Abstract
Objectives
Temporomandibular joint ankylosis (TMJA) is the partial or complete inability to open the mouth due to intra- or extra-articular fibrous, bony or fibro-osseous tissue proliferation. Surgical procedures such as gap arthroplasty, condylectomy or wide extra-articular osteotomy have been recommended to treat this condition; these techniques are challenging, time-consuming and have been occasionally associated with postoperative recurrence, severe periarticular neurovascular iatrogenic trauma and death. Segmental mandibulectomy had previously been recommended as an alternative option for unilateral TMJA, but the location of mandibulectomy and extent of bone removal from the mandible region have not been mentioned in the literature. This study aimed to validate the area of the mandibular body (rostral, middle or caudal) and amount of bony tissue that should be osteotomized during a segmental mandibulectomy for treatment of unilateral TMJA in cats.
Methods
In this block study, 30 cadaver heads of domestic shorthair cats were randomly divided into three groups of 10 specimens each based on the mandibular region that would undergo segmental mandibulectomy (rostral, middle and caudal). The size of the removed mandibular segment and pre- and postoperative vertical range of mandibular motion were compared for statistical purposes.
Results
A significant statistical difference was observed between the pre- and postoperative vertical range of mandibular motion between the rostral, middle and caudal segmental mandibulectomies (P <0.001). The mean postoperative recovered range of mandibular motion for the rostral, middle and caudal segmental mandibulectomies was 50.4%, 81.9% and 90.4%, respectively.
Conclusions and relevance
The caudal segmental mandibulectomy showed the highest postoperative vertical range of mandibular motion. The removal of a minimum of 1.2 cm of the caudal mandibular body was required to achieve nearly full recovery of presurgical mouth opening in the specimens of this study. The caudal segmental mandibulectomy may eliminate the risk of iatrogenic periarticular neurovascular damage inherent to more invasive surgeries performed at the temporomandibular joint area. When performed unilaterally, the caudal segmental mandibