Abstract
Objectives
Feline chronic gingivostomatitis (FCGS) is an oral disease. Cats with FCGS experience intense oral pain. Some cats remain refractory to current therapies based on dental extraction and adjuvant medical treatment; it is therefore necessary to investigate alternative therapeutic targets involved in inflammatory mechanisms and pain, namely the endocannabinoid system (ECS). The present study investigated the expression of cannabinoid receptors type 1 (CB1R) and 2 (CB2R), and cannabinoid-related receptors G protein-coupled receptor 55 (GPR55), transient receptor potential ankyrin 1 (TRPA1) and serotonin 1a receptor (5-HT1aR), in the oral mucosa of healthy cats to determine whether there was altered expression and distribution in cats with FCGS.
Methods
Samples of caudal oral mucosa were collected from eight control cats (CTRL cats) and from eight cats with FCGS (FCGS cats). Tissue samples were processed using an immunofluorescence assay with cat-specific antibodies, and the immunolabelling of the receptors studied was semiquantitatively evaluated.
Results
The mucosal epithelium of the CTRL cats showed CB1R, TRPA1 and 5-HT1aR immunoreactivity (IR), while CB2R and GPR55 IR were generally not expressed. In the CTRL cats, the subepithelial inflammatory cells expressed CB2R, GPR55 and 5-HT1aR IR. In the FCGS cats, all the receptors studied were markedly upregulated in the epithelium and inflammatory infiltrate.
Conclusions and relevance
Cannabinoid and cannabinoid-related receptors are widely expressed in the oral mucosa of healthy cats and are upregulated during the course of FCGS. The presence of cannabinoid and cannabinoid-related receptors in healthy tissues suggests the possible role of the ECS in the homeostasis of the feline oral mucosa, while their overexpression in the inflamed tissues of FCGS cats suggests the involvement of the ECS in the pathogenesis of this disease, with a possible role in the related inflammation and pain. Based on the present findings, ECS could be considered a potential therapeutic target for patients with FCGS.
Keywords: FCGS, immunohistochemistry, oral mucosa, CB1R, CB2R, GPR55, TRPA1, 5-HT1aR
Introduction
The endocannabinoid system (ECS) is an extensive and complex endogenous signalling network composed of endocannabinoid ligands (anandamide and 2-arachidonoylglycerol), cannabinoid and cannabinoid-related receptors, and enzymes involved in the synthesis and degradation of endocannabinoids. 1 In addition to endocannabinoids, cannabinoid and cannabinoid-related receptors can also be modulated by exogenous mediators, such as the phytocannabinoid compounds derived from Cannabis sativa, in particular delta 9-tetrahydrocannabinol and cannabidiol (CBD),2,3 and by synthetic cannabinoids. 4
Various studies, primarily concerning humans and laboratory animals, have demonstrated how the ECS is involved in multiple physiological processes, contributing to the homeostasis of various organs, and how its dysregulation seems to be associated with several pathological conditions.1,5–7 In humans, it has been demonstrated that the ECS is involved in inflammatory and neuropathic pain, modulating the inflammatory response by means of the suppression of immune cell migration and the activation of immune cell apoptosis. 8
Unfortunately, to the best of our knowledge, studies regarding the ECS of cats are very limited. The presence of cannabinoid/cannabinoid-related receptors has been demonstrated in the cerebral arterial muscle cells (cannabinoid receptor type 1 [CB1R]), 9 the ovary and the oviduct (CB1R), 10 the skin (CB1R, cannabinoid receptor type 2 [CB2R] and peroxisome proliferator-activated receptor alpha [PPARα]) and the gastrointestinal tract (CB1R, CB2R, G protein-coupled receptor 55 [GPR55], PPARα, transient receptor potential ankyrin 1 [TRPA1] and serotonin 1a receptor [5-HT1aR]) of healthy cats.11,12 Furthermore, overexpression of CB1R and CB2R was observed in the skin of cats affected by hypersensitivity dermatitis, suggesting that the ECS plays a role in the inflammatory processes of the skin in this species. 11 These findings suggested that, in the cat, the ECS may also play an important role in the maintenance of tissue homeostasis and could be involved in the pathogenesis of various feline inflammatory diseases, including inflammatory conditions involving the oral mucosa.
Feline chronic gingivostomatitis (FCGS) is a severe inflammatory disease, which is characterised by bilateral hyperplastic or ulcerative lesions across the oral mucosa, characteristically affecting the palatoglossal folds and the buccal mucosa overlying the premolar and molar arches. Microscopically, these lesions present an abundant lymphoplasmacytic infiltrate and a variable quantity of mast cells, Mott cells and neutrophils. 13 FCGS affects cats of any age, does not have any sex or breed predisposition, 13 and is more prevalent in multi-cat, overcrowded environments. 14 Affected cats suffer from intense oral pain, which leads to hypersalivation, dysphagia, anorexia and weight loss, and has a serious negative impact on quality of life. 15 FCGS is considered a multifactorial disease in which a dysregulation of immune response to the oral commensal microbiota plays an important role, and could potentially be exacerbated by viral infections.16,17 It has also been demonstrated that FCGS affects other parts of the digestive system, such as the oesophagus. 18
Dental extractions (premolar and molar extractions or full-mouth extractions, including retained roots) remain the standard of care for FCGS, accompanied by adjuvant medical treatment intended to suppress the inflammatory response, control pain and inhibit microbial growth (ie, immunosuppressive or immunomodulatory drugs, anti-inflammatory drugs, antibiotics). 15 Nevertheless, a consistent number of patients are refractory to the aforementioned treatments,15,19,20 and, consequently, in recent years, research has shifted focus to innovative alternative therapies,17,21–24 highlighting the fact that different therapeutic targets should be investigated.
Thus, the aim of the present study was to characterise the expression pattern of the cannabinoid receptors (CB1R and CB2R), and three cannabinoid-related receptors (GPR55; TRPA1; 5-HT1aR) in the caudal oral mucosa of healthy cats and cats with FCGS in order to assess the usefulness of non-psychotropic cannabinoid molecules as a possible alternative therapeutic approach.
Materials and methods
Inclusion criteria
Cats presenting at the Companion Animal Clinic of the Aristotle University of Thessaloniki (Greece) with clinical signs, and macroscopic and microscopic alterations of the caudal oral cavity compatible with FCGS (FCGS cats), were prospectively enrolled (Table 1). Animals treated with glucocorticoids, antibiotics or non-steroidal anti-inflammatory drugs in the 15 days prior to presentation, and cats with concurrent metabolic diseases were excluded from the study. For each case, the intensity of the macroscopic oral inflammation was semi-quantitatively assessed according to a modified version of the validated Stomatitis Disease Activity Index (SDAI), scoring eight sites of the oral mucosa (Table 2), 25 while the inflammation at the sampling point (local inflammation index [LII]) was assessed using a semiquantitative score system (0 = normal; 1 = mild; 2 = moderate; 3 = severe). Cats with no history of oral disease, which died spontaneously or were euthanased for humane reasons owing to various diseases, and that had a macroscopic appearance of normal oral mucosa, were included as controls (CTRL cats) (Table 1).
Table 1.
Signalment, local inflammation index (LII), Stomatitis Disease Activity Index (SDAI) and microscopic inflammation degree (MID) of the caudal oral mucosa of the cats with feline chronic gingivostomatitis (FCGS), and signalment and cause of death of the control cats (CTRL)
| FCGS cats | Breed | Sex | Age (years) | LII | SDAI | MID |
|---|---|---|---|---|---|---|
| FCGS 1 | DSH | FS | 6 | 3 | 21 | 3 |
| FCGS 2 | DSH | FS | 3 | 3 | 15 | 2 |
| FCGS 3 | DSH | MC | 3 | 2 | 15 | 2 |
| FCGS 4 | DSH | FS | 7 | 2 | 16 | 2 |
| FCGS 5 | DSH | MC | 3.5 | 3 | 16 | 2 |
| FCGS 6 | DSH | FS | 5 | 3 | 23 | 2 |
| FCGS 7 | DSH | MC | 4.5 | 3 | 23 | 2 |
| FCGS 8 | DSH | FS | 2.5 | 3 | 20 | 3 |
| CTRL cats | Breed | Sex | Age (years) | Cause of death | ||
| CTRL 1 | DSH | F | 2 | Pneumothorax | ||
| CTRL 2 | DSH | F | 3 | Diaphragmatic hernia | ||
| CTRL 3 | DSH | M | 4 | Pulmonary oedema | ||
| CTRL 4 | DSH | F | 2 | Abdominal haemorrhage | ||
| CTRL 5 | DSH | MC | 5 | Pleural effusion | ||
| CTRL 6 | DSH | FS | 5 | Aortic thromboembolism | ||
| CTRL 7 | DSH | F | 1.5 | Diaphragmatic hernia | ||
| CTRL 8 | DSH | M | 6 | Cranioencephalic trauma | ||
DSH = domestic shorthair; FS = spayed female; MC = castrated male; F = intact female; M = intact male
Table 2.
Modified Stomatitis Disease Activity Index (SDAI) 25
| SDAI | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Maxillary buccal mucosal inflammation | ||||
| Mandibular buccal mucosal inflammation | ||||
| Maxillary attached gingival inflammation | ||||
| Mandibular attached gingival inflammation | ||||
| Inflammation lateral to palatoglossal folds | ||||
| Molar salivary gland inflammation | ||||
| Oropharyngeal inflammation | ||||
| Sublingual and lingual inflammation | ||||
| Total score (maximum = 24) | ||||
Grading systems adopted for the semiquantitative assessment of the oral cavity inflammation. Degree of inflammation: 0 = none; 1 = mild; 2 = moderate; 3 = severe
Sample collection and processing
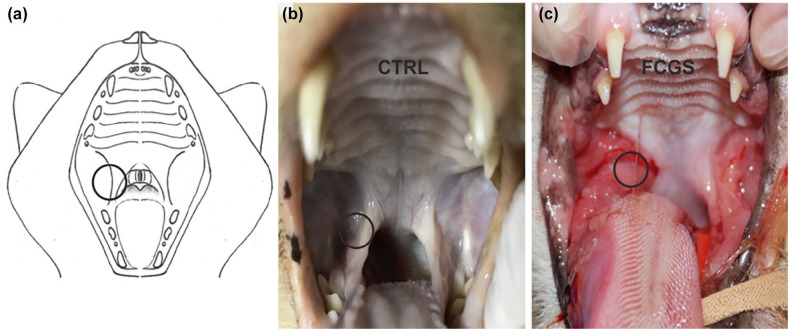
In the FCGS group, one biopsy sample of the palatoglossal folds was collected with a sterile 6 mm biopsy punch for diagnostic purposes (Figure 1).
Figure 1.
(a) Image representing the feline oral cavity. The circle represents the area from which the oral mucosa samples were collected. (b) Photograph showing the oral cavity of a 4-year-old intact male domestic shorthair (DSH) control cat with no evident lesions of the mucosa (post mortem). (c) Photograph showing the oral cavity of a 6-year-old neutered female DSH cat affected by feline chronic gingivostomatitis (FCGS) exhibiting marked inflammation, ulceration and hyperplasia of the caudal oral mucosa
Sampling was carried out prior to any anti-inflammatorydrug administration and under general anaesthesia, using the same protocol in all cats: 0.05 mg/kg acepromazine maleate and 0.1 mg/kg butorphanol intramuscularly for premedication, propofol intravenously for induction and 0.5–2% isoflurane delivered in oxygen for maintenance.
In the CTRL group, the collection of tissue occurred within 1 h of death, and in the same anatomical site as the FCGS group (Figure 1).
The samples were fixed overnight in 2% paraformaldehyde plus 0.2% picric acid, in 0.1 M sodium phosphate buffer (pH 7.0) at +4°C. After being washed in phosphate-buffered saline (PBS; 0.15 M NaCl in 0.01 M sodium phosphate buffer, pH 7.2), the tissues were immersed in PBS plus sodium azide 0.1% for 48 h (+ 4°C) and were then preserved in PBS–sodium azide 0.1% plus sucrose 30% (+4°C). Subsequently, all samples were frozen in liquid nitrogen, and 14 μm-thick cryosections were obtained.
Histology
Tissue cryosections from each case were stained with haematoxylin and eosin for histopathological examination in order to confirm FCGS. The type and degree of inflammation were additionally assessed using a semi-quantitative scoring system (microscopic inflammation degree [MID]) as follows: 0 = normal mucosa; 1 = mild superficial lymphoplasmacytic infiltrate, no mucosal ulceration; 2 = moderate superficial-intermediate lymphoplasmacytic infiltrate, with or without ulceration; 3 = severe superficial and deep lymphoplasmacytic infiltrate with ulceration.
Immunofluorescence
The cryosections were hydrated in PBS and processed for immunostaining, and were observed as recently described. 12 The antibody panel applied is detailed in Table 3.
Table 3.
Primary and secondary antibodies used in this study
| Host | Code | Dilution | Source | |
|---|---|---|---|---|
| Primary antibody | ||||
| CB1R | Rabbit | Ab23703 | 1:100 | Abcam |
| CB2R | Rabbit | Ab45942 | 1:200 | Abcam |
| GPR55 | Rabbit | NB110-55498 | 1:200 | Novus Biologicals |
| 5-HT1aR | Rabbit | AB85615 | 1:100 | Abcam |
| TRPA1 | Rabbit | AB58844 | 1:100 | Abcam |
| Secondary antibody | ||||
| Anti-Rabbit F(ab′)2 FITC | Goat | AB98430 | 1:300 | Abcam |
CB1R = cannabinoid receptor 1; CB2R = cannabinoid receptor 2; GPR55 = G protein-coupled receptor 55; 5-HT1aR = serotonin 1a receptor; TRPA1 = transient receptor potential ankyrin 1
Owing to the small dimensions of the biopsies, it was not possible to process all the tissue samples for each marker; the number (n) of subjects used for each receptor and for each group (CTRL and FCGS) is indicated in brackets.
The slides were observed using a Nikon Eclipse Ni microscope equipped with the appropriate filter cubes to distinguish the fluorochrome employed. The images were recorded with a Nikon DS-Qi1Nc digital camera and NIS elements software BR 4.20.01 (Nikon Instruments Europe). The figure panels were prepared using Corel Draw (Corel Photo Paint and Corel Draw).
Specificity of the primary antibodies
The specificity of the primary antibodies used in the present study was previously tested on feline tissues using Western blot analysis.11,12
The specificity of the secondary antibody was tested by omission of the primary antibodies.
Results
Demographics
Eight domestic shorthair (DSH) cats were included in the FCGS group. There were three (37.5%) castrated males and five (62.5%) spayed females. Median age was 4.3 years (range 2.5–7 years). The main clinical signs included dysphagia, ptyalism, inappetence and weight loss. The median SDAI was 18.6 (range 15–23). Six cats had a score of 3 according to the LII, while the remaining two cats had a score of 2 (Table 1).
The CTRL group was composed of eight DSH cats: four intact females (50%), two intact males (25%), one spayed female (12.5%) and one castrated male (12.5%). Median age was 3.6 years (range 1.5–6 years). The causes of death are detailed in Table 1.
Histopathological examination
The caudal oral mucosa of the CTRL cats exhibited no histological signs of inflammation (MID = 0).
Histologically, all the FCGS cats had a mild-to-severe subepithelial inflammatory infiltrate, mainly composed of lymphocytes and plasma cells. Neutrophilic and macrophagic infiltration admixed to granulation tissue was observed in association with an ulcerated mucosa. Six cases (75%) were classified as score 2 (according to the MID) and two (25%) as score 3 (Table 1).
CB1R immunoreactivity
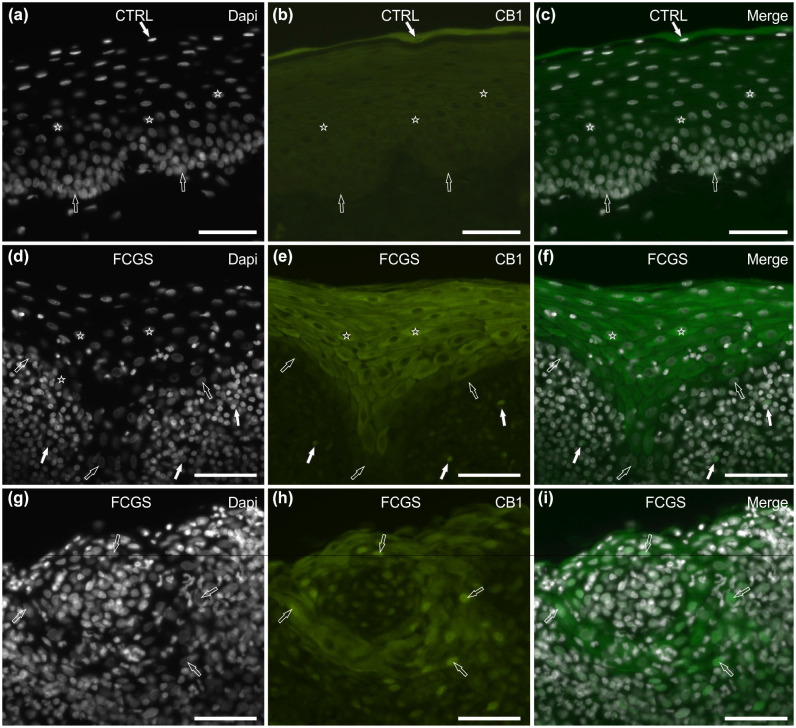
In 8/8 (100 %) CTRL cats tested, superficial epithelial cells showed bright cytoplasmic CB1R immunoreactivity (IR) (Figure 2a–c), while labelling was faint to moderate in the intermediate layers and undetectable in the basal layer. No CB1R IR was observed in the cells of the subepithelial connective tissue.
Figure 2.
Photomicrographs of cryosections of feline caudal oral mucosa showing cannabinoid receptor 1 (CB1R) immunoreactivity (IR) in tissues of the (a–c) control cats (CTRL) and (d–i) the cats affected by feline chronic gingivostomatitis (FCGS). (a–c) The white arrow indicates a 4′,6-diamidino-2-phenylindole (DAPI)-labelled nucleus of a superficial epithelial cell, displaying bright cytoplasmic CB1 immunoreactivity (IR); the stars indicate the DAPI-labelled nuclei of the epithelial cells of the intermediate layers, showing moderate cytoplasmatic CB1R IR; the open arrows indicate the DAPI-labelled nuclei of the basal layer cells, which were CB1R negative. (d–f) The stars represent the DAPI-labelled nuclei of the epithelial cells, displaying bright cytoplasmatic CB1R IR (intermediate layers); the open arrows indicate the DAPI-labelled nuclei of the basal layer cells, which were CB1R negative; the white arrows indicate the DAPI-labelled nuclei of the immunocytes localised in the lamina propria/submucosa, showing bright membrane CB1R IR. (g–i) The open arrows indicate the DAPI-labelled nuclei of the epithelial cells with a round organisation, expressing moderate cytoplasmic and bright nuclear CB1R IR. Bar = 50 µm
Of 8/8 (100%) FCGS cats tested, CB1R IR was brightly expressed in the cytoplasm of the epithelial cells, with the exclusion of the cells of the basal layer (Figure 2d–f). Nuclear CB1R IR was also observed in cats with severe inflammation (LII = 3) (Figure 2g–i). Inflammatory cells within the mucosal epithelium and subepithelial connective tissue showed scattered moderate membranous positivity (Figure 2d–f).
CB2R IR
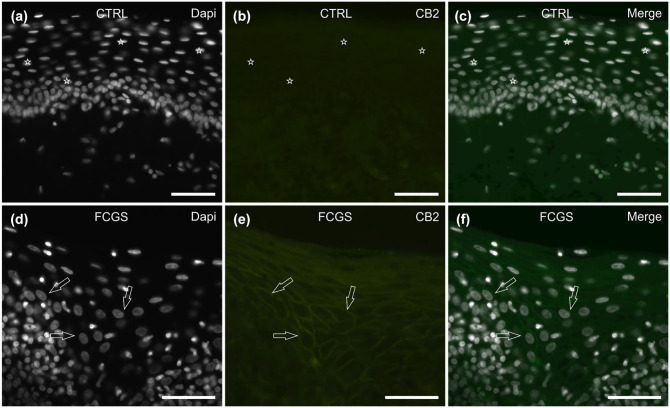
Of 7/7 (100 %) CTRL cats tested, CB2R IR was undetectable in the epithelial cells (Figure 3a–c), while only a few scattered inflammatory cells, localised in the subepithelial connective tissue, expressed moderate cytoplasmic CB2R IR.
