Abstract
Objectives
This study aimed to characterize the cytologic, pathologic and immunohistochemical (IHC) aspects of feline giant-cell sarcoma.
Methods
Biopsy and necropsy reports from the Department of Veterinary Pathology were retrieved, and 13 cases of pleomorphic sarcoma (PS) were selected according to the established epidemiologic, pathologic and IHC criteria. All samples were fixed in 10% formalin, routinely processed for histology, and stained with hematoxylin and eosin. Samples also underwent IHC testing for vimentin, ionized calcium-binding adaptor molecule 1 (Iba-1), desmin, actin and S-100.
Results
The mean age of the affected cats was 9.5 years, and females were over-represented. Most neoplasms were observed in the flank, lateral thorax, limbs and interscapular region, and were >2 cm in diameter. Cytology analysis revealed highly cellular preparations with three distinct populations (spindle cells, small round cells and multinucleated giant cells) in a dense eosinophilic stroma. Histologically, PS was composed of a combination of these three populations. IHC labeling for vimentin and Iba-1 was strongly positive for spindle cells and multinucleated giant cells, respectively. Desmin/actin showed variable labeling among the samples. S-100 was negative in all samples.
Conclusions and relevance
PS is a neoplasm of mesenchymal origin, also known as malignant fibrous histiocytoma. The predominant subtype in this study that affected the cats was the giant-cell type, characterized by the presence of multinucleated giant cells among spindle-shaped cells. These findings are similar to those described in human patients; thus, a comparison between the neoplasms seen in these species might be useful, and the knowledge of biologic behavior and overall treatment approach for humans could be extrapolated to cats.
Keywords: Cytology, pathology, injection-site sarcoma, immunohistochemistry
Introduction
Pleomorphic sarcoma (PS), previously known as malignant fibrous histiocytoma (MFH) or anaplastic sarcoma with giant cells, is a mesenchymal neoplasm that affects humans, 1 cats, 2 dogs 3 and rats. 4 The neoplasm is composed of spindle (fibroblast/myofibroblast-like) cells often arranged in a storiform pattern interspersed with rounded cells (histiocyte-like) and multinucleated giant cells. In human and animal medicine, neoplasms are divided into histologic subtypes according to the pattern and the cell type.2,3 However, it has been suggested that this is not a single neoplasm with different variants, but a collection of several neoplasms that are histologically and immunohistochemically diverse. In cats, two subtypes have been described: giant-cell and storiform-pleomorphic.2,3
PS is one of the most common soft tissue sarcomas seen in people, affecting mainly the lower and upper extremities, and can be induced by radiation and metallic implants such as prostheses.5,6 In rats, tumors can be experimentally induced by chemicals and materials (metals). 4 PS in cats is considered as a histologic variant of the entity ‘feline injection-site sarcoma’ (FISS), which comprises tumors that arise as a result of vaccination, drug application or surgical material placement.7,8
Reports describing clinical behavior, diagnosis and treatment of this type of tumor in cats are scarce, and appropriate diagnostic criteria are essential to proceed with treatment. Therefore, this study aimed to describe the cytologic, pathologic and immunohistochemical (IHC) aspects of giant-cell PS in cats.
Materials and methods
The records from the Department of Veterinary Pathology of the Federal University of Rio Grande do Sul, from January 2007 to December 2019, were searched for biopsy samples, based on the combinations of keywords such as ‘feline’, ‘cats’, ‘sarcoma’, ‘injection-site’, ‘giant-cell’, ‘malignant fibrous-histiocytoma’, ‘fibrosarcoma’ and ‘undifferentiated sarcoma’ on their diagnosis. Selected samples included excisional or incisional biopsies referred mainly by small animal practitioners in the metropolitan region of Porto Alegre, Brazil. Thirteen cases with the revised morphologic criteria for PS were selected, of which eight were biopsy specimens, four were biopsy-associated with cytologic examination and one was derived only from cytologic diagnosis. Information related to the breed, age and sex of the cats, as well as anatomic location, size and peripheral tissue invasion of tumors, was obtained through the evaluation of histopathologic reports.
