Abstract
Streptococcus canis was isolated from 31 milk samples from 11 cows in a dairy herd (with 49 lactating cows) affected by subclinical mastitis in north Rhine-Westphalia, Germany. Thirty-one isolates from the infected udder quarters were further characterized for their phenotypic and molecular properties. Most isolates (83.9%) produced α-galactosidase, and all were negative for β-d-glucuronidase. Amplification of the 16S rRNA gene by the PCR method and digestion with the restriction enzymes RsaI, MspI, and AvaII yielded species-specific patterns. Additional identification by species-specific amplification of the 16S rRNA gene, the 16S-23S rRNA gene intergenic spacer region, the CAMP factor-encoding gene cfg, and the internal fragments of the sodA gene was consistent with S. canis. Macrorestriction analysis of the chromosomal DNA by pulsed-field gel electrophoresis showed that the S. canis isolates originated from a single clone or were very closely related.
Streptococcus canis (Lancefield serogroup G) belongs to the pyogenic group of streptococci. This group includes S. pyogenes, S. agalactiae, S. dysgalactiae subsp. dysgalactiae (serogroups C and L), S. dysgalactiae subsp. equisimilis (serogroup G), S. equi subsp. equi, S. equi subsp. zooepidemicus, S. uberis, S. parauberis, S. porcinus, S. iniae, and S. hyointestinalis (17, 31). In 1986, Devriese et al. (9) characterized the beta-hemolytic group G streptococci isolated from cows and dogs and suggested that this group of bacteria be named S. canis. The beta-hemolytic group G streptococci mainly consist of S. canis, S. dysgalactiae subsp. equisimilis, S. anginosus (S. milleri), and S. intestinalis. S. canis was isolated mainly from dogs and cats, and in rare cases it was also isolated from the udders of lactating cows and other animal species (7, 8, 10, 22, 28, 40). Isolation of S. canis from humans has also been reported sporadically (6, 35, 41). In contrast, S. dysgalactiae subsp. equisimilis and S. anginosus were predominantly isolated from human infections (13, 32), and S. intestinalis was mainly isolated from pigs (30). The two beta-hemolytic species S. canis and S. dysgalactiae subsp. equisimilis can be differentiated by their biochemical properties, including acid production from trehalose and lactose, esculin hydrolysis, fibrinolysin production, and enzyme activities, including α-galactosidase, β-galactosidase, and β-d-glucuronidase activities and CAMP-like factor phenomena (6, 10). Species identification and differentiation can be achieved by molecular biology-based methods with rRNA genes (4). Whatmore et al. (41) amplified the internal fragments of the sodA gene (sodAint), which encodes a manganese-dependent enzyme (manganese-dependent superoxide dismutase), and the mutS gene, which encodes the DNA mismatch repair protein of different Streptococccus species. Täpp et al. (36) described the genotyping relationships of 50 Streptococcus species, including S. canis, by sequencing the RNase P RNA gene, rnpB. Oligonucleotides for the species-specific 16S rRNA gene, the 16S-23S rRNA gene intergenic spacer region (ISR), as well as the CAMP factor-encoding gene cfg could also be used for amplification (14, 19, 20, 21). The molecular aspects of the virulence factors of S. canis, such as M and M-like proteins, streptolysin O, and streptokinase, were studied previously (11).
In dogs, S. canis is associated with infections of the urogenital and respiratory tracts and with otitis externa (7, 27), while in cats it is associated with lymphadenitis and arthritis (22, 34). S. canis infections have been described in humans with meningitis, peritonitis, neonatal septicemia, adult septicemia, and cellulitis (6, 35, 41). Subclinical mastitis due to S. canis seems to be very rare, and only a relatively small number of reports so far have described the isolation of Streptococcus group G or S. canis from bovine mammary gland infections (2, 5, 12, 16, 26, 27, 38).
In the present study, we isolated S. canis from milk samples collected from a dairy herd in which a high percentage of animals were affected by subclinical mastitis. These strains were characterized for their phenotypic and molecular properties.
CASE REPORT
Udder quarter milk samples from 15 cows on a dairy farm located in north Rhine-Westphalia, Germany, were received in November 2002 for routine bacteriological and cytological analysis at our institute because subclinical mastitis (elevated somatic cell counts [SCCs]) had been diagnosed by the veterinarian in charge of the farm and the animals had reduced levels of milk production. Initial bacteriological analysis of these samples on blood agar revealed the frequent and massive growth of Lancefield serogroup G streptococci, with variable esculin hydrolysis patterns for the isolates in most milk samples. Therefore, an atypical outbreak of subclinical mastitis was suspected. In order to clarify the infection situation on this farm, udder quarter milk samples from all 49 lactating cows (196 samples) were collected by members of the institute 1 week later for laboratory analysis: after teat disinfection with 70% alcohol, 10 ml of milk was collected in a test tube containing boric acid (Nerbe Plus, Winsen-Luhe, Germany). The samples were transported to the laboratory in a cooling box (<10°C).