Figure 3.
Photomicrographs of cryosections of feline caudal oral mucosa showing cannabinoid receptor 2 (CB2R) immunoreactivity (IR) in tissues of (a–c) the control cats (CTRL) and (d–f) the cats affected by feline chronic gingivostomatitis (FCGS). (a–c) The stars indicate 4′,6-diamidino-2-phenylindole (DAPI)-labelled nuclei of epithelial cells, which were CB2R negative. (d–f) The open arrows indicate the DAPI-labelled nuclei of the intermediate-layer epithelial cells, showing moderate membrane CB2R IR. Bar = 50 µm
Of the 6/6 (100%) FCGS cats (tested, faint-to-moderate CB2R IR was expressed by the membrane of the mucosal epithelial cells (Figure 3d–f). Inflammatory cells localised in the submucosa showed bright and diffuse cytoplasmic CB2R IR expression (data not shown).
GPR55 IR
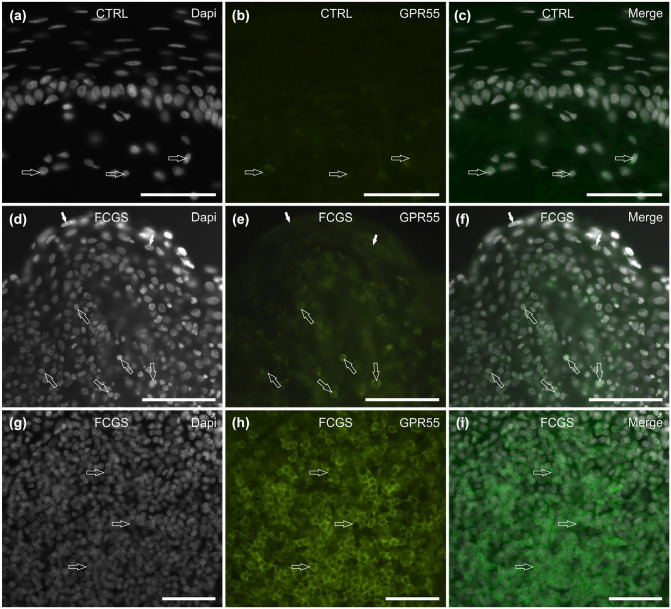
Of the 6/6 (100%) CTRL cats tested, GPR55 IR was not expressed by the mucosal epithelial cells. Faint-to-moderate GPR55 IR was expressed by the cytoplasm of the inflammatory cells localised in the subepithelial connective tissue (Figure 4a–c).
Figure 4.
Photomicrographs of cryosections of feline caudal oral mucosa showing G protein-coupled receptor 55 (GPR55) immunoreactivity (IR) in tissues of the (a–c) control cats (CTRL) and (d–i) the cats affected by feline chronic gingivostomatitis (FCGS). (a–c) The arrows indicate three inflammatory cells scattered in the subepithelial connective tissue, showing faint GPR55 IR. (d–f) The open arrows indicate some inflammatory cells in the subepithelial connective tissue, showing bright GPR55 IR; the white arrows indicate the superficial layer epithelial cells, showing moderate GPR55 IR. (g–i) The arrows indicate a nodule of inflammatory cells, expressing bright GPR55 IR. Bar: (a–f) 100 µm; (g–i) 50 µm
Of the 6/6 (100%) FCGS cats tested, moderate GPR55 IR was expressed by the cytoplasm of the superficial epithelial cells (Figure 4d–f). In the subepithelial connective tissue of all the cats, bright GPR55 IR was expressed by the cell membrane of many inflammatory cells, which were sparsely distributed (Figure 4d–f) and organised in nodules (Figure 4g–i).
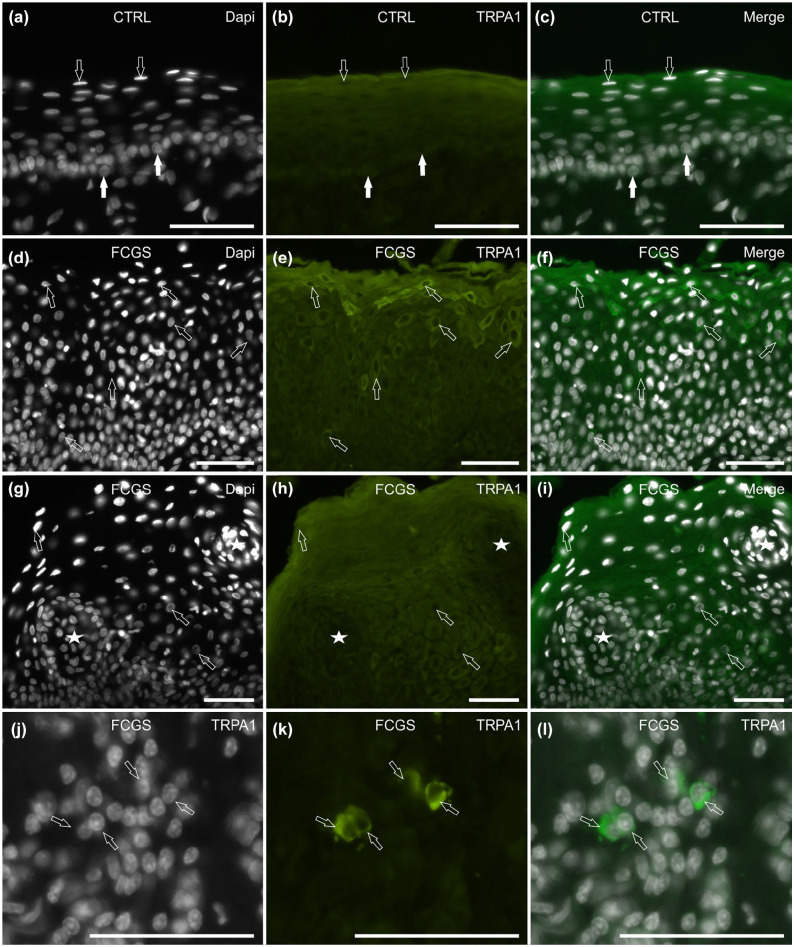
TRPA1 IR
Of the 7/7 (100%) CTRL cats tested, the mucosal epithelial cells showed different degrees of cytoplasmic TRPA1 IR; the superficial epithelial cells displayed moderate-to-bright TRPA1 IR, whereas the cells of the basal and the intermediate layers showed faint-to-moderate immunolabelling (Figure 5a–c). In the subepithelial connective tissue, no inflammatory cells displayed TRPA1 IR.
