Abstract
Practical relevance:
Chronic kidney disease (CKD) is a highly prevalent disorder of senior cats. CKD is frequently diagnosed in association with hypertension, and the two conditions have an intermingled cause-and-effect relationship. Hypertensive target organ damage (TOD) to the eye, brain, heart and kidney significantly impacts the welfare of cats suffering from this comorbidity. Hypertension also drives proteinuria, which is an independent risk factor for progression and mortality in cats with CKD. Blood pressure monitoring and institution of effective antihypertensive treatment, where indicated, is therefore crucial in effective management of the feline CKD patient. Current guidelines recommend a target systolic blood pressure of <160 mmHg to minimise risk of TOD. Both amlodipine besylate and telmisartan are effective antihypertensive agents for use in these patients.
Clinical challenges:
Clinical signs of hypertension may not be apparent to owners of affected cats until severe hypertensive TOD is present. Despite this, blood pressure monitoring in cats with CKD is still infrequently performed, and hypertension likely remains underdiagnosed in this population.
Evidence base:
This review is based upon evaluation of the currently available published literature, including relevant consensus statements. There is a large body of evidence supporting the association between hypertension and CKD in cats. However, significant aspects, such as the mechanisms behind this association, and effect of hypertension and antihypertensive treatment on mortality and progression of CKD, remain unclear. Further research is therefore required in order to improve understanding of these conditions.
Keywords: CKD, hypertension, blood pressure, renal
Introduction
Chronic kidney disease (CKD) is defined as the presence of structural or functional abnormalities of one or both kidneys for an extended period (usually longer than 3 months). CKD is a hugely important cause of morbidity and mortality in the feline population; as a cause of death it ranked second only to trauma in one large study of cats in the UK. 1 Prevalence increases with age, and 28–50% of senior cats are reported to be affected by azotaemic CKD.2,3 While various factors have been implicated that could contribute to the initiation of CKD in affected cats, including ageing, ischaemia, dietary factors and routine vaccination, the underlying aetiology of the disease is not fully understood. 4 Accordingly, the majority of cats are found to have non-specific renal lesions on histopathology, characterised by chronic tubulointerstitial inflammation and fibrosis. 5
CKD is one of the most common underlying causes of systemic hypertension in cats, and approximately three-quarters of cats presenting with hypertensive target organ damage (TOD) have evidence of abnormal renal function on further evaluation. 6 A significant proportion of cats with CKD are hypertensive at diagnosis. The reported prevalence varies depending on the population assessed and the definition of hypertension used, but is between approximately 20% and 65%.7–11 Furthermore, those cats with CKD that are normotensive at diagnosis are significantly more likely than cats with normal renal function to go on to develop hypertension at a later time point. 7 Therefore, it follows that it is essential for cats presenting with signs referable to hypertensive TOD to be evaluated for kidney disease, and for cats presenting with kidney disease to be evaluated for hypertension. The aim of this article is to review the current understanding of the relationship between these two conditions, and the latest recommendations regarding their diagnosis and management.
The kidney and regulation of blood pressure
In healthy animals, systolic blood pressure (SBP) is maintained within a relatively narrow optimal range in order to ensure adequate perfusion of the vital organs without leading to pathological damage to tissues. What constitutes a ‘normal’ blood pressure in cats can be challenging to determine, but one large study of 780 apparently healthy cats found a median SBP of 120.6 mmHg (interquartile range 110.4–132.4), 12 and current American College of Veterinary Internal Medicine (ACVIM) consensus guidelines suggest a cut-off of <140 mmHg for defining normotension. 13 As in humans, blood pressure in apparently healthy cats also increases with age. 7 The major body systems involved in maintaining this status quo are the kidney, via direct control of circulating fluid volume alongside indirect neurohormonal effects on systemic vascular resistance, and the cardiovascular system, via alterations in heart rate and force of contraction. The cardiovascular system has traditionally been thought of as responsible for minute-to-minute control of blood pressure, whereas the kidney is viewed as primarily responsible for longer term regulation.
The main mechanisms by which the kidney senses alterations in blood pressure and circulating fluid volume are the renal afferent arteriolar stretch receptors, which detect renal perfusion pressure, and the macula densa at the start of the distal convoluted tubule, which detects the rate of chloride ion delivery in tubular fluid. If these mechanisms are functioning correctly, a perceived decrease in SBP will be accompanied by a decrease in renal sodium excretion, leading to an increase in total body sodium (the major determinant of extracellular fluid volume), an increased circulating blood volume and a subsequent increase in SBP. The opposite sequence of events occurs when a perceived increase in SBP is sensed, and therefore SBP and renal sodium excretion can be considered linked in a feedback loop. This occurs intrinsically via the phenomenon of pressure natriuresis, but is also augmented by the renin-angiotensin-aldosterone system (RAAS).
The RAAS acts as a sodium-conserving system, which is activated when renal blood flow is reduced, as when low flow in the macula densa is sensed. The initial step in activation of the RAAS is the release of renin from juxtaglomerular cells located in the walls of the afferent arteriole. Renin cleaves the prohormone angiotensinogen to form angiotensin I (AT I), which is then converted by angiotensin-converting enzyme (ACE) to angiotensin II, the major bioactive product of the system. In turn, angiotensin II stimulates the release of aldosterone from the adrenal medulla. Angiotensin II has direct effects on the proximal tubule to increase sodium retention, on vascular smooth muscle to cause vasoconstriction, and potentiates the sympathetic nervous system. Aldosterone acts on the distal tubule to increase sodium retention and is also a potent vasoconstrictor. The overall effect of RAAS activation is therefore to increase circulating fluid volume, raise total peripheral resistance and increase venous return, thereby increasing systemic blood pressure.
Pathophysiology of hypertension in CKD
The mechanisms described above enable the kidney to maintain blood pressure within an optimal range in healthy animals, despite alterations in dietary sodium intake and hydration status. In cats with CKD, there is a dysregulation of these systems which favours the development or exacerbation of systemic hypertension. The exact nature of these disturbances is multifactorial, complex and not fully understood, and much is extrapolated from human medicine while not yet being proven in cats. Furthermore, the disease processes may not be entirely analogous. The prevalence and severity of hypertension are related to severity of CKD in humans. 14 Generally, this has not been found to be the case in cats, but conflicting reports do exist.7,8,10,15 The pathophysiology is more extensively reviewed elsewhere, but currently it is thought that activation of the RAAS, increased sympathetic tone and disruption of endothelial cell function represent the most significant contributors to blood pressure dysregulation. 16
Increased RAAS activation is an expected physiological compensatory response to progressive loss of nephrons during CKD, as angiotensin II works to increase single nephron glomerular filtration rate (GFR) via preferential constriction of the efferent arteriole. In CKD this response is thought to become maladaptive, resulting in proteinuria and potentially driving disease progression and the development of hypertension. 17 There is somewhat conflicting evidence of RAAS activation in hypertensive cats with CKD, which suggests significant variation in plasma concentration of various components of the RAAS between individuals and study populations.18–20 However, there is evidence to suggest that cats with hypertension secondary to CKD may have elevated plasma aldosterone concentrations, an increased plasma aldosterone:renin ratio and decreased plasma renin activity.18,19 These studies measuring plasma concentrations of RAAS components are complicated by uncertainties as to whether assessment of the systemic RAAS reflects the state of the intrarenal RAAS, as the two systems can substantially differ. 21 Further investigation into the state of intrarenal RAAS activation in cats with CKD is therefore necessary.
Increased sympathetic activity driven by dysfunctional renal afferent nerve activity is also thought to contribute to development of hypertension in CKD. Excessive sympathetic discharge leads to chronic vasoconstriction and pathological structural changes within the walls of the vasculature in human patients with CKD, as well as activating the RAAS. 22 Further study is required to fully elucidate the role of sympathetic nervous system activation in hypertensive cats, as there is currently a paucity of literature on the subject.
Endothelial dysfunction is also believed to contribute to development of hypertension in patients with CKD. This is characterised by an impaired ability of the endothelial layer of blood vessels to release vasodilator substances, such as nitric oxide (NO), which acts to counterbalance vasoconstrictor substances. In CKD, chronic overstimulation of the vasculature by mediators such as angiotensin II and aldosterone is thought to contribute to this state. Studies attempting to detect the presence of endothelial dysfunction indirectly via measurement of plasma NO metabolites have not documented a significant difference in hypertensive vs non-hypertensive cats with CKD.23,24 No studies utilising more direct methods of assessing endothelial dysfunction have been performed in cats to date.
The kidney as a target of end organ damage in hypertension
While CKD is a potential cause of secondary systemic hypertension, the kidney is also a target organ that can be damaged by systemic hypertension. The normal kidney is able to autoregulate renal blood flow, and is protected from hypertensive TOD via afferent arteriolar vasoconstriction. In CKD there is a loss of functioning nephrons, which results in afferent arteriolar vasodilation, thereby exposing the glomerular capillaries to increased arterial pressures. The consequence of this glomerular capillary hypertension is increased protein loss across the glomerular filtration barrier. Increased protein in the filtrate is thought to be intrinsically toxic to renal tubular epithelial cells, driving inflammation and further loss of functional nephrons. 25 Systemic hypertension is a well-documented risk factor for development of proteinuria in cats,15,26 and, in turn, proteinuria is associated with an increased likelihood of disease progression and shorter survival times in cats with CKD.26,27
It is challenging to tease out any direct deleterious effects of hypertension on the kidney outside of its effect on proteinuria. In multi-variable analyses, hypertension has not been identified as a risk factor for progression and mortality in cats with CKD independent of proteinuria.15,26 The effect of antihypertensive treatment on kidney TOD and progression of CKD is also unclear. In one study, treatment with amlodipine resulted in a significant reduction in urine protein:creatinine ratio (UPC) in hypertensive cats (58% of which were also azotaemic at diagnosis), suggesting a positive effect; however, adequacy of blood pressure control was not independently associated with survival. 15 At present, it therefore appears that hypertension is most significantly involved in driving the progression of CKD through its role in the development and exacerbation of proteinuria. Indeed, renal lesions independently associated with hypertension alone in post-mortem studies of cats with CKD appear to be relatively mild. 5
Clinical signs
Clinical signs of CKD are insidious in nature, and may have been present for an extended period of time prior to presentation. Commonly reported clinical signs include polydipsia, polyuria, poor appetite and weight loss in the early stages, with additional signs such as lethargy and vomiting becoming more prevalent with deterioration of renal function. 28
Clinical signs of hypertension may not be apparent to owners of affected cats until severe hypertensive TOD is present. The organs most vulnerable to hypertensive TOD are the eyes, brain, heart and kidneys. The eye is commonly the first organ to manifest clinical signs of hypertensive TOD, purely because changes are evident to the owner, with blindness due to hyphaema or retinal detachment being a common initial presenting complaint in hypertensive cats. 29
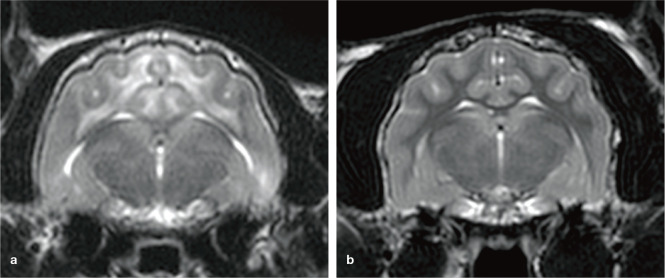
Neurological signs are also common sequelae to systemic hypertension, affecting a reported 14–46% of cats.29,30 These can manifest secondarily to hypertensive encephalopathy or due to vascular accidents (Figure 1). Clinical presentation will vary depending on the area of the central nervous system affected, but can include diffuse forebrain signs (such as seizures), vestibular disease and acute spinal cord disease. 31
Figure 1.
Transverse T2-weighted MRI of the brain of (a) a cat with hypertensive encephalopathy and (b) an unaffected control cat. Note the diffuse, symmetrical, poorly defined hyperintensity throughout the white matter tracts of the cerebrum in the cat with hypertensive encephalopathy (a), consistent with vasogenic oedema. Courtesy of Joe Fenn
Hypertension can also result in left ventricular hypertrophy, altering diastolic and systolic myocardial function. 30 This does not typically result in clinical signs in isolation, but can increase the risk of cardiovascular complications in patients with pre-existing heart disease or other haemodynamic stressors. 32 One study reports a presumed cardiovascular cause of death (congestive heart failure or collapse and sudden death) in 21% of cats with hypertension, 33 although other studies have failed to demonstrate a link between echocardiographic abnormalities and survival. 30
Diagnosis
The diagnosis of CKD in most feline patients is based upon persistently elevated fasting serum creatinine or symmetric dimethylarginine (SDMA), which reflects a decrease in GFR, in combination with inappropriately dilute urine. Patients without evidence of functional impairment can also be diagnosed with CKD through detection of abnormalities identified by renal palpation or imaging. Once a diagnosis has been made, a staging system based upon fasting serum creatinine concentration and SDMA, developed by the International Renal Interest Society (IRIS), is used to classify the severity of CKD; the four IRIS stages are defined in Table 1. 34 Cats are then substaged dependent on the presence or absence of proteinuria and hypertension. Substaging of proteinuria should be based upon UPC (Table 2), rather than less specific methods such as urine dipsticks and, as discussed earlier, may be of particular relevance in patients suffering from hypertension. 34
Table 1.
IRIS staging of CKD based upon blood creatinine and SDMA concentrations 34
| IRIS stage | Creatinine (µ mol/l) | SDMA (µg/dl) |
|---|---|---|
| 1 | <140 | <18 |
| 2 | 140–250 | 18–25 |
| 3 | 251–440 | 26–38 |
| 4 | >440 | >38 |
IRIS = International Renal Interest Society; CKD = chronic kidney disease; SDMA = symmetric dimethylarginine
Table 2.
IRIS substaging by degree of proteinuria 34
| UPC value | Substage |
|---|---|
| <0.2 | Non-proteinuric |
| 0.2–0.4 | Borderline proteinuric |
| >0.4 | Proteinuric |
IRIS = International Renal Interest Society; UPC = urine protein:creatinine ratio
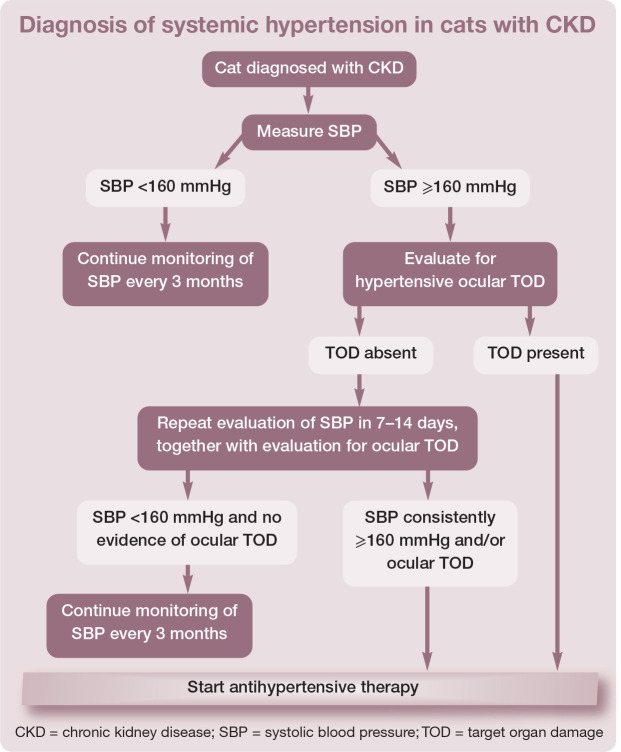
All cats diagnosed with CKD should be evaluated for the presence of hypertension at the point of diagnosis, and regularly thereafter (every 3–6 months). One recent study performed in the UK found that only 25% of cats diagnosed with CKD had blood pressure measured, and therefore it is likely that hypertension associated with CKD remains significantly underdiagnosed. 35 Cats that are considered healthy, but at risk of developing CKD and hypertension due to their life stage, should also undergo routine blood pressure monitoring. Current ISFM guidelines recommend monitoring every 12 months for cats aged 7–10 years, and every 6–12 months for cats aged 11 years or older. 36 Proactive monitoring of this population is vital, as hypertensive TOD is associated with significant morbidity and one UK epidemiological study documented improved survival in hypertensive cats diagnosed prior to clinical manifestations of disease. 37
Figure 2.

Blood pressure monitoring should take place in a quiet room, after the cat has had time to acclimatise to the clinic surroundings, and ideally in the presence of the owner. Courtesy of Nicola Lötter
When there is evidence of TOD, measurement of SBP on a single occasion (five to seven readings averaged) is sufficient to confirm diagnosis. All cats with suspected systemic hypertension should be evaluated via ophthalmoscopy for evidence of ocular TOD, since owners rarely perceive ocular abnormalities until they are very advanced. Prevalence of ocular lesions in studies of hypertensive cats varies depending on the population, but can be as high as 100%. 29 Lesions include bullous retinal detachment (focal, multifocal or total), retinal haemorrhages, intraocular haemorrhage and tapetal hyperreflectivity. 42 Indirect ophthalmoscopy is the most useful methodology for detecting ocular TOD, and is superior to direct ophthalmoscopy for identifying subtle or focal retinal lesions. Indirect ophthalmoscopy is also practically easier, allowing for a general assessment of the fundus and identification of hypertensive lesions in most cats. Diagnosis of hypertensive retinopathy via fundic examination has recently been reviewed elsewhere. 43
In patients with CKD (or other predisposing conditions) where TOD is not evident, repeated measurement of SBP on at least two occasions, 7–14 days apart, is required to confirm hypertension. An algorithm for the diagnosis of systemic hypertension in cats with CKD is shown in the box. Current ACVIM guidelines recommend treatment when hypertensive TOD is noted, and in those patients where SBP is persistently ≥160 mmHg. 13 These guidelines also provide a classification system for diagnosis of hypertension based upon the risk of developing TOD (Table 3). 13
Table 3.
ACVIM guidelines for the classification of hypertension based upon risk of TOD 13
| SBP (mmHg) | Classification |
|---|---|
| <140 | Normotensive (minimal TOD risk) |
| 140–159 | Prehypertensive (low TOD risk) |
| 160–179 | Hypertensive (moderate TOD risk) |
| >180 | Severely hypertensive (high TOD risk) |
ACVIM = American College of Veterinary Internal Medicine; SBP = systolic blood pressure; TOD = target organ damage
General principles in the treatment of CKD
CKD is not a reversible condition, and treatment is aimed at slowing progression and minimising the clinical impact of the disease on the patient. Dietary protein and phosphate restriction is a mainstay of therapy and the only therapeutic intervention where there is evidence for a beneficial effect on survival time.44,45 This is best accomplished via feeding one of a number of commercially available renal diets, which in addition to protein and phosphate restriction, are also restricted in sodium content, and may be supplemented with omega-3-polyunsaturated fatty acids, antioxidants, fibre, vitamin D and potassium. 46 IRIS have published targets for serum phosphate in cats, depending on their stage of CKD (Table 4), and if these are not met by dietary management alone, then the addition of an intestinal phosphate binder should be considered. 47
Table 4.
Serum phosphate targets according to IRIS stage of CKD 47
| IRIS stage | Target serum phosphate concentration (mmol/l) |
|---|---|
| 1 | 0.90–1.5 |
| 2 | 0.90–1.5 |
| 3 | 0.90–1.6 |
| 4 | 0.90–1.90 |
IRIS = International Renal Interest Society; CKD = chronic kidney disease
Proteinuria is also associated with disease progression and, as discussed earlier, may in some circumstances be secondary to systemic hypertension. Treatment of proteinuria has not yet been demonstrated to improve survival in cats as it has in humans with CKD, and there is some controversy over whether proteinuria plays a causal role in progression or whether it is merely a biomarker of more severe disease. 48 Despite this, a strong body of evidence exists from experimental models demonstrating that increased protein in the glomerular filtrate has intrinsic renal toxicity. Thus antiproteinuric therapy is recommended in cats with overt proteinuria (UPC >0.4) and persistent borderline proteinuria (UPC 0.2–0.4). Drugs used in the treatment of the proteinuric, hypertensive feline patient with CKD are discussed later.
Clinical signs of nausea and hyporexia may become more apparent with increasing severity of disease, in which case treatment with an antiemetic, such as maropitant, and appetite stimulant, such as mirtazapine, may be indicated.49,50 The prevalence of anaemia also increases with disease severity, and treatment with the synthetic erythropoietin analogue darbepoetin should be considered if this is affecting quality of life – which typically occurs with a packed cell volume <20%. Darbepoetin therapy has been associated with the development of hypertension, 51 and cats should have blood pressure monitored prior to every dose administered, with antihypertensive therapy instituted accordingly. Finally, consideration should be given to maintaining an appropriate hydration status. In advanced stages of disease, subcutaneous fluid therapy may be required.
The effect of instituting therapies known to delay the progression of CKD, such as commercially available renal diets, on the incidence of hypertension is uncertain, as there is not yet a proven relationship between severity of kidney disease and SBP in cats.
General principles in the treatment of hypertension
The goal of antihypertensive therapy is to reduce SBP to below 160 mmHg in order to prevent the occurrence of TOD, and to attempt to minimise, or reverse, TOD when it is already evident. Early initiation of antihypertensive treatment is critical in the latter scenario. In the case of ocular TOD, the retina will only reattach after a reduction in blood pressure. The chances of successfully regaining vision are substantially improved with prompt treatment in humans; however, the feline retina may potentially regain function even with prolonged detachment. 42 In cats affected with hypertensive encephalopathy, early treatment can reverse neurological signs and prevent progression of central nervous system lesions.52,53 Treatment is often very rewarding, with 57% of blind eyes regaining some vision in one study, 42 and cats treated for hypertensive encephalopathy having a very good chance of returning to a normal neurological state. 53
For most cats diagnosed with hypertension at a routine consultation and started on antihypertensive therapy, blood pressure monitoring should be performed 7–14 days after instigating treatment or making any dose adjustments. Once SBP is within the target range, ongoing monitoring should be performed every 3 months. However, for cats presenting as a hypertensive emergency, for example with hypertensive encephalopathy, hospitalisation may be considered for closer evaluation of blood pressure; if this is not feasible, sooner evaluation within 24–72 h may be recommended. 36
Pharmacological agents used in the treatment of cats with CKD and hypertension
Amlodipine besylate
Amlodipine besylate is a calcium channel blocker that acts on the peripheral vasculature to lower systemic vascular resistance. It has a slow onset of action, with a statistically significant antihypertensive effect evident by day 5 in one experimental study. 54 Amlodipine is the most commonly used drug for the treatment of feline hypertension, and has been licensed for this use in Europe since 2014. A large volume of literature exists that indicates that amlodipine, at a dosage of 0.125–0.25 mg/kg PO q24h (typically 0.625 mg or 1.25 mg per cat based on tablet size), decreases SBP by 30–70 mmHg in the majority of hypertensive cats.15,33,54–57 There is evidence that cats with severe hypertension (SBP >200 mmHg) require higher dosages than cats with more moderate disease, and these patients may benefit from commencing treatment at the higher dosage (ie, 1.25 mg/cat PO q24h) from the outset. 55 For those cats starting on the lower dosage of amlodipine (0.625 mg/cat PO q24h), if adequate control of hypertension (SBP <160 mmHg) has not been achieved at re-examination then the dosage can be increased to 1.25 mg/cat PO q24h.
The use of transdermal amlodipine besylate has been reported in the literature but a licensed product is not available. Limited pharmacokinetic/pharmacodynamic data suggest substantially reduced bioavailability with transdermal vs oral administration; in a pilot study only 50% of cats were able to maintain SBP <180 mmHg with transdermal application, with therapy monitored over a short period of 7 days. 57 Further data is therefore required before the use of transdermal amlodipine can be widely recommended for the management of hypertension in cats.
Rarely, licensed dosages of amlodipine may fail to adequately control hypertension. In these situations, compliance of both cat and owner should be carefully evaluated but further dosage increments may be required up to a total of 0.5 mg/kg q24h, although it should be noted that this is outside licensing regulations. Alternatively, consideration can be given in this situation to the introduction of a second antihypertensive agent. Studies have documented a significant reduction in proteinuria with control of hypertension when administering amlodipine besylate; therefore, control of hypertension can be beneficial prior to sub-staging for proteinuria in cats with CKD, and concurrent antiproteinuric therapy may not be indicated once hypertension is controlled. 15
In hypertensive emergencies dosing can be repeated every 4–8 h if necessary, up to a maximum of 2.5 mg/cat in the first 24 h. 36 Very few adverse effects are reported, although they can include gingival hyperplasia. If SBP <120 mmHg and clinical signs of hypotension are present, a decrease in dose may be required. 58 If the dose is decreased in this manner then repeat SBP measurement should be performed after 7–10 days to assess the adequacy of the lower dose. 13
No change in GFR or renal parameters is anticipated when therapy with amlopdine is started and it is therefore an antihypertensive agent that can be used in cats at all stages of CKD and also those with acute kidney injury.
Telmisartan
The selective AT I receptor blocker telmisartan is licensed for use in Europe and the USA for the treatment of systemic hypertension and management of proteinuria in cats with CKD.59–61 Studies on the use of telmisartan in the management of hypertension have revealed a mean decrease of approximately 20–25 mmHg in the SBP of treated cats.60,61 The licensed dosage for telmisartan depends on the indication for use, with a higher starting dosage of 2 mg/kg PO q24h recommended for systemic hypertension in cats compared with the licensed dosage of 1 mg/kg PO q24h for an antiproteinuric effect. As with all antihypertensive therapy, response should be monitored carefully in order to achieve a target SBP of <160 mmHg. Blood pressure should be rechecked 7–10 days after commencing therapy; however, caution should be exercised when considering dosage increases during this period as SBP may continue to decrease throughout the first 14 days of treatment. 62 Down-titration of telmisartan dosage may be required if SBP falls to <140 mmHg after 4 weeks.
Telmisartan may be considered as an initial monotherapy if significant proteinuria is present, or as an additional medication in cats that remain proteinuric after successful blood pressure control with amlodipine. When combined antihypertensive therapy is used, cats should be monitored carefully for the development of hypotension.
Telmisartan has been demonstrated to be an effective treatment for proteinuria, and in cats with CKD (that were normotensive at baseline) telmisartan at the lower dosage of 1 mg/kg PO q24h significantly decreased UPC at all time points assessed. 59
Telmisartan should never be administered to any cat that is clinically dehydrated or hypovolaemic due to the role of the RAAS in preserving renal perfusion in these states. It should therefore be considered contraindicated as either an antiproteinuric or antihypertensive agent in cats with acute kidney injury and should be used with caution in those cats with either advanced IRIS stage 3 or IRIS stage 4 CKD.
ACE inhibitors
ACE inhibitors as a monotherapy are not recommended for the treatment of feline hypertension. In one study of cats with experimentally induced CKD and mild systemic hypertension, benazepril decreased systemic arterial blood pressure by only 10–15 mmHg, 63 and both benazepril and enalapril fail to decrease SBP below 170 mmHg in the majority of clinical cases. 64 Despite this, there is evidence to suggest that the addition of benazepril as an adjunct to amlodipine could improve blood pressure control in cases where amlodipine monotherapy fails to decrease SBP to <160 mmHg. 65
Benazepril has been demonstrated to reduce proteinuria in cats with CKD, and therefore also has utility in the treatment of non-hypertensive cats with renal proteinuria, and in cats with hypertension controlled by amlodipine that remain proteinuric. 48 Similar to telmisartan, benazepril should never be administered to dehydrated or hypovolaemic patients.
Other treatments
Hydralazine, a direct arterial vasodilator, has been used in the treatment of hypertensive emergencies in cats, such as occurs after renal transplantation, at a dosage of 2.5 mg/cat PO or SC, q24h or q12h. 52 Continuous or very frequent blood pressure monitoring is required with the use of this medication.
Other agents that have been considered for the treatment of hypertensive emergencies in cats include nitroprusside, acepromazine and fenoldopam, although there is a lack of data to support recommendations for their use at present. Dietary sodium restriction has not been shown to have a beneficial effect in the treatment of hypertension in cats, unlike some populations of human patients with salt-sensitive hypertension. 66
Key Points
CKD and systemic hypertension in cats often occur in association with one another and have an intermingled cause-and-effect relationship. The precise mechanisms behind this association are multifactorial and have not yet been fully elucidated.
Due to the potential morbidity associated with hypertensive TOD, it is imperative that patients with CKD have blood pressure measured at diagnosis, and regularly thereafter. Similarly, feline patients with evidence of hypertensive TOD should be evaluated for the presence of CKD via serum biochemistry and urinalysis.
While antihypertensive treatment does not independently increase survival time in cats with CKD, it has been shown to decrease proteinuria, which is a risk factor for both mortality and disease progression in these patients.
Further research is required into the relationship between CKD and hypertension, and it is likely that along with our increased understanding, guidelines for diagnosis and treatment of these two disease entities in cats will continue to evolve in the future.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not specifically required for publication in JFMS.
Informed consent: This work did not involve the use of animals (including cadavers) and therefore informed consent was not required. For any animals or people individually identifiable within this publication, informed consent (verbal or written) for their use in the publication was obtained from the people involved.
References
- 1. O’Neill DG, Church DB, McGreevy PD, et al. Longevity and mortality of cats attending primary care veterinary practices in England. J Feline Med Surg 2015; 17: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartlett PC, Van Buren JW, Neterer M, et al. Disease surveillance and referral bias in the veterinary medical database. Prev Vet Med 2010; 94: 264–271. [DOI] [PubMed] [Google Scholar]
- 3. Marino CL, Lascelles BD, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2014; 16: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown CA, Elliott J, Schmiedt CW, et al. Chronic kidney disease in aged cats: clinical features, morphology, and proposed patho-geneses. Vet Pathol 2016; 53: 309–326. [DOI] [PubMed] [Google Scholar]
- 5. Chakrabarti S, Syme HM, Brown CA, et al. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol 2013; 50: 147–155. [DOI] [PubMed] [Google Scholar]
- 6. Maggio F, DeFrancesco TC, Atkins CE, et al. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985–1998). J Am Vet Med Assoc 2000; 217: 695–702. [DOI] [PubMed] [Google Scholar]
- 7. Bijsmans ES, Jepson RE, Chang YM, et al. Changes in systolic blood pressure over time in healthy cats and cats with chronic kidney disease. J Vet Intern Med 2015; 29: 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Syme HM, Barber PJ, Markwell PJ, et al. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc 2002; 220: 1799–1804. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi DL, Peterson ME, Graves TK, et al. Hypertension in cats with chronic renal failure or hyperthyroidism. J Vet Intern Med 1990; 4: 58–62. [DOI] [PubMed] [Google Scholar]
- 10. Hori Y, Heishima Y, Yamashita Y, et al. Relationship between indirect blood pressure and various stages of chronic kidney disease in cats. J Vet Med Sci 2018; 80: 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stiles J, Polzin D, Bistner DI. The prevalence of retinopathy in cats with systemic hypertension and chronic renal failure or hyperthyroidism. J Am Anim Hosp Assoc 1994; 30: 564–572. [Google Scholar]
- 12. Payne JR, Brodbelt DC, Luis Fuentes V. Blood pressure measurements in 780 apparently healthy cats. J Vet Intern Med 2017; 31: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Acierno MJ, Brown S, Coleman AE, et al. ACVIM consensus statement: guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2018; 32: 1803–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horowitz B, Miskulin D, Zager P. Epidemiology of hypertension in CKD. Adv Chronic Kidney Dis 2015; 22: 88–95. [DOI] [PubMed] [Google Scholar]
- 15. Jepson RE, Elliott J, Brodbelt D, et al. Effect of control of systolic blood pressure on survival in cats with systemic hypertension. J Vet Intern Med 2007; 21: 402–409. [DOI] [PubMed] [Google Scholar]
- 16. Elliott J. Physiology of blood pressure regulation and pathophysiology of hypertension. In: Elliot J, Syme HM, Jepson RE. (eds). Hypertension in the dog and cat. Switzerland: Springer, 2020, pp 3–10. [Google Scholar]
- 17. Ames MK, Atkins CE, Pitt B. The renin–angiotensin–aldosterone system and its suppression. J Vet Intern Med 2019; 33: 363–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jepson RE, Syme HM, Elliott J. Plasma renin activity and aldosterone concentrations in hypertensive cats with and without azotemia and in response to treatment with amlodipine besylate. J Vet Intern Med 2014; 28: 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen J, Henik RA, Brownfield M, et al. Plasma renin activity and angiotensin I and aldos-terone concentrations in cats with hypertension associated with chronic renal disease. Am J Vet Res 1997; 58: 535–540. [PubMed] [Google Scholar]
- 20. Mishina M, Watanabe T, Fujii K, et al. Noninvasive blood pressure measurements in cats: clinical significance of hypertension associated with chronic renal failure. J Vet Med Sci 1998; 60: 805–808. [DOI] [PubMed] [Google Scholar]
- 21. Yang T, Xu C. Physiology and pathophysi-ology of the intrarenal renin–angiotensin system: an update. J Am Soc Nephrol 2017; 28: 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sata Y, Head GA, Denton K, et al. Role of the sympathetic nervous system and its modulation in renal hypertension. Front Med (Lausanne) 2018; 5: 82. DOI: 10.3389/fmed.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jepson RE, Syme HM, Vallance C, et al. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, l-arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J Vet Intern Med 2008; 22: 317–324. [DOI] [PubMed] [Google Scholar]
- 24. Bijsmans ES, Jepson RE, Syme HM, et al. Nitric oxide in feline chronic kidney disease and hypertension. Proceedings of the British Small Animal Veterinary Association congress; April 2015, Birmingham. British Small Animal Veterinary Association, p 491 [Google Scholar]
- 25. Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17: 2974–2984. [DOI] [PubMed] [Google Scholar]
- 26. Syme HM, Markwell PJ, Pfeiffer D, et al. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006; 20: 528–535. [DOI] [PubMed] [Google Scholar]
- 27. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med 2012; 26: 275–281. [DOI] [PubMed] [Google Scholar]
- 28. Elliott J, Barber PJ. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39: 78–85. [DOI] [PubMed] [Google Scholar]
- 29. Littman MP. Spontaneous systemic hypertension in 24 cats. J Vet Intern Med 1994; 8: 79–86. [DOI] [PubMed] [Google Scholar]
- 30. Chetboul V, Lefebvre HP, Pinhas C, et al. Spontaneous feline hypertension: clinical and echocardiographic abnormalities, and survival rate. J Vet Intern Med 2003; 17: 89–95. [DOI] [PubMed] [Google Scholar]
- 31. Matiasek K, Matiasek LA, Rosati M. Hypertension and the central nervous system. In: Elliott JS, Syme HM, Jepson RE. (eds). Hypertension in the dog and cat. Switzerland:Springer, 2020, pp 241–264. [Google Scholar]
- 32. Coleman AE, Brown SA. Hypertension and the heart and vasculature. In: Elliot J, Syme HM, Jepson RE. (eds). Hypertension in the dog and cat. Switzerland: Springer, 2020, pp 187–215. [Google Scholar]
- 33. Elliott J, Barber PJ, Syme HM, et al. Feline hypertension: clinical findings and response to antihypertensive treatment in 30 cases. J Small Anim Pract 2001; 42: 122–129. [DOI] [PubMed] [Google Scholar]
- 34. Elliot J, White J; International Renal Interest Society. IRIS staging system. http://iris-kidney.com/education/staging_system.html (2019, accessed on April 7, 2021). [Google Scholar]
- 35. Conroy M, Brodbelt DC, O’Neill D, et al. Chronic kidney disease in cats attending primary care practice in the UK: a VetCompass™ study. Vet Rec 2019; 184: 526. DOI: 10.1136/vr.105100. [DOI] [PubMed] [Google Scholar]
- 36. Taylor SS, Sparkes AH, Briscoe K, et al. ISFM consensus guidelines on the diagnosis and management of hypertension in cats. J FelineMed Surg 2017; 19: 288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Conroy M, Chang YM, Brodbelt D, et al. Survival after diagnosis of hypertension in cats attending primary care practice in the United Kingdom. J Vet Intern Med 2018; 32: 1846–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jepson RE, Hartley V, Mendl M, et al. A comparison of CAT Doppler and oscillomet-ric Memoprint machines for non-invasive blood pressure measurement in conscious cats. J Feline Med Surg 2005; 7: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pereira JS, Fragoso S, Beck A, et al. Improving the feline veterinary consultation: the usefulness of Feliway spray in reducing cats’ stress. J Feline Med Surg 2016; 18: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Haaften KA, Forsythe LRE, Stelow EA, et al. Effects of a single preappointment dose of gabapentin on signs of stress in cats during transportation and veterinary examination. J Am Vet Med Assoc 2017; 251: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 41. Zeugswetter FK, Tichy A, Weber K. Radial vs coccygeal artery Doppler blood pressure measurement in conscious cats. J Feline Med Surg 2018; 20: 968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Young WM, Zheng C, Davidson MG, et al. Visual outcome in cats with hypertensive chorioretinopathy. Vet Ophthalmol 2019; 22: 161–167. [DOI] [PubMed] [Google Scholar]