Fine-needle aspiration (FNA) was performed in four cases using a standard 10 ml syringe and a 25 G needle. The cytology was performed during the clinical consultation or before the cats entered the surgery. Slides were air-dried and then stained with fast Panoptic (Romanowsky-type stain), staying for 1 min in each color. For histopathology, wax-embedded tissue samples were cut into sections and stained with hematoxylin and eosin. Samples were analyzed in a double-blinded manner by two veterinary pathologists. During the histologic evaluation, the following criteria were used: histologic location; invasion of adjacent tissue; necrosis; inflammatory infiltrate (lymphocytes and macrophages); presence and quantification of multinucleated cells; and cellular pleomorphism. Mitotic count was assessed randomly through the evaluation of 10 high-power fields (× 400). Additionally, selected tissue samples were also stained with Masson’s trichrome to evaluate the collagen production according to its staining intensity (mild, moderate and marked).
IHC analyses were performed on serial sections of the neoplasms, using the peroxidase-labeled universal polymer method (MACH 4, Universal HRP-Polymer; Biocare Medical) for the following antibodies: vimentin; desmin; smooth muscle actin; ionized calcium-binding adaptor molecule 1 (Iba-1); and S-100. Positive controls consisted of canine benign peripheral nerve sheath tumors (vimentin and S-100), feline pulmonary Langerhans cell histiocytosis (Iba-1) and canine large intestine (desmin and smooth muscle actin). For negative controls, phosphate buffered saline was used to replace the primary antibodies in each IHC assay. The chromogens used for visualization were 3,3-diaminobenzidine (Sigma). Sections were counterstained with Harris’s hematoxylin. Table 1 describes the antibodies and IHC protocols applied.
Table 1.
Antibodies and immunohistochemical protocols
| Antibody specificity | Clone | Dilution | Antigen retrieval | Source |
|---|---|---|---|---|
| Vimentin | Monoclonal (V9) | 1:200 | Heat (Dakocitomaker), 3 mins in pH 6.0 citrate buffer for 125°C | Zymed |
| Iba-1 | Polyclonal antibody (Wako no. 019-1974) | 1:1000 | Heat, 10 mins in pH 6.0 citrate buffer | Dako |
| Desmin | Monoclonal (D33) | 1:300 | Heat, 10 mins in pH 9.0 Tris EDTA | Dako |
| Smooth muscle actin | Monoclonal Clone 1A4 | 1:50 | Heat, 20 mins in pH 9.0 Tris EDTA | Dako |
| S-100 | Monoclonal (Code IR50461-2) | Ready to use | Heat, 10 mins in pH 6.0 citrate buffer | Dako |
Iba-1 = ionized calcium-binding adaptor molecule 1
The intensity of histochemical labeling was evaluated as mild, moderate and marked. The IHC reaction for each neoplasm was assessed by two pathologists, and categorized as (–), no labeling in cells; (1+), 10–20% of positive cells; (2+), <50% of positive cells; and (3+), >50% of positive cells.
Results
In the 12-year period, 13 cases of feline PS were diagnosed. Mean age was 9.5 years (range 5–13), and most cats were female (n = 10/13 [77%]). Apart from one cat that was a Siamese, all other cats were of mixed breed. Tumors were seen in the flank (n = 5 [38.5%]), lateral thorax (n = 4 [30.7%]), limbs (n = 2 [15.4%]) and interscapular region (n = 2 [15.4%]). All neoplasms were firmly adhered to the adjacent tissues. Grossly, tumors presented themselves as white, firm-to-soft and expansile multilobulated (n = 6 [46.2%]) or single masses (n = 5 [38.5%]). In terms of size, masses ranged from 2.0 cm in diameter to larger than 15.0 × 4.0 cm. A clinical history of vaccination near the origin of the tumor was available in only three cases. Information regarding the outcome of these cats were only available for four cases.
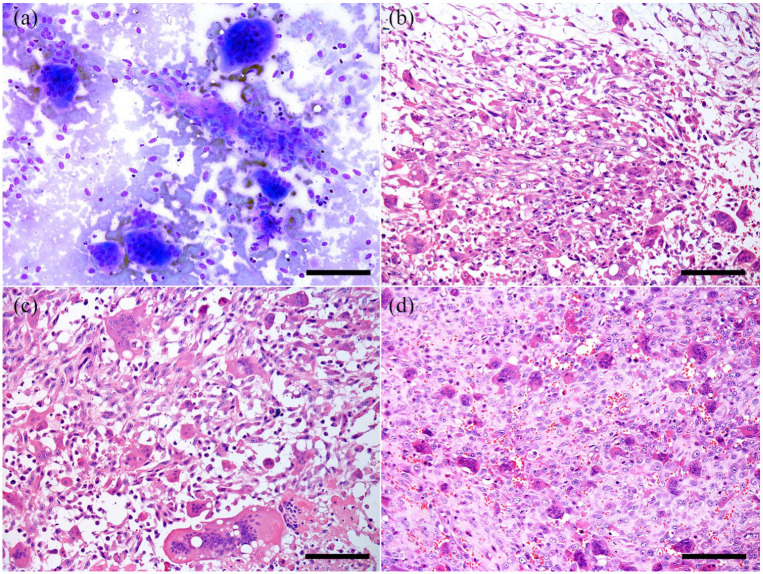
Cytologic analysis (FNA) was performed in four cases, and all samples collected were similar. The aspirated material had high viscosity. The samples exhibited highly cellular preparation, composed of three distinct populations of neoplastic cells interspersed with a small amount of eosinophilic matrix (Figure 1a). All cell types demonstrated a linear arrangement (windrowing) with an eosinophilic background. The first population was characterized by spindle-shaped cells with basophilic cytoplasm, elongated nuclei, open chromatin and multiple nucleoli. The second population comprised cells with an intermediate shape between spindloid and round, and consisted of multinucleated giant cells with deeply basophilic cytoplasm, sometimes with vacuoles, ranging from 30 to 45 nuclei per cell, and peripheral chromatin with multiple nucleoli. These two populations had numerous criteria for malignancy, including nuclear molding, high nuclear-to-cytoplasmic ratio (1:1–2), and marked variation in cellular and nuclear size with marked karyomegaly. The third, and scarce, population was characterized by round cells, with peripheral nuclei that resembled histiocytes. A small number of lymphocytes and plasma cells were also observed between the neoplastic cells in the test samples.
Figure 1.
Feline giant-cell pleomorphic sarcoma. (a) Cytologic characteristics of the tumor exhibiting spindle cells interspersed with eosinophilic stroma, multinucleated giant cells and a small number of rounder mononuclear histiocytoid cells. Romanowsky stain, bar = 120 μm. (b) Histopathology of the tumor with numerous spindle-shaped cells and a small amount of multinucleated giant cells. Hematoxylin and eosin (H&E), bar = 200 μm. (c) Numerous multinucleated giant cells containing up to 40 nuclei. H&E, bar = 200 μm. (d) Numerous round histiocytoid cells interspersed with few multinucleated giant cells. H&E, bar = 200 μm
Histologically, PS was characterized by spindle-shaped cells (Figure 1b) mixed with numerous multinucleated giant cells (Figure 1c) and round cells (Figure 1d), in an interwoven manner, supported by a fibrovascular stroma. This stroma, which was identified by HE and confirmed through Masson’s trichrome (Figure 2a), was mild (25% of cases), moderate (25% of cases) and marked (50% of cases). In 50% (n = 6/12) of cases, the neoplasms were restricted to the subcutaneous tissue, and invaded the dermis and the muscular layer in 33.4% (n = 4/12) and 41.7% (n = 5/12) of the cases, respectively. The spindle-shaped cells had a faintly basophilic cytoplasm with indistinct cell borders, nuclei varying in size and shape (round-to-elongated) with dispersed chromatin, and multiple large nucleoli. The giant cells exhibited multiple nuclei (up to 30–45) of varying size and shape ranging from 25 µm to 100 µm. In all cases, anisocytosis and anisokaryosis were marked, and 8–14 mitosis per 10 high-power fields were observed in two of the cellular populations (round and spindle-shaped cells). Additionally, multifocal areas of necrosis were observed in all masses, which corresponded to the soft areas of the tumor seen grossly, and were commonly associated with multifocal and discrete hemorrhage and deposition of fibrin. Cellular infiltrates were also seen in all samples, and lymphocytes were the predominant cell type. A small number of macrophages and plasma cells were also present. In 6/12 cases (50%) epidermal ulceration with deposition of fibrin was also observed. None of the cats showed any evidence of vascular or lymphatic invasion.
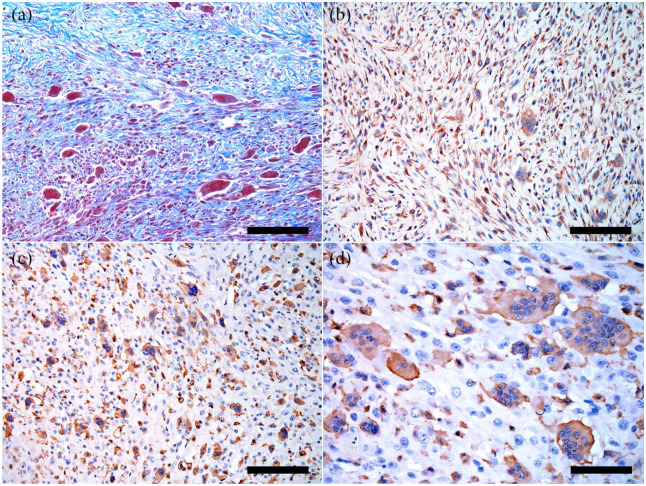
Figure 2.
Feline giant-cell pleomorphic sarcoma. (a) Masson’s trichrome exhibiting marked staining in stroma and spindle cells. Masson’s trichrome, bar = 200 μm. (b) Marked vimentin labeling in multinucleated giant cells and spindle cells. 3,3-diaminobenzidine (DAB), bar = 200 μm. (c) Marked Iba-1 labeling for multinucleated giant cells and round cells. DAB, bar = 200 μm. (d) Marked Iba-1 labeling for multinucleated giant cells, and few round cells. DAB, bar = 200 μm
IHC labeling for vimentin (Figure 2b) was strongly positive and cytoplasmic in spindle cells, and moderately positive for multinucleated giant cells. The labeling for Iba-1 was strongly positive in small round cells (histiocyte-like) (Figure 2c) and the membrane/cytoplasm of multinucleated giant cells (Figure 2d). In contrast, desmin and smooth muscle actin showed variable labeling among samples. S-100 was negative in all slides (Table 2).
Table 2.
Immunohistochemistry results
| Case | Masson’s trichrome (stroma) | Vimentin | Smooth muscle actin | Desmin | S-100 | Iba-1 |
|---|---|---|---|---|---|---|
| 1 | 1+ | 2+ | 1+ | – | – | 3+ |
| 2 | 3+ | 2+ | – | 1+ | – | 1+ |
| 3 | 1+ | 1+ | – | – | – | 3+ |
| 4 | 3+ | 1+ | – | – | – | 1+ |
| 5 | 1+ | 2+ | – | 1+ | – | 2+ |
| 6 | 3+ | 3+ | 1+ | 1+ | – | 2+ |
| 7 | 3+ | 2+ | 1+ | – | – | 1+ |
| 8 | 2+ | 1+ | 1+ | – | – | 2+ |
| 9 | 3+ | 3+ | 1+ | – | – | 3+ |
| 10 | 2+ | 3+ | 1+ | – | – | 2+ |
| 11 | 3+ | 3+ | 1+ | – | – | 3+ |
| 12 | 2+ | 3+ | 2+ | – | – | 2+ |
Iba-1 = ionized calcium-binding adaptor molecule 1; (–) = absent; (1+) = mild labeling; (2+) = moderate labeling; (3+) = marked labeling
The outcomes of most animals were unavailable owing to lack of contact information for the owners; however, the four cats that had this information were euthanized due to relapse of the tumor or poor prognosis. All of these cats presented tumor relapse that varied from 2 to 8 months after the first tumor excision.
Discussion
PS is a soft tissue neoplasm that affects humans and animals.4,2,9 In humans, it is considered to be the most common soft tissue tumor in adults, but it can also be diagnosed in juvenile patients, and occurs mostly in the retroperitoneum and extremities.9,10 As described in this study and the literature, mature adult cats were the most affected (mean age 9.5 years). 2 Although females were over-represented in our study, there was no sex predilection. The present study also demonstrated the absence of a breed predisposition; however, most cats were of no defined breed, which is consistent with the population of domestic cats in this geographic area. 11
The most common locations described in the literature are the interscapular region, flank and lateral thorax, consistent with sites of vaccination and drug application, which leads to the assumption that most diagnosed PSs are histologic variants of FISS.2,7 Similar findings were reported in the present study. In humans, PS can occur secondary to lesions caused by radiation exposure12,13 and metallic fixation devices. 5 Radiation used as a cancer treatment is usually insufficient to kill viable cells but can induce cell damage and genetic mutations, which generates a disorganized reparative proliferation that can lead to tumor development. 13 PS is the most commonly diagnosed radiation-induced neoplasm located in the neck or head, and this type of tumor generally is characterized by poorer prognosis than those that are not radiation-induced. 14
FISSs are related to the development of tumors after vaccination 15 and substance application, such as cisplatin. 16 Cases associated with surgical materials, such as non-absorbable surgical sutures, 17 surgical sponges 18 and foreign body implants, such as microchips and orthopedic devices,8,19 have also been described. The latency period between exposure to the inducing agent and development of the tumor is variable for both humans and cats; however, in many cases, there is a long latency period, with the tumor developing months to years after the original insult, 20 which correlates with the average age of the cats in the present study.
The histogenesis of these sarcomas remains unclear in humans and animals. 4 Clinically, the neoplasm is characterized by a rapidly enlarging and well-circumscribed mass, firmly adherent to the adjacent tissues, which is usually painless.13,14 The present study also reports similar findings. Histopathologic assessment is the most reliable tool for the diagnosis of PS, 21 as these tumors can present with extensive areas of necrosis because of their fast growth, and these areas can lead to an inconclusive cytologic diagnosis. 22 In our study, the mitotic rate was considered high; however, the literature is scarce regarding PS this feature in this species PS.2,3
Cytologic preparations of PS consist of multinucleated giant cells, neoplastic mesenchymal cells and, less commonly, a low number of smaller round cells that resemble histiocytes along with few inflammatory cells. The multinucleated giant cells may have a characteristic appearance and usually contain >30 nuclei.2,3 FNA was available for four of the evaluated cases, and all samples were highly cellular and composed of abundant spindle-shaped cells interspersed with giant multinucleated cells and round cells, allowing the presumed diagnosis of PS with multinucleated giant cells through this examining tool.
For a definitive diagnosis, it is necessary to consider the location of the tumor, high cellular pleomorphism, a high mitotic index, the presence of large numbers of multinucleated giant cells and different histologic patterns.2,21 As described in our cases, all tumors demonstrated multinucleated giant cells admixed with spindle cells and a variable amount of mononuclear histiocytoid cells, as well as distinct malignant characteristics, areas of necrosis and inflammatory infiltrate.14,22 Fibrovascular proliferation, which is demonstrated distinctly with Masson’s trichrome stain, was also observed interspersed between necrotic and inflammatory areas.
As seen in this study, the IHC analysis of PS is compatible with a fibroblastic/myofibroblastic phenotype, with positivity for vimentin and variable labeling for smooth muscle actin and desmin for spindle cells. 23 The multinucleated giant cells showed variable labeling for vimentin and Iba-1, which is in accordance with Madewell et al, 24 who performed an ultrastructural study and suggested that it is not possible to identify if these giant cells were neoplastic or only reactive; however, their pleomorphic appearance suggests their malignancy. The previous terminology, MFH, determined that the neoplasm was composed at some level of histiocytes or cells derived from histiocytes, and the positivity for Iba-1 confirmed the histiocytic origin of giant multinucleated cells and round histiocyte-like cells. Expression of Iba-1 occurs in all subpopulations of the macrophage/monocyte lineage, both inflammatory and neoplastic histiocytes, as well as normal histiocytic cells. 25 In all our cases, there was a variable amount of labeling between samples; however, the cells that demonstrated labeling were round histiocyte-like cells and multinucleated giant cells, which confirms their macrophage/monocyte lineage, although it is not clear whether these cells have neoplastic or inflammatory behavior.