Figure 5.
Photomicrographs of cryosections of feline caudal oral mucosa showing transient receptor potential ankyrin 1 (TRPA1) immunoreactivity (IR) in tissues of the (a–c) control cats (CTRL) and the (d–l) cats affected by feline chronic gingivostomatitis (FCGS). (a–c) In the CTRL cats, the epithelial cells expressed different degrees of TRPA1 IR. The white arrows indicate the basal layer cells, expressing faint-to-moderate immunolabelling; the open arrows indicate the superficial layer cells, which expressed moderate-to-bright TRPA1 IR. (d–f) The arrows indicate some mucosal epithelial cells showing bright TRPA1 IR; they were more concentrated in the superficial layers. (g–i) The stars indicate two large inflammatory infiltrates encircled by epithelial cells (arrows) expressing TRPA1 IR. (j–l) The arrows indicate some inflammatory cells scattered in the subepithelial connective tissue, showing bright TRPA1 IR. Bar = 50 µm
Of the 8/8 (100%) FCGS cats tested, moderate-to-bright TRPA1 IR was expressed by the cytoplasm of the mucosal epithelial cells, with the brightest immunolabelling expressed by some cells of the superficial layers (Figure 5d–f) and by the cells encircling the large inflammatory infiltrate (Figure 5g–i). In the subepithelial connective tissue, bright TRPA1 IR was expressed by the cytoplasm of some inflammatory cells (Figure 5j–l).
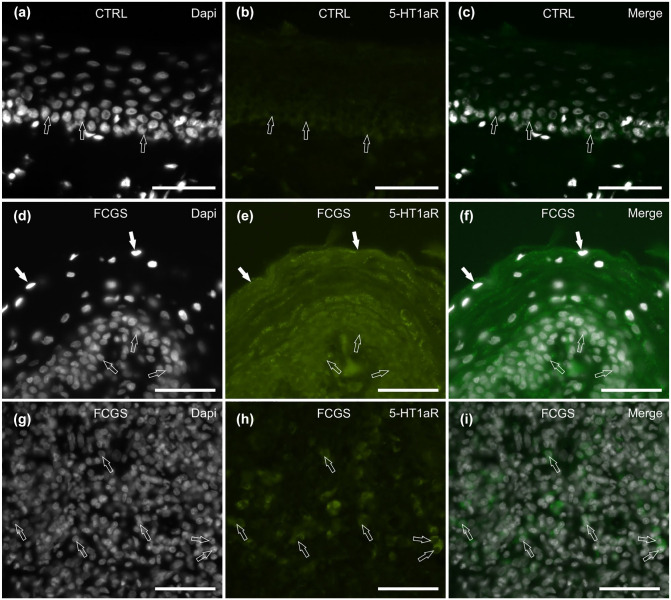
5-HT1aR IR
In 3/4 (75%) CTRL cats tested, faint cytoplasmic 5-HT1aR IR was displayed only by the deepest layers of the mucosal epithelial cells (Figure 6a–c). In the subepithelial connective tissue, only a few inflammatory cells expressed faint cytoplasmic 5-HT1aR IR (data not shown).
Figure 6.
Photomicrographs of cryosections of feline caudal oral mucosa showing serotonin 1a receptor (5-HT1aR) immunoreactivity (IR) in the (a–c) control and the (d–l) inflamed (feline chronic gingivostomatitis [FCGS]) tissues. (a–c) The arrows indicate the epithelial cells of the mucosal basal layer, expressing faint 5-HT1aR IR. (d–f) In the mucosa of the FCGS cats, the epithelial cells of the superficial (white arrows) and deepest (open arrows) layers show bright granular 5-HT1aR IR. (g–i) In the subepithelial connective tissue, some inflammatory cells (arrows) show bright 5-HT1aR IR. Bar = 50 µm
In 3/8 (37.5%) FCGS cats tested, all the cell layers of the mucosal epithelium showed moderate-to-bright granular cytoplasmic 5-HT1aR IR (Figure 6d–f), while in the remaining cats (n = 5/8; 62.5%), the epithelium was 5-HT1aR negative. In all the cats, some inflammatory cells distributed within the epithelium and in the subepithelial connective tissue displayed moderate-to-bright cytoplasmic 5-HT1aR IR (Figure 6g–i).
Semiquantitative analysis
The intensity of the expressions of the cannabinoid and cannabinoid-related receptors investigated and semi-quantitatively evaluated in the CTRL and the FCGS cats are summarised in Table 4.
Table 4.
Semiquantitative evaluation of the density of cannabinoid receptor 1 (CB1R), cannabinoid receptor 2 (CB2R), G protein-coupled receptor 55 (GPR55), transient receptor potential ankyrin 1 (TRPA1) and serotonin 1a receptor (5-HT1aR) immunoreactivity in different cellular elements of the caudal oral mucosa of control cats (CTRL) and cats with feline chronic gingivostomatitis (FCGS)
| CB1R | CB2R | GPR55 | TRPA1 | 5-HT1aR | |
|---|---|---|---|---|---|
| CTRL cats | |||||
| Superficial epithelial cells | C +++ | – | – | C++/+++ | – |
| Intermediate epithelial cells | C+/++ | – | – | C+/++ | – |
| Basal epithelial cells | – | – | – | C+/++ | C + |
| Immunocytes | – | C ++ | M+/++ | – | C + |
| FCGS cats | |||||
| Superficial epithelial cells | C + | M + | C ++ | C +++ | −/C++/+++ |
| Intermediate epithelial cells | C
+++
N +++ |
M+/++ | – | C ++ | C++/+++ (3/8)
– (5/8) |
| Basal epithelial cells | – | – | – | C ++ | C++/+++ (3/8)
– (5/8) |
| Immunocytes | C
++
M ++ |
C +++ | M ++ | C +++ | C++/+++ |
Immunoreactive cells were graded as: – negative; + faintly stained; ++ moderately stained; and +++ brightly stained
C = cytoplasmic; M = membrane; N = nuclear
Discussion
As already mentioned, studies regarding the ECS of the cat are very limited and, to the best of our knowledge, there are no studies concerning the distribution of cannabinoid receptors in feline oral cavity tissues.