- 43. Carter J. Hypertensive ocular disease in cats: a guide to fundic lesions to facilitate early diagnosis. J Feline Med Surg 2019; 21: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elliott J, Rawlings JM, Markwell PJ, et al. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract 2000; 41: 235–242. [DOI] [PubMed] [Google Scholar]
- 45. Ross SJ, Osborne CA, Kirk CA, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc 2006; 229: 949–957. [DOI] [PubMed] [Google Scholar]
- 46. Scherk MA, Laflamme DP. Controversies in veterinary nephrology: renal diets are indicated for cats with International Renal Interest Society chronic kidney disease stages 2 to 4: the con view. Vet Clin North Am Small Anim Pract 2016; 46: 1067–1094. [DOI] [PubMed] [Google Scholar]
- 47. International Renal Interest Society. Treatment recommendations for CKD in cats. http://www.iris-kidney.com/pdf/IRIS_CAT_Treatment_Recommendations_2019.pdf (2019, accessed April 21, 2021). [Google Scholar]
- 48. King JN, Gunn-Moore DA, Tasker S, et al. ; Benazepril in Renal insufficiency in Cats Study Group. Tolerability and efficacy of benazepril in cats with chronic kidney disease. J Vet Intern Med 2006; 20: 1054–1064. [DOI] [PubMed] [Google Scholar]
- 49. Quimby JM, Lunn KF. Mirtazapine as an appetite stimulant and antiemetic in cats with chronic kidney disease: a masked placebo-controlled crossover clinical trial. Vet J 2013; 197: 651–655. [DOI] [PubMed] [Google Scholar]
- 50. Quimby JM, Brock WT, Moses K, et al. Chronic use of maropitant for the management of vomiting and inappetence in cats with chronic kidney disease: a blinded, placebo-controlled clinical trial. J Feline Med Surg 2015; 17: 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chalhoub S, Langston CE, Farrelly J. The use of darbepoetin to stimulate erythro-poiesis in anemia of chronic kidney disease in cats: 25 cases. J Vet Intern Med 2012; 26: 363–369. [DOI] [PubMed] [Google Scholar]
- 52. Kyles AE, Gregory CR, Wooldridge JD, et al. Management of hypertension controls postoperative neurologic disorders after renal transplantation in cats. Vet Surg 1999; 28: 436–441. [DOI] [PubMed] [Google Scholar]
- 53. O’Neill J, Kent M, Glass EN, et al. Clinicopathologic and MRI characteristics of presumptive hypertensive encephalopathy in two cats and two dogs. J Am Anim Hosp Assoc 2013; 49: 412–420. [DOI] [PubMed] [Google Scholar]
- 54. Mathur S, Syme H, Brown CA, et al. Effects of the calcium channel antagonist amlodipine in cats with surgically induced hypertensive renal insufficiency. Am J Vet Res 2002; 63: 833–839. [DOI] [PubMed] [Google Scholar]
- 55. Bijsmans ES, Doig M, Jepson RE, et al. Factors influencing the relationship between the dose of amlodipine required for blood pressure control and change in blood pressure in hypertensive cats. J Vet Intern Med 2016; 30: 1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huhtinen M, Derre G, Renoldi HJ, et al. Randomized placebo-controlled clinical trial of a chewable formulation of amlodipine for the treatment of hypertension in client-owned cats. J Vet Intern Med 2015; 29: 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Helms SR. Treatment of feline hypertension with transdermal amlodipine: a pilot study. J Am Anim Hosp Assoc 2007; 43: 149–156. [DOI] [PubMed] [Google Scholar]
- 58. Desmet L, van der Meer J. Antihyper-tensive treatment with telmisartan in a cat with amlodipine-induced gingival hyperplasia. JFMS Open Rep 2017; 3. DOI: 10.1177/2055116917745236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sent U, Gossl R, Elliott J, et al. Comparison of efficacy of long-term oral treatment with telmisartan and benazepril in cats with chronic kidney disease. J Vet Intern Med 2015; 29: 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Glaus TM, Elliott J, Herberich E, et al. Efficacy of long-term oral telmisartan treatment in cats with hypertension: results of a prospective European clinical trial. J Vet Intern Med 2019; 33: 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Coleman AE, Brown SA, Traas AM, et al. Safety and efficacy of orally administered telmisar-tan for the treatment of systemic hypertension in cats: results of a double-blind, placebo-controlled, randomized clinical trial. J Vet Intern Med 2019; 33: 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Coleman AE, Brown SA, Stark M, et al. Evaluation of orally administered telmisartan for the reduction of indirect systolic arterial blood pressure in awake, clinically normal cats. J Feline Med Surg 2019; 21: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brown SA, Brown CA, Jacobs G, et al. Effects of the angiotensin converting enzyme inhibitor benazepril in cats with induced renal insufficiency. Am J Vet Res 2001; 62: 375–383. [DOI] [PubMed] [Google Scholar]
- 64. Steele JL, Henik RA, Stepien RL. Effects of angiotensin-converting enzyme inhibition on plasma aldosterone concentration, plasma renin activity, and blood pressure in spontaneously hypertensive cats with chronic renal disease. Vet Ther 2002; 3: 157–166. [PubMed] [Google Scholar]
- 65. Elliot J, Fletcher M, Souttar K, et al. Effect of concomitant amlodipine and benazepril therapy in the management of feline hypertension [abstract]. J Vet Intern Med 2004; 18: 788. [Google Scholar]
- 66. Buranakarl C, Mathur S, Brown SA. Effects of dietary sodium chloride intake on renal function and blood pressure in cats with normal and reduced renal function. Am J Vet Res 2004; 65: 620–627. [DOI] [PubMed] [Google Scholar]