MATERIALS AND METHODS
Identification of bacteria.
All samples were subjected to total SCC determination by the direct microscopic method according to the reference method of Prescott-Breed in order to confirm the subclinical infection status of the samples collected. A total of 0.1 ml from each milk sample was plated onto sheep blood agar containing 1% esculin (Oxoid, Wessel, Germany). The plates were incubated under aerobic conditions for 24 to 48 h at 37°C. Detection of Staphylococcus aureus and coagulase-negative staphylococci (CoNS) was achieved by conventional methods, including hemoylsis patterns on blood agar, the coagulase tube test (BD BBL, Darmstadt, Germany), and detection of clumping factor and protein A by latex agglutination with a Staphaurex test system (Genzyme Virotech, Rüsselsheim, Germany).
Colonies that showed hemolysis and that were found to be facultatively anaerobic, gram-positive, and catalase-negative cocci (the genera Streptococcus and Enterococcus) were cultivated on esculin blood agar (Oxoid). Esculin-hydrolyzing cultures were further cultivated on kanamycin esculin azide agar (KAA; Merck, Darmstadt, Germany) in order to differentiate Streptococcus from Enterococcus species. The Lancefield group was detected from fresh cultures by a latex agglutination test for the identification of streptococcal groups A, B, C, D, F, and G, according to the instructions of the manufacturer (Streptococcal Grouping kit; Oxoid). Group G isolates were studied together with five control strains of S. canis isolated from cows (n = 3) and dogs (n = 2), which were obtained from our institute's strain collection. On the basis of cultural ability and growth patterns, 31 isolates were obtained from 11 cows. All isolates were identified as S. canis and were subjected to further characterization.
Biochemical test and phenotypic characterization.
The carbohydrate fermentation test was performed by using phenol-red broth (Merck) containing 1% glucose, inulin, lactose, maltose, mannitol, raffinose, ribose, saccharose, salicin, sorbitol, mannitol, and trehalose. Other characteristics of the isolates studied included hydrolysis of esculin, arginine, and sodium hippurate. The α-galactosidase, β-galactosidase, β-d-glucuronidase, and pyrrolidonyl aminopeptidase activities of the isolates were determined with commercial test kits from Rosco Diagnostics (Freiburg, Germany) and Merck or as described in the literature (9). All isolates were subcultured in brain heart infusion broth (BHI; Oxoid) containing 6.5% NaCl at 37°C and BHI without salt at 10 and 45°C, respectively. Synergistic CAMP-like hemolytic activities with beta-hemolytic S. aureus were tested on sheep blood agar plates (Oxoid), as described by Isenberg (23). All isolates suspected of being S. canis were identified by using the API 20 Strep identification system (bio-Mérieux, Nürtingen, Germany).
Susceptibilities to antimicrobial agents.
The antibiotic sensitivity test was performed as follows: four to five identical colonies were incubated in 3 ml of Todd-Hewitt broth (Oxoid) for 2 h at 37°C. Then, 0.1 ml of the bacterial suspension was plated on Mueller-Hinton agar (Merck), followed by addition of antibiotic disks (enrofloxacin, 5 μg; trimethoprim-sulfamethoxazole, 25 μg; tetracycline, 30 μg; gentamicin, 10 μg; amoxicillin-clavulanic acid, 30 μg; lincomycin at 60 μg with neomycin at 15 μg; colistin sulfate, 10 μg; penicillin G, 10 IU [all from Oxoid]; and cefoperazone, 10 μg [Pfizer, Karlsruhe, Germany]).
Identification of bacteria by PCR amplification methods.
Extraction of bacterial genomic DNA was performed as described previously (18). PCR amplification of the gene encoding the 16S rRNA of all isolates was performed by using oligonucleotide primers AR1 and AmII (1, 4), followed by restriction fragment length polymorphism (RFLP) analysis with the restriction enzymes RsaI, MspI, and AvaII (25). Species-specific PCR was performed by using oligonucleotide primer pairs can-I and can-II for amplification of the species-specific fragment of the 16S rRNA gene. Oligonucleotide primers c-I and c-II were used to amplify the species-specific fragment of ISR (21). Oligonucleotide primers camp-canis-I and camp-canis-II, specific for the cfg gene of S. canis, were also used (19). The sequence of the sodAint gene, previously described by Whatmore et al. (41) (National Center for Biotechnology Information GenBank accession number AJ413204), was used to design the species-specific oligonucleotide primers canis-sod-I and canis-sod-II by using the computer program OLIGO 4 (OLIGO Primer Analysis Software, version 4.0; National Biosciences Inc., Plymouth, Mass.). In order to amplify the sodAint gene, a final volume of 30 μl was prepared as previously described by Hassan et al. (19, 21) for the 16S rRNA, ISR, and cfg genes. The tubes were then subjected to 30 cycles in a thermal cycler (iCycler; Bio-Rad, Munich, Germany) with the following program: 94°C for 25 s, 60°C for 20 s, and 72°C for 25 s. The final cycle was followed by an extension step at 72°C for 5 min. All oligonucleotide primers used in the present study are summarized in Table 1. The PCR products were analyzed by 2% agarose gel electrophoresis (Biozym, Hessisch-Oldendorf, Germany) in Tris-acetate electrophoresis buffer (0.04 mol of Tris per liter, 0.001 mol of EDTA [pH 7.8] per liter). The molecular marker GeneRuler 50 and a 100-bp DNA ladder (MBI Fermentas) were used. The Streptococcus species used as controls in all PCR amplification reactions in this study included S. pyogenes (n = 2); S. agalactiae (n = 3) reference strains 090 (serotype Ia), H36B (serotype Ib), and 18RS 21 (serotype II); S. dysgalactiae subsp. dysgalactiae serogroup C (n = 2); S. dysgalactiae subsp. equisimilis serogroup G (n = 2); S. dysgalactiae subsp. dysgalactiae serogroup L (n = 2); S. equi subsp. equi (strain CF32); S. equi subsp. zooepidemicus (strain W60); S. canis isolates from cows (n = 3) and dogs (n = 2); S. uberis reference strains NCDO 2038 and NCDO 2086; S. parauberis reference strain NCDO 2020 and strain M3; S. porcinus reference strain ATCC 12390; S. phocae reference strain NCTC 12719; S. suis (strain 11538); and Enterococcus faecalis. All strains were taken from our institute's strain collection.
TABLE 1.
Oligonucleotide primer pairs used for amplification of the 16S rRNA gene, the species-specific part of the 16S rRNA gene, the ISR, the cfg gene, and the sodAint gene of S. canis
| Target gene | Oligonucleotide primer | Sequence (5′-3′) | PCR product size (bp) | Reference(s) |
|---|---|---|---|---|
| 16S rRNA | AR1 | GAGAGTTTGATCCTGGCTCAGGA | 1,430 | 2, 6 |
| AmII | CGGGTGTTACAAACTCTCGTGGT | |||
| 16S rRNA | canis I | AGTGCTTAACACATGTTAAGAA | 1,320 | 25 |
| canis II | GATAGATGATTGGGGTGAAG | |||
| ISR | c-I | TAAACCGAAAACGCTGTAAGTATTA | 215 | 25 |
| c-II | ACCATTAGTTAGTGGGTTCCCCC | |||
| cfg | camp-canis-I | CAATTAACTAATAAGGTAGAACAG | 238 | 25 |
| camp-canis-II | CTCTCTCAAAACGGGTG | |||
| sodAint | canis-sod-I | AGAATTATTGGCAGATGTCACTA | 363 | Present study |
| canis-sod-II | TTTCAAGTTGTCCTTCCTTATTG |
PFGE.
Macrorestriction analysis of chromosomal DNA was performed as described by Soedarmanto and Lämmler (33). DNA fingerprinting was carried out by preparation of the whole bacterial DNA of the S. canis isolates in agarose gel plugs and subsequent digestion of the bacterial DNA with the SmaI restriction enzyme and with the pulse time suggested by Baseggio et al. (3). The fragment pattern was finally studied by pulsed-field gel electrophoresis (PFGE). Interpretation of the restriction patterns was performed as described by Tenover et al. (37).
RESULTS
A massive situation of subclinical mastitis was found in the cow population on the dairy farm under study. More than half of the animals had elevated cell counts of >105/ml, and at least one udder quarter of 27 of the cows was bacteriologically positive. The dominant pathogenic species, isolated from 31 udder quarters, was S. canis, but other major (S. uberis, S. aureus, Enterococcus spp.) and minor (CoNS) pathogens responsible for mastitis were also found at various frequencies and in some cases as a mixed flora in single samples. The S. canis isolates were found on one or more quarters of a total of 11 cows. The SCCs of the quarters infected with S. canis only (n = 12) ranged from 3.3 × 105 to 1.4 × 106/ml, and the udders were therefore clearly abnormal in terms of udder health. Most of the quarters (n = 19) with mixed infections (S. canis and other bacteria) also had elevated SCCs (>1.0 × 105/ml), with a maximum of 2.0 × 106/ml. The other quarter milk samples from which no S. canis isolates could be isolated (n = 67) were positive mostly for CoNS (n = 58), some together with S. uberis or S. aureus, and the proportion of quarters with SCCs >1.0 × 105/ml was 65.7% (maximum, 3.5 × 106/ml).
All 31 presumptive S. canis isolates were gram-positive, catalase-negative cocci and showed complete beta-hemolysis. In the first cultivation, all 31 S. canis isolates appeared to be esculin negative. However, in the second subculture all isolates exhibited an esculin-positive reaction. None of the 31 isolates grew on KAA. Serotyping showed that all 31 isolates were Lancefield serogroup G. All isolates hydrolyzed glucose, maltose, ribose, and saccharose. They were uniformly negative for inulin, lactose, mannitol, raffinose, salicin, sorbitol, and trehalose. All isolates hydrolyzed arginine, but none of the isolates hydrolyzed sodium hippurate. A total of 83.9% (26 of 31) of the isolates showed α-galactosidase activity. All isolates yielded positive reactions for β-galactosidase and pyrrolidonyl aminopeptidase, whereas all were negative for β-d-glucuronidase as well as pyrrolidonyl arylamidase activities with the API 20 Strep system.
None of the isolates showed growth in BHI containing 6.5% NaCl at 37°C or in BHI at 10°C, but 17 strains (54.8%) grew in BHI at 45°C. Synergistic CAMP-like hemolytic activities were determined on sheep blood agar plates and could be observed for all strains. All isolates were identified as S. canis by the API 20 Strep system (software version 3.3.3) with digit code 4273405 (99.9% identity). The results are summarized in Table 2.
TABLE 2.
Phenotypic and genotypic properties of 31 S. canis isolates from cows with subclinical mastitis in comparison with those of five S. canis control strains
| Property | Reactiona
|
|
|---|---|---|
| S. canis (n = 31) | S. canis control strains (n = 5) | |
| Growth on: | ||
| KAA | − | − |
| 6.5% NaCl | − | − |
| Beta-hemolysis | + | + |
| Carbohydrate fermentation | ||
| Glucose, maltose, ribose, saccharose | + | + |
| Inulin, lactose, mannitol, raffinose, salicin, sorbit, mannit, trehalose | − | − |
| Hydrolysis of: | ||
| Esculin | +b | + |
| Arginine | + | + |
| Sodium hippurate | − | − |
| Enzyme activity | ||
| α-Galactosidase | +c | + |
| β-Galactosidase | + | + |
| β-d-Glucuronidase | − | − |
| Pyrrolidonyl arylamidase in API 20 Strep system | − | − |
| CAMP-like factor on sheep blood agar plates | + | + |
| Specific PCR | ||
| cfg gene | + | + |
| sodAint gene | + | + |
+, 100% positive reaction; −, 0% positive reaction.
Positive after second subculture.
Of the isolates tested, 83.9% were positive.
The antibiotic sensitivity test showed that all S. canis isolates were sensitive to penicillin G, cefoperazone, amoxicillin-clavulanic acid, and lincomycin-neomycin. All were sensitive or at least of intermediate sensitivity to trimethoprim-sulfamethoxazole and enrofloxacin. All isolates were resistant or at least of intermediate sensitivity to tetracycline and gentamicin, and all were resistant to colistin sulfate. In general, only minor differences in antibiotic sensitivities, as estimated from the inhibition zone diameters, were observed between isolates.
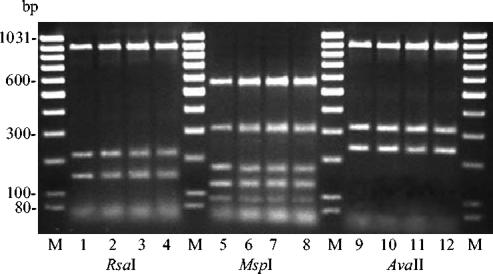
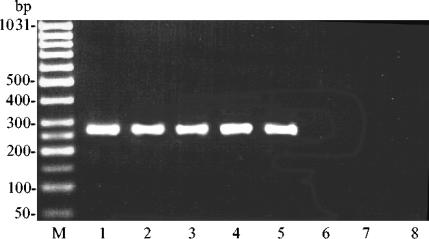
Further characterization of the 16S rRNA gene by PCR-RFLP analysis revealed that all isolates displayed an amplicon size of 1,430 bp. Digestion of this amplicon with the restriction enzymes RsaI, MspI, and AvaII gave the following results. Digestion with RsaI yielded three fragments of 900, 210, and 145 bp, respectively. Digestion with MspI revealed a uniform restriction pattern with fragment sizes of 570, 310, 170, 130, and 90 bp, while digestion with AvaII gave fragments of 900, 310, and 230 bp (Fig. 1). Amplification of the S. canis 16S rRNA gene, the ISR, and the cfg gene with species-specific oligonucleotide primers revealed amplicons of 1,320, 215, and 238 bp, respectively (data not shown). All isolates were positive for the sodAint gene, with a specific amplicon of 363 bp (Fig. 2), while no amplicon was obtained for any of the non-S. canis streptococcal control strains.
FIG. 1.
Typical fragments of the 16S rRNA gene of S. canis isolated from milk samples after digestion with different restriction enzymes. Lanes 1 to 4, PCR fragments after digestion with RsaI; lanes 5 to 8, PCR fragments after digestion with MspI; lanes 9 to 12, PCR fragments after digestion with AvaII; lanes M, GeneRuler 100-bp DNA ladder (MBI Fermentas).
FIG. 2.
A 263-bp amplicon (lanes 1 to 5) of the sodAint gene of S. canis isolated from milk samples obtained by PCR with species-specific oligonucleotide primers canis-sod-I and canis-sod-II. Lanes 6 to 8, S. agalactiae, S. dysgalactiae, and S. uberis, respectively, which served as negative controls; lane M, GeneRuler 50-bp DNA ladder (MBI Fermentas).
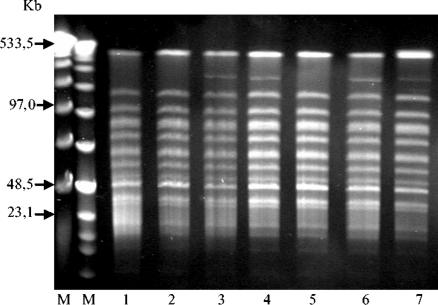
As shown in Fig. 3, the patterns obtained by PFGE of genomic DNA revealed that 77.4% of the S. canis isolates were identical. At the same time, these isolates were closely related to the remaining seven isolates (derived from the same clone, according to Tenover et al. [37]). The PFGE patterns of the closely related isolates differed by only one fragment at 450 kb. This heterogeneity of some of the phenotypic and genotypic properties among the S. canis isolates (single isolates and closely related clones) may be due to evolutionary processes.
FIG. 3.
PFGE restriction patterns of chromosomal DNA from S. canis isolated from seven milk samples (lanes 1 to 7, respectively) after digestion with endonuclease SmaI. Lanes M (left and right), a 50- to 1,000-kb ladder (Lambda Ladder PFG Marker; BioLabs, Schwalbach, Germany) and a 0.1- to 200-kb ladder (Low Range PFG Marker; BioLabs), respectively, which served as size markers.
DISCUSSION
Subclinical mastitis in cows is a major source of economic loss in the dairy production industry throughout the world. The prevailing pathogens (at various frequencies) isolated from the milk of cows with subclinical mastitis are staphylococci (mostly S. aureus), enterococci (mostly E. faecalis), and streptococcal species, including S. agalactiae, S. dysgalactiae subsp. dysgalactiae, and S. uberis.
Subclinical mastitis caused by serogroup G streptococci is very rare, with a reported incidence of isolation of <1% (38). The origin of such an infection is not clear, but S. canis has been described as a contagious cause of subclinical mastitis, with cow-to-cow transmission (15). It is speculated that infection may also result from transmission from other animals, such as dogs and cats. In combination with the SCCs, the bacteriological results suggest that S. canis was the predominant pathogen at least in 11 of 27 cases, although other, more common mastitis pathogens may have played a role in some cases.