The present study found CB1R to be distributed in the healthy oral mucosa epithelium, which is in line with the expression of CB1R reported in the human oral cavity, specifically in the epithelium of the tongue and periodontal soft tissues.26,27 The presence of CB1R in the healthy feline oral mucosal epithelium suggests that it could play a role in the maintenance of oral mucosa homeostasis and, as has been proposed for the human epidermal barrier, may be involved in barrier permeability, and epithelial cell differentiation and proliferation. 28 A marked upregulation of CB1R was observed throughout the mucosal epithelium of the FCGS cats, in parallel with the presence of numerous CB1R immunoreactive inflammatory cells. It is plausible to consider that some of these CB1R immunoreactive inflammatory cells may be mast cells as we have recently shown that mast cells of the cat intestine express CB1R IR. 12 These CB1R immunoreactive cells might also be macrophages, as the expression by these cells has been demonstrated in the healing of granular tissue of rats with periodontal injury. 29 Taken together, these findings suggest that CB1R could be involved in the modulation of the inflammatory response associated with FCGS.
It has been hypothesised that CB1R could play a role in various human diseases as its expression increases during inflammatory processes in several anatomical sites, such as the tongue, periodontal ligament structures, gastrointestinal tract, liver and pancreas.26,27,30–32 It has recently been shown that CB1R is upregulated in the skin epithelium of cats affected by hypersensitivity dermatitis, 11 supporting the hypothesis that CB1R may be involved in inflammatory diseases of various organs in cats.
In the present study, CB2R was generally not expressed by the oral mucosal epithelial cells in the healthy feline population. In FCGS cats, a substantial change in the degree of expression and distribution of CB2R IR, which was distributed through all the epithelial layers, was noted.
In the healthy cats, very few immunocytes localised in subepithelial connective tissue expressed faint CB2R IR. This finding was in line with the theory that, in humans, CB2R is linked to immune events and has a function in maintaining immunological homeostasis. 33 A recent study showed that CB2R IR was expressed by macrophages of the feline intestine. 12 In the FCGS population, CB2R IR was strongly expressed by the inflammatory cells. The data in the present study are consistent with previous studies, which demonstrated an upregulation of CB2R IR in the skin epithelium and subepidermal inflammatory infiltrate of cats affected by hypersensitivity dermatitis. 11 It is therefore possible that CB2R could play a role during inflammatory insults of various organs in the cat. Numerous studies have demonstrated that the activation of CB2R seemed to have an anti-inflammatory effect by means of the reduction of the cytokine secretion,34–36 suggesting that CB2R agonists could be used in FCGS patients to reduce the inflammation.
One of the most interesting findings of the present study was the density of immunocytes expressing bright GRP55 IR in the subepithelial connective tissue of the FCGS cats. In other species, GPR55 has already been identified in a large number of cell types, such as macrophages, plasma cells, neutrophils, natural killer cells, monocytes and T lymphocytes.37–42 In a previous study, it was demonstrated that feline intestinal immunocytes and macrophages showed GPR55 IR. 12 It has been shown that, in humans, GPR55 regulates intra-epithelial lymphocyte migration, at least in the damaged intestine. 43 The role played by this receptor during inflammation is not yet clear; however, it has been shown that, in mice, the administration of GPR55 antagonists seemed to reduce intestinal inflammation and decrease pro-inflammatory cytokines and leukocyte recruitment,38,42,44 suggesting a pro-inflammatory role of this receptor.
TRPA1 has not only been identified in rodent sensory neurons, but also in non-neuronal cells of various mammalian species. In sensory neurons, TRPA1 acts as a cold-sensing channel, which can be activated by noxious cold temperatures (<18°C), and several chemicals, such as mustard oil, cinnemaldehyde, menthol and curcumin. 45 In non-neuronal cells, TRPA1 has been identified in rat salivary glands, 46 the porcine lung epithelium 47 and also in cat intestinal goblet cells. 12 In the present study, TRPA1 IR was expressed by the mucosal epithelial cells of the CTRL cats, in particular in the superficial layers. It has been shown that, in humans, TRPA1 may stimulate the release of pro-inflammatory mediators from skin keratinocytes with a possible role in maintaining the integrity of the immune response, stimulating cutaneous inflammation and altering the expression of the genes involved in the control of the keratinocyte differentiation, proliferation and apoptosis.48,49 In the present study, TRPA1 IR was upregulated in the mucosal epithelial layers and markedly expressed by the immunocytes of the FCGS cats. These last findings were consistent with those of other researchers who identified TRPA1 upregulation in keratinocytes and macrophages in the human oral lichen planus, a chronic inflammatory disease of unknown aetiology. 50
We observed 5-HT1aR IR in the mucosal epithelial cells and immunocytes of the CTRL cats, with an upregulation of this receptor in the FCGS cats. The expression of this receptor has already been observed in the epithelial cells of heathy feline intestines, 12 suggesting a role of 5-HT1aR in cellular homeostasis and secretion. The 5-HT1aR has also been found in the human epidermis and mast cells, and in mice macrophages.51–53 In humans, 5-HT1aR IR is highly expressed in skin mast cells during atopic dermatitis; 52 we suggest that agonists of the 5-HT1aR might be of value in the treatment of these patients.
Considering the general upregulation of the cannabinoid and cannabinoid-related receptors in the oral tissues of the FCGS cats, it seemed appropriate to consider the hypothetical role played by the components of C sativa (eg, CBD) and other molecules that interact with the ECS (eg, palmythoiletanolamide [PEA]) in modulating the inflammation during FCGS. In vitro studies have supported the fact that CBD suppresses T-cell function and that PEA could directly inhibit T-cell (CD8 and CD4) responses by reducing their production of lymphokines, such as tumour necrosis factor alpha and interferon gamma.54–56 Other studies have indicated that cannabinoids act as immunosuppressive and anti-inflammatory agents, and have been shown to alleviate a wide range of immune-mediated disorders in humans, such as multiple sclerosis, rheumatoid arthritis and inflammatory bowel disease. 57 The use of bioactive molecules derived from C sativa for the treatment of oral inflammation, or their general use in dentistry, has been poorly investigated and, at present, their effects on oral diseases are not well known. There are studies supporting CBD as a therapeutic agent for human burning mouth syndrome and oral mucositis.26,58 Moreover, phytocannabinoids have been reported to have antimicrobial properties against Gram-positive and Gram-negative bacterial species, and it has been demonstrated that CBD is effective against dental plaque-associated bacteria in humans. 59 At present, to the best of our knowledge, no clinical studies regarding the use of cannabinoids in cats are available whereas there is a growing body of literature in dogs. 60
Nonetheless, considering cannabinoid properties, mechanisms of action and favourable results in treating other complex diseases of different species, it is believed that they could exert a positive therapeutic effect on some diseases that are still challenging for veterinarians, such as FCGS, by reducing the associated inflammation/pain and controlling dental plaque bacteria.