The terminology MFH is considered obsolete in both human medicine and veterinary medicine; however, it is still present in the latest World Health Organization classification of human tumors of soft tissue and bone as a synonym for PS. 26 In veterinary medicine, the nomenclature currently used is PS, and for cats, the most common subtype is the giant cell. 2 When considering our descriptions of PS in cats, this neoplasm may be comparable with the human subtype, regarding its pathologic and IHC features, plus possible inductive factors. Although some characteristics of these tumors are alike, it is necessary to carry out further studies regarding the biologic behavior, owing to a lack of information regarding the cat’s outcome, plus the prognosis, and potential therapies of neoplasms in cats.
Conclusions
PS is an uncommon skin or subcutaneous neoplasm in cats, usually affecting mature adult animals (mean age 9.5 years), and occurs in the interscapular region, flank, limbs and the lateral thorax. This neoplasm is one of the variants of FISS; therefore, in some cases, PS can be related to drug injections. Similar features, such as epidemiologic and pathologic data, are seen in PSs in humans, making a comparison between species possible. The use of Iba-1 made it possible to determine that the multinucleated giant cells and round histiocyte-like cells present in the neoplasm are, in fact, from monocyte/macrophage lineage. However, it is necessary to carry out more research to determine whether these cells are neoplastic or inflammatory.
Acknowledgments
This study was supported by the Conselho Nacional de Pesquisa (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Footnotes
Accepted: 16 October 2020
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was funded by Conselho Nacional de Pesquisa (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), project number 31728 (Compesq UFRGS).
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Bianca S de Cecco  https://orcid.org/0000-0003-4827-4011
https://orcid.org/0000-0003-4827-4011
Fernando F Argenta  https://orcid.org/0000-0002-1186-3632
https://orcid.org/0000-0002-1186-3632
Fernanda VA da Costa  https://orcid.org/0000-0002-1031-7728
https://orcid.org/0000-0002-1031-7728
References
- 1. O’Brien JE, Stout AP. Malignant fibrous xanthomas. Cancer 1964; 17: 1445–1455. [DOI] [PubMed] [Google Scholar]
- 2. Garma-Aviña A. Malignant fibrous histiocytoma of the giant cell type in a cat. J Comp Pathol 1987; 97: 551–557. [DOI] [PubMed] [Google Scholar]
- 3. Gleiser CA, Raulston GL, Jardine JH, et al. Malignant fibrous histiocytoma in dogs and cats. Vet Pathol 1979; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 4. Kotera T, Katou-Ichikawa C, Tennakoon AH, et al. Rat malignant fibrous histiocytoma (MFH)-derived cloned cell lines (MT-8 and MT-9) show different differentiation in mesenchymal stem cell lineage. Exp Toxicol Pathol 2015; 67: 499–507. [DOI] [PubMed] [Google Scholar]
- 5. Lee YS, Pho RWH, Nather A. Malignant fibrous histiocytoma at site of metal implant. Cancer 1984; 54: 2286–2289. [DOI] [PubMed] [Google Scholar]
- 6. Sabesan T, Xuexi W, Yongfa Q, et al. Malignant fibrous histiocytoma: outcome of tumours in the head and neck compared with those in the trunk and extremities. Br J Oral Max Surg 2006; 44: 209–212. [DOI] [PubMed] [Google Scholar]