Eberhart and Guss (12) described intramammary infections caused by serogroup G streptococci isolated from 13 of 35 dairy cows in Pennsylvania, but they did not identify the species. Watts et al. (38) also described an outbreak of bovine mastitis in a dairy herd caused by Streptococcus group G characterized by weak hemolysis and esculin-negative reaction. Watts (39) reported that S. canis group G streptococci were isolated in low numbers from bovine mammary glands. That investigator identified 8 S. canis isolates among the 317 isolates recovered from bovine mammary glands. Four of these isolates exhibited beta-hemolysis, four other isolates showed alpha-hemolysis, and all isolates were negative for trehalose fermentation.
The present study provides data on the phenotypic and genotypic properties of 31 S. canis isolates from 11 dairy cows with subclinical mastitis, which seems to be one of the largest outbreaks of S. canis infection documented so far. The phenotypic characteristics, based on 18 individual biochemical tests and a commercial identification test (API 20 Strep system), correspond to those reported for S. canis by other investigators (9, 33). It is interesting that on primary culture all S. canis isolates appeared to be esculin negative. Meanwhile, the isolates uniformly gave positive results for esculin after they were subcultured. This effect could affect the routine diagnosis of mastitis. The probability of identification of all 31 isolates was 99.9%, on the basis of the biochemical reaction implemented with the API 20 Strep test kit. Beta-hemolysis on blood agar and carbohydrate fermentation were consistent with earlier descriptions of S. canis (9, 10, 33). The results reported for human isolates of S. canis (9, 41) were also in agreement with our findings and suggest that production of the enzymes α-galactosidase, β-galactosidase, and β-d-glucuronidase, in combination with information obtained from serogrouping analysis, seems to be a suitable criterion for the differentiation of S. canis. The antibiotic resistance patterns of the S. canis isolates found in this study are generally in agreement with the findings of Soedarmanto and Lämmler (33).
Identification of S. canis by PCR-RFLP analysis of the 16S rRNA gene demonstrated that all isolates had species-specific restriction profiles compared with the profiles of the other streptococcal species (S. agalactiae; S. dygalactiae Lancefield serogroups C, L, and G; S. uberis; and S. parauberis) (data not shown). The results of restriction analysis with the RsaI, MspI, and AvaII enzymes indicated no intraspecies variations among the isolates (1, 24, 25).
For further characterization, several species-specific oligonucleotide primers were successfully used in PCRs for the 16S rRNA gene, ISR, and the cfg gene. Comparable investigations were carried out by Hassan et al. (19), who studied the S. canis genes encoding 16S rRNA and ISR. In addition, the sodAint gene of S. canis was amplified with species-specific oligonucleotide primers in this study to identify all S. canis strains. To the best of our knowledge, this is the first report of the amplification of this gene for the identification of S. canis. The sodAint genes of S. canis and various streptococcal strains have been sequenced and aligned by Poyart et al. (29) and Whatmore et al. (41). Those groups of investigators found obvious variations in sequence homology values within a range of approximately 64.1 to 90.1% among 27 different streptococci species and homology values of 98.9% between S. equi subsp. equi and S. equi subsp. zooepidemicus. Thus, the sodAint gene seems to be a suitable tool for the differentiation of S. canis from other Streptococcus species.
The PFGE restriction patterns showed that 24 isolates were of a predominant clone and that the remaining isolates displayed a strong clonal relationship to that clone. The difference of one fragment size could be due to a spontaneous mutation or the result of the repeated subculturing of S. canis isolates over time (37).
The potential contagiousness of this microorganism should be emphasized, considering the high percentage of infected animals in the herd that we studied. Although S. canis is rare, it could present a problem for all animals in a herd, even when it is isolated from only one cow. Therefore, some effort was put into careful species identification, as described above. In terms of workload and costs, PCR-RFLP and PCR amplification of species-specific genes of S. canis are not routine methods for the diagnosis of mastitis, but they may help with the unambiguous identification of S. canis strains isolated from dairy cow herds.