Conclusions
The present study described for the first time the expression of cannabinoid and cannabinoid-related receptors in the oral mucosa of healthy cats and in cats affected by FCGS. These findings support the hypothesis that the ECS in cats may play a role in the maintenance of oral mucosa homeostasis. Furthermore, considering the general upregulation of the target receptors of this system in the epithelium and in the inflammatory infiltrate of the oral mucosa of the FCGS cats, it seemed reasonable that the ECS could be involved in the pathogenesis of this disease, representing an interesting potential therapeutic target. Phytocannabinoids and endocannabinoid-like molecules, as well as inhibitors of the enzymes involved in endocannabinoid degradation (‘entourage effect’), could be useful in modulating the inflammation and pain associated with FCGS. The findings in the present study could represent a starting point for additional immunohistochemical and clinical research.
Acknowledgments
The authors would like to thank Professor Chrysostomos Dovas, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki (Greece), for his collaboration.
Footnotes
Accepted: 5 October 2020
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Financial support was provided by Formula Swiss AG (Swiss).
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Giorgia Galiazzo  https://orcid.org/0000-0002-8132-1310
https://orcid.org/0000-0002-8132-1310
Serafeim Papadimitriou  https://orcid.org/0000-0001-7956-501X
https://orcid.org/0000-0001-7956-501X
Roberto Chiocchetti  https://orcid.org/0000-0003-4448-6199
https://orcid.org/0000-0003-4448-6199
References
- 1. Tóth KF, Ádám D, Birò T, et al. Cannabinoid signaling in the skin: therapeutic potential of the “c(ut)annabinoid” system. Molecules 2019; 24: 918. DOI: 10.3390/mol ecules24050918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ligresti A, De Petrocellis L, Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev 2016; 96: 1593–1659. [DOI] [PubMed] [Google Scholar]
- 3. Maroon J, Bost J. Review of the neurolgical benefits of phytocannabinoids. Surg Neurol Int 2018; 9: 91. DOI: 10.4103/sni.sni_45_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giacoppo S, Mandolino G, Galuppo M, et al. Cannabinoids: new promising agents in the treatment of neurological diseases. Molecules 2014; 19: 18781–18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov 2008; 7: 438–455. [DOI] [PubMed] [Google Scholar]
- 6. Kupczyk P, Reich A, Szepietowski JC. Cannabinoid system in the skin – a possible target for future therapies in dermatology. Exp Dermatol 2009; 18: 669–679. [DOI] [PubMed] [Google Scholar]
- 7. Hasenoehrl C, Taschler U, Storr M, et al. The gastrointestinal tract – a central organ of cannabinoid signaling in health and disease. Neurogastroenterol Motil 2016; 28: 1765–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donvito G, Nass SR, Wilkerson JL, et al. The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology 2018; 43: 52–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gebremedhin D, Lange AR, Campbell WB, et al. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol 1999; 276: H2085–H2093. [DOI] [PubMed] [Google Scholar]
- 10. Pirone A, Lenzi C, Briganti A, et al. Spatial distribution of cannabinoid receptor 1 and fatty acid amide hydrolase in the cat ovary and oviduct. Acta Histochem 2017; 119: 417–422. [DOI] [PubMed] [Google Scholar]
- 11. Miragliotta V, Ricci PL, Albanese F, et al. Cannabinoid receptor types 1 and 2 and peroxisome proliferator-activated receptor-α: distribution in the skin of clinically healthy cats and cats with hypersensitivity dermatitis. Vet Dermatol 2018; 29: 316–e111. [DOI] [PubMed] [Google Scholar]
- 12. Stanzani A, Galiazzo G, Giancola F, et al. Localization of cannabinoid and cannabinoid related receptors in the cat gastrointestinal tract. Histochem Cell Bio 2020; 153: 339–356. [DOI] [PubMed] [Google Scholar]
- 13. Rolim VM, Pavarini SP, Campos FS, et al. Clinical, pathological, immunohistochemical and molecular characterization of feline chronic gingivostomatitis. J Feline Med Surg 2017; 19: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peralta S, Carney PC. Feline chronic gingivostomatitis is more prevalent in shared households and its risk correlates with the number of cohabiting cats. J Feline Med Surg 2020; 21: 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winer JN, Arzi B, Verstraete FJM. Therapeutic management of feline chronic gingivostomatitis: a systematic review of the literature. Front Vet Sci 2016; 3: 54. DOI: 10.3389/fvets.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dolieslager SM, Bennett D, Johnston N, et al. Novel bacterial phylotypes associated with the healthy feline oral cavity and feline chronic gingivostomatitis. Res Vet Sci 2013; 94: 428–432. [DOI] [PubMed] [Google Scholar]
- 17. Lee DB, Verstraete FJM, Arzi B. An update on feline chronic gingivostomatitis. Vet Clin North Am Small Anim Pract 2020; 50: 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kouki M, Papadimitriou SA, Psalla D, et al. Chronic gingivostomatitis with esophagitis in cats. J Vet Intern Med 2017; 31: 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hennet P. Chronic gingivo-stomatitis in cats: long-term follow-up of 30 cases treated by dental extractions. J Vet Dent 1997; 14: 15–21. [Google Scholar]
- 20. Bellei E, Dalla F, Masetti L, et al. Surgical therapy in chronic feline gingivostomatitis (FCGS). Vet Res Commun 2008; 32: S231–S234. [DOI] [PubMed] [Google Scholar]
- 21. Arzi B, Mills-Ko E, Verstraete FJM, et al. Therapeutic efficacy of fresh, autologous mesenchymal stem cells for severe refractory gingivostomatitis in cats. Stem Cells Transl Med 2016; 5: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arzi B, Clark KC, Sundaram A, et al. Therapeutic efficacy of fresh, allogeneic mesenchymal stem cells for severe refractory feline chronic gingivostomatitis. Stem Cells Transl Med 2017; 6: 1710–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Squarzoni P, Bani D, Cialdai F, et al. NIR laser therapy in the management of feline stomatitis. SM Dermatolog J 2017; 3: 1021. [Google Scholar]
- 24. Matsumoto H, Teshima T, Iizuka Y, et al. Evaluation of the efficacy of the subcutaneous low recombinant feline interferon-omega administration protocol for feline chronic gingivitis-stomatitis in feline calicivirus-positive cats. Res Vet Sci 2018; 121: 53–58. [DOI] [PubMed] [Google Scholar]
- 25. Lommer MJ. Efficacy of cyclosporine for chronic, refractory stomatitis in cats: A randomized, placebo-controlled, double-blinded clinical study. J Vet Dent 2013; 30: 8–17. [DOI] [PubMed] [Google Scholar]