- 7. Hartmann K, Day MJ, Thiry E, et al. Feline injection-site sarcoma: ABCD guidelines on prevention and management. J Feline Med Surg 2015; 17: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cecco BS, Henker LC, Souza MJ, et al. Fibrosarcoma arising after femur osteosynthesis with pin and plate placement in a cat. Semin Ciênc Agrár 2019; 40: 2805–2812. [Google Scholar]
- 9. Li X, Liu R, Shi T, et al. Primary pulmonary malignant fibrous histiocytoma: case report and literature review. J Thorac Dis 2017; 9: 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amjad M, Bari A. Malignant fibrous histiocytoma: an uncommon soft tissue sarcoma. J Pak Assoc Dermatol 2009; 19: 243–246. [Google Scholar]
- 11. Withoeft JA, Cristo TG, Biezus G, et al. Causes of death and euthanasia in domestic cats in the Santa Catarina plateau (1995–2015). Pesq Vet Bras 2019; 39: 192–200. [Google Scholar]
- 12. Makimoto Y, Yamamoto S, Takano H, et al. Imaging findings of radiation-induced sarcoma of the head and neck. Br J Radiol 2007; 80: 790–797. [DOI] [PubMed] [Google Scholar]
- 13. Satomi T, Watanabe M, Kaneko T, et al. Radiation-induced malignant fibrous histiocytoma of the maxilla. Odontology 2011; 99: 203–208. [DOI] [PubMed] [Google Scholar]
- 14. Henderson MT, Hollmig T. Malignant fibrous histiocytoma: changing perceptions and management challenges. J Am Acad Dermatol 2012; 67: 1335–1341. [DOI] [PubMed] [Google Scholar]
- 15. Doddy FD, Glickman LT, Glickman NW. Feline fibrosarcomas at vaccination sites and non-vaccination sites. J Comp Pathol 1996; 114: 165–174. [DOI] [PubMed] [Google Scholar]
- 16. Martano M, Morello E, Iussich S, et al. A case of feline injection-site sarcoma at the site of cisplatin injections. J Feline Med Surg 2012; 14: 751–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buracco P, Martano M, Morello E, et al. Vaccine-associated-like fibrosarcoma at the site of a deep nonabsorbable suture in a cat. Vet J 2002; 163: 105–107. [DOI] [PubMed] [Google Scholar]
- 18. Haddad JL, Goldschmidt MH, Patel RT. Fibrosarcoma arising from the site of a retained surgical sponge in a cat. Vet Clin Pathol 2010; 39: 241–246. [DOI] [PubMed] [Google Scholar]
- 19. Daly MK, Saba CF, Crochik SS, et al. Fibrosarcoma adjacent to the site of microchip implantation in a cat. J Feline Med Surg 2008; 10: 202–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel SG, See AC, Williamson PA, et al. Radiation induced sarcoma of the head and neck. Head Neck 1999; 21: 346–354. [DOI] [PubMed] [Google Scholar]
- 21. Rosenberg AE. Malignant fibrous histiocytoma: past, present, and future. Skeletal Radiol 2003; 32: 613–618. [DOI] [PubMed] [Google Scholar]
- 22. Couto SS, Griffey SM, Duarte PC, et al. Feline vaccine-associated fibrosarcoma: morphologic distinctions. Vet Pathol 2002; 39: 33–41. [DOI] [PubMed] [Google Scholar]
- 23. Hendrick MJ, Brooks JJ. Postvaccinal sarcomas in the cat: histology and immunohistochemistry. Vet Pathol 1994; 31: 126–129. [DOI] [PubMed] [Google Scholar]
- 24. Madewell BR, Griffey SM, McEntee MC, et al. Feline vaccine-associated fibrosarcoma: an ultrastructural study of 20 tumors (1996–1999). Vet Pathol 2001; 38: 196–202. [DOI] [PubMed] [Google Scholar]
- 25. Pierezan F, Mansell J, Ambrus A, et al. Immunohistochemical expression of ionized calcium binding adapter molecule 1 in cutaneous histiocytic proliferative, neoplastic and inflammatory disorders of dogs and cats. J Comp Pathol 2014; 151: 347–351. [DOI] [PubMed] [Google Scholar]
- 26. Nascimento AF, Raut CP. Diagnosis and management of pleomorphic sarcomas (so-called ‘MFH’) in adults. J Surg Oncol 2008; 97: 330–339. [DOI] [PubMed] [Google Scholar]