [Google Scholar]
- 49. Walzer C, Hittmair KM, Walzer-Wagner C. Ultra-sonographic identification and characterisation of splenic nodular lipomatosis or myelolipomas in cheetahs (Acinonyx jubatus). Vet Radiol Ultrasound 1996; 37: 289–292. [Google Scholar]
- 50. Sandler CH, Langham RF. Myelolipomas in the spleen of a cat. J Am Vet Med Assoc 1972; 160: 1101–1103. [PubMed] [Google Scholar]
- 51. Cardy RH, Bostrom RE. Multiple splenic myelolipomas in a cheetah (Acinonyx jubatus). Vet Pathol 1978; 15: 556–558. [DOI] [PubMed] [Google Scholar]
- 52. Sander CH, Langham RF. Myelolipoma of the spleen in a cat. J Am Vet Med Assoc 1972; 160: 1101–1103. [PubMed] [Google Scholar]
- 53. Friedenberg SG, Balakrishnan N, Guillaumin J, et al. Splenic vasculitis, thrombosis, and infarction in a febrile dog infected with Bartonella henselae. J Vet Emerg Crit Care (San Antonio) 2015; 25: 789–794. [DOI] [PubMed] [Google Scholar]
- 54. Hardie EM, Vaden SL, Spaulding K, et al. Splenic infarction in 16 dogs: a retrospective study. J Vet Intern Med 1995; 9: 141–148. [DOI] [PubMed] [Google Scholar]
- 55. Mai W. The hilar perivenous hyperechoic triangle as a sign of acute splenic torsion in dogs. Vet Radiol Ultrasound 2006; 47: 487–491. [DOI] [PubMed] [Google Scholar]
- 56. Ramirez GA, Altimira J, Garcia-Gonzalez B, et al. Intrapancreatic ectopic splenic tissue in dogs and cats. J Comp Pathol 2013; 148: 361–364. [DOI] [PubMed] [Google Scholar]
- 57. Torner K, Staudacher M, Steiger K, et al. Clinical and pathological data of 17 non-epithelial pancreatic tumors in cats. Vet Sci 2020; 7. DOI: 10.3390/vetsci7020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liffman R, Courtman N. Fine needle aspiration of abdominal organs: a review of current recommendations for achieving a diagnostic sample. J Small Anim Pract 2017; 58: 599–609. [DOI] [PubMed] [Google Scholar]
- 59. Christopher MM. Cytology of the spleen. Vet Clin North Am Small Anim Pract 2003; 33: 135–152. [DOI] [PubMed] [Google Scholar]
- 60. O’Keefe DA, Couto CG. Fine-needle aspiration of the spleen as an aid in the diagnosis of splenomegaly. J Vet Intern Med 1987; 1: 102–109. [DOI] [PubMed] [Google Scholar]
- 61. Mahoney P. Spleen. In: Barr F, Gaschen L. (eds). BSAVA manual of canine and feline ultrasonography. Gloucestershire: British Small Animal Veterinary Association, 2012, pp 100–109. [Google Scholar]
- 62. Leblanc CJ, Head LL, Fry MM. Comparison of aspiration and nonaspiration techniques for obtaining cytologic samples from the canine and feline spleen. Vet Clin Pathol 2009; 38: 242–246. [DOI] [PubMed] [Google Scholar]
- 63. Raskin RE, Meyer D. Canine and feline cytology: a color atlas and interpretation guide. St Louis, MO: Elsevier Health Sciences, 2015. [Google Scholar]
- 64. Skeldon N, Dewhurst E. The perceived and actual diagnostic utility of veterinary cytological samples. J Small Anim Pract 2009; 50: 180–185. [DOI] [PubMed] [Google Scholar]
- 65. Watson AT, Penninck D, Knoll JS, et al. Safety and correlation of test results of combined ultrasound-guided fine-needle aspiration and needle core biopsy of the canine spleen. Vet Radiol Ultrasound 2011; 52: 317–322. [DOI] [PubMed] [Google Scholar]
- 66. Leveille R, Partington BP, Biller DS, et al. Complications after ultrasound-guided biopsy of abdominal structures in dogs and cats: 246 cases (1984–1991). J Am Vet Med Assoc 1993; 203: 413–415. [PubMed] [Google Scholar]