REFERENCES
- 1.Abdulmawjood, A., and C. Lämmler. 1999. Amplification of 16S ribosomal RNA gene sequences for the identification of streptococci of Lancefield group B. Res. Vet. Sci. 67:159-162. [DOI] [PubMed] [Google Scholar]
- 2.Barnum, D. A., and D. S. Fuller. 1958. Report of an outbreak of chronic mastitis in cattle caused by a Streptococcus of Lancefield's group G. Can. J. Comp. Med. 17:465-472. [PMC free article] [PubMed] [Google Scholar]
- 3.Baseggio, N., P. D. Mansell, J. W. Browning, and G. F. Browning. 1997. Strain differentiation of isolates of streptococci from bovine mastitis by pulsed-field gel electrophoresis. Mol. Cell. Probes 11:349-354. [DOI] [PubMed] [Google Scholar]
- 4.Bentley, R. W., J. A. Leigh, and M. D. Collins. 1991. Intrageneric structure of Streptococcus based on comparative analysis of small-subunit rRNA sequences. Int. J. Syst. Bacteriol. 41:487-494. [DOI] [PubMed] [Google Scholar]
- 5.Bergner-Rabinowitza, S., M. Fernea, G. Ziv, S. Fleidermana, A. Saran, and M. Winkler. 1981. Group G type X: a new antigenic combination in streptococci isolated from cases of bovine mastitis in Israel. Vet. Microbiol. 6:383-387. [Google Scholar]
- 6.Bert, F., and N. Lambert-Zechovsky. 1997. Septicemia caused by Streptococcus canis in a human. J. Clin. Microbiol. 35:777-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bornand, V. 1992. Bacteriology and mycology of otitis externa in dogs. Schweiz. Arch. Tierheilkd. 134:341-348. [PubMed] [Google Scholar]
- 8.Corning, B. F., J. C. Murphy, and J. G. Fox. 1991. Group G streptococcal lymphadenitis in rats. J. Clin. Microbiol. 29:2720-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devriese, L. A., J. Hommez, R. Kilpper-Bälz, and K. Schleifer. 1986. Streptococcus canis sp. nov.: a species of group G streptococci from animals. Int. J. Syst. Bacteriol. 36:422-425. [Google Scholar]
- 10.Devriese, L. A. 1991. Streptococcal ecovars associated with different animal species: epidemiological significance of serogroups and biotypes. J. Appl. Bacteriol. 71:478-483. [DOI] [PubMed] [Google Scholar]
- 11.DeWinter, L. M., D. E. Low, and J. F. Prescott. 1999. Virulence of Streptococcus canis from canine streptococcal toxic shock syndrome and necrotizing fasciitis. Vet. Microbiol. 70:95-110. [DOI] [PubMed] [Google Scholar]
- 12.Eberhart, R. J., and S. B. Guss. 1970. Group G streptococci in the udders of a Pennsylvania dairy herd. J. Am. Vet. Med. Assoc. 157:1195-1199. [PubMed] [Google Scholar]
- 13.Efstratiou, A. 1989. Outbreaks of human infection caused by pyogenic streptococci of Lancefield groups C and G. J. Med. Microbiol. 29:207-219. [DOI] [PubMed] [Google Scholar]
- 14.Forsman, P., A. Tilsala-Timisjärvi, and T. Alatossava. 1997. Identification of staphylococcal and streptococcal causes of bovine mastitis using 16S-23S rRNA spacer regions. Microbiology 143:3491-3500. [DOI] [PubMed] [Google Scholar]
- 15.Fox, L. K., and J. M. Gay. 1993. Contagious mastitis. Vet. Clin. N. Am. Food Anim. Pract. 9:475-487. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton, C. A., and D. M. Stark. 1970. Occurrence and characterization of Lancefield group G streptococci in bovine mastitis. Am. J. Vet. Res. 31:397-398. [PubMed] [Google Scholar]
- 17.Hardie, J. M. 1986. Genus Streptococcus, p. 1043-1071. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. The Williams & Wilkins Co., Baltimore, Md. [Google Scholar]
- 18.Hassan, A. A., I. U. Khan, A. Abdulmawjood, and C. Lämmler. 2001. Evaluation of PCR methods for rapid identification and differentiation of Streptococcus uberis and Streptococcus parauberis. J. Clin. Microbiol. 39:1618-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan, A. A., I. U. Khan, A. Abdulmawjood, and C. Lämmler. 2003. Development of PCR assays for detection of Streptococcus canis. FEMS Microbiol. Lett. 219:209-214. [DOI] [PubMed] [Google Scholar]
- 20.Hassan, A. A., I. U. Khan, A. Abdulmawjood, and C. Lämmler. 2003. Inter- and intraspecies variations of the 16S-23S rDNA intergenic spacer region of various streptococcal species. Syst. Appl. Microbiol. 26:97-103. [DOI] [PubMed] [Google Scholar]
- 21.Hassan, A. A., I. U. Khan, and C. Lämmler. 2003. Identification of Streptococcus dysgalactiae strains of Lancefield's group C, G and L by polymerase chain reaction. J. Vet. Med. 50:161-165. [DOI] [PubMed] [Google Scholar]
- 22.Iglauer, F., I. Kunstýř, R. Mörstedt, H. Farouq, M. Wullenweber, and S. Damsch. 1991. Streptococcus canis arthritis in a cat breeding colony. J. Exp. Anim. Sci. 34:59-65. [PubMed] [Google Scholar]
- 23.Isenberg, H. D. 1992. Clinical microbiology procedures handbook, vol. 1, p. 20-21. American Society for Microbiology, Washington, D.C. [Google Scholar]
- 24.Jayarao, B. M., Jr., J. J. E. Doré, G. A. Baumbach, K. R. Matthews, and S. P. Oliver. 1991. Differentiation of Streptococcus uberis from Streptococcus parauberis by polymerase chain reaction and restriction fragment length polymorphism analysis of the 16S ribosomal DNA. J. Clin. Microbiol. 29:2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayarao, B. M., Jr., J. J. E. Doré, and S. P. Oliver. 1992. Restriction fragment length polymorphism analysis of 16S ribosomal DNA of Streptococcus and Enterococcus species of bovine origin. J. Clin. Microbiol. 30:2235-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klastrup, O. 1963. Mastitis control in Denmark. Procedures and experiences. Bull. Off. Int. Epizoot. 60:501-511. [Google Scholar]
- 27.McDonald, T., J., and J. S. McDonald. 1976. Streptococci isolated from bovine intramammary infections. Am. J. Vet. Res. 37:377-381. [PubMed] [Google Scholar]
- 28.Olson, P. S. 1975. Streptococcus canis: an isolate from a canine uterus. Vet. Med. Small Anim. Clin. 70:933-934. [PubMed] [Google Scholar]
- 29.Poyart, C., G. Quesne, S. Coulon, P. Berche, and P. Trieu-Cuot. 1998. Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J. Clin. Microbiol. 36:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson, I. M., J. M. Stromley, V. H. Varel, and E. P. Cato. 1988. Streptococcus intestinalis, a new species from the colons and feces of pigs. Int. J. Syst. Bacteriol. 38:245-248. [Google Scholar]
- 31.Schleifer, K. H., and R. Klipper-Bälz. 1987. Molecular and chemotaxonomic approches to the classification of streptococci, enterococci and lactococci: a review. Syst. Appl. Microbiol. 10:1-19. [Google Scholar]
- 32.Singh, K. P., A. Morris, S. D. Lang, D. M. MacCulloch, and D. A. Bremner. 1988. Clinically significant Streptococcus anginosus (Streptococcus milleri) infections: a review of 186 cases. N. Z. Med. J. 101:813-816. [PubMed] [Google Scholar]
- 33.Soedarmanto, I., and C. Lämmler, 1996. Comparative studies on streptococci of serological group G isolated from various origins. J. Vet. Med. 43:513-523. [DOI] [PubMed] [Google Scholar]
- 34.Swindle, M. M., O. Narayan, M. Luzarraga, and D. L. bobbie. 1980. Contagious streptococcal lymphadenitis in cats. J. Am. Vet. Med. Assoc. 177:829-830. [PubMed] [Google Scholar]
- 35.Takeda, N., K. Kikuchi, R. Asano, T. Harada, K. Totsuka T. Sumiyoshi, T. Uchiyama, and S. Hosoda. 2001. Recurrent septicemia caused by Streptococcus canis after a dog bite. Scand. J. Infect. Dis. 33:927-928. [DOI] [PubMed] [Google Scholar]
- 36.Täpp, J., M. Thollesson, and B. Herrmann. 2003. Phylogenetic relationships and genotyping of the genus Streptococcus by sequence determination of the RNase P RNA gene, rnpB. Int. J. Syst. Evol. Microbiol. 53:1861-1871. [DOI] [PubMed] [Google Scholar]
- 37.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watts, J. L., S. C. Nickerson, and J. W. Pankey. 1984. A case study of Streptococcus group G infection in a dairy herd. Vet. Microbiol. 9:571-597. [DOI] [PubMed] [Google Scholar]
- 39.Watts, J. L. 1988. Characterization and identification of streptococci isolated from bovine mammary glands. J. Dairy Sci. 71:1616-1624. [DOI] [PubMed] [Google Scholar]
- 40.Watts, J. R., P. J. Wright, and K. C. Whithear. 1996. Uterine, cervical and vaginal microflora of the normal bitch throughout the reproductive cycle. J. Small Anim. Pract. 37:54-60. [DOI] [PubMed] [Google Scholar]
- 41.Whatmore, A. M., K. H. Engler, G. Gudmundsdottir, and A. Efstratiou. 2001. Identification of isolates of Streptococcus canis infecting humans. J. Clin. Microbiol. 39:4196-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]