- 26. Borsani E, Majorana A, Cocchi MA, et al. Epithelial expression of vanilloid and cannabinoid receptors: a potential role in burning mouth syndrome pathogenesis. Histol Histopathol 2014; 29: 523–533. [DOI] [PubMed] [Google Scholar]
- 27. Konermann A, Jäger A, Held SAE, et al. In vivo and in vitro identification of endocannabinoid signaling in periodontal tissues and their potential role in local pathophysiology. Cell Mol Neurobiol 2017; 37: 1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roelandt T, Heughebaert C, Bredif S, et al. Cannabinoid receptors 1 and 2 oppositely regulate epidermal permeability barrier status and differentiation. Exp Dermatol 2012; 21: 688–693. [DOI] [PubMed] [Google Scholar]
- 29. Kozono S, Matsuyama T, Biwasa KK, et al. Involvement of the endocannabinoid system in periodontal healing. Biochem Biophys Res Comun 2010; 394: 928–933. [DOI] [PubMed] [Google Scholar]
- 30. Lee Y, Jo J, Chung HY, et al. Endocannabinoids in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 2016; 311: G655–G666. [DOI] [PubMed] [Google Scholar]
- 31. Maccarrone M, Bab I, Bíró T, et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci 2015; 36: 277–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Michler T, Storr M, Kramer J, et al. Activation of cannabinoid receptor 2 reduces inflammation in acute experimental pancreatitis via intra-acinar activation of p38 and MK2-dependent mechanisms. Am J Physiol Gastrointest Liver Physiol 2013; 304: G181–G192. [DOI] [PubMed] [Google Scholar]
- 33. Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med 2009; 11: e3. DOI: 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cabral GA, Staab A. Effects on the immune system. Handb Exp Pharmacol 2005; 168: 385–423. [DOI] [PubMed] [Google Scholar]
- 35. Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol 2005; 5: 400–411. [DOI] [PubMed] [Google Scholar]
- 36. Lunn CA, Reich EP, Bober L. Targeting the CB2 receptor for immune modulation. Expert Opin Ther Targets 2006; 10: 653–663. [DOI] [PubMed] [Google Scholar]
- 37. Balenga NAB, Henstridge CM, Kargl J, et al. Pharmacology, signaling and physiological relevance of the G protein-coupled receptor 55. Adv Pharmacol 2011; 62: 251–277. [DOI] [PubMed] [Google Scholar]
- 38. Stančić A, Jandl K, Hasenöhrl C, et al. The GPR55 antagonist CID16020046 protects against intestinal inflammation. J Neurogastroenterol Motil 2015; 27: 1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiurchiù V, Leuti A, Maccarrone M. Cannabinoid signaling and neuroinflammatory diseases: a melting pot for the regulation of brain immune responses. J Neuroimmune Pharmacol 2015; 10: 268–280. [DOI] [PubMed] [Google Scholar]
- 40. Lanuti M, Talamonti E, Maccarrone M, et al. Activation of GPR55 receptors exacerbates oxLDL-induced lipid accumulation and inflammatory responses, while reducing cholesterol efflux from human macrophages. PLoS One 2015; 10: e0126839. DOI: 10.1371/journal.pone.0126839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Galiazzo G, Giancola F, Stanzani A, et al. Localization of cannabinoid receptors CB1, CB2, GPR55, and PPARα in the canine gastrointestinal tract. Histochem Cell Biol 2018; 150: 187–205. [DOI] [PubMed] [Google Scholar]
- 42. Grill M, Hasenoehrl C, Kienzl M, et al. Cellular localization and regulation of receptors and enzymes of the endocannabinoid system in intestinal and systemic inflammation. Histochem Cell Biol 2019; 151: 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sumida H, Lu E, Chen H, et al. GPR55 regulates intraepithelial lymphocyte migration dynamics and susceptibility to intestinal damage. Sci Immunol 2017; 2: eaao1135. DOI: 10.1126/sciimmunol.aao1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tuduri E, López M, Dieguez C, et al. GPR55 and the regulation of glucose homeostasis. Int J Biochem Cell Biol 2017; 88: 204–207. [DOI] [PubMed] [Google Scholar]
- 45. Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: a gatekeeper for inflammation. Annu Rev Physiol 2013; 75: 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ogawa Y. Immunocytochemistry of myoepithelial cells in the salivary glands. Prog Histochem Cytochem 2003; 38: 343–426. [DOI] [PubMed] [Google Scholar]
- 47. Büch TRH, Schäfer EAM, Demmel MT, et al. Functional expression of the transient receptor potential channel TRPA1, a sensor for toxic lung inhalants, in pulmonary epithelial cells. Chem Biol Interact 2013; 206: 462–471. [DOI] [PubMed] [Google Scholar]
- 48. Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br J Pharmacol 2012; 166: 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ho JC, Lee CH. TRP channels in skin: from physiological implications to clinical significances. Biophysics 2015; 11: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kun J, Perkecz A, Knie L, et al. TRPA1 receptor is upregulated in human oral lichen planus. Oral Dis 2017; 23: 189–198. [DOI] [PubMed] [Google Scholar]
- 51. Ritter M, El-Nour H, Hedblad MA, et al. Serotonin and its 5-HT1 receptor in human mastocytosis. Immunopharm Immunot 2012; 34: 679–685. [DOI] [PubMed] [Google Scholar]
- 52. Rasul A, El-Nour H, Lonne-Rahm SB, et al. Serotonergic markers in atopic dermatitis. Acta Derm Venereol 2016; 96: 732–736. [DOI] [PubMed] [Google Scholar]
- 53. Freire-Garabal M, Núñez MJ, Balboa J, et al. Serotonin upregulates the activity of phagocytosis through 5-HT1A receptors. Br J Pharmacol 2003; 139: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu HY, Chu RM, Wang CC, et al. Cannabidiol-induced apoptosis in primary lymphocytes is associated with oxidative stress-dependent activation of caspase-8. Toxicol Appl Pharmacol 2008; 226: 260–270. [DOI] [PubMed] [Google Scholar]
- 55. Kaplan BLF, Springs AEB, Kaminski NE. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem Pharmacol 2008; 76: 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chiurchiù V, van der Stelt M, Centonze D, et al. The endocannabinoid system and its therapeutic exploitation in multiple sclerosis: clues for other neuroinflammatory diseases. Prog Neurobiol 2018; 160: 82–100. [DOI] [PubMed] [Google Scholar]
- 57. Katz D, Katz I, Porat-Katz BS, et al. Medical cannabis: another piece in the mosaic of autoimmunity? Clin Pharmacol Ther 2017; 101: 230–238. [DOI] [PubMed] [Google Scholar]
- 58. Cuba LF, Salum FG, Cherubini K, et al. Cannabidiol: an alternative therapeutic agent for oral mucositis? J Clin Pharm Ther 2017; 42: 245–250. [DOI] [PubMed] [Google Scholar]
- 59. Stahl V, Vasudevan K. Comparison of the efficacy of cannabinoids versus commercial oral care products in reducing bacterial content from dental plaque: a preliminary observation. Cereus 2020; 12: e6809. DOI: 10.7759/cureus.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Della Rocca G, Di Salvo A. Hemp in veterinary medicine: from feed to drug. Front Vet Sci 2020; 7: 387. DOI: 10.3389/fvets.2020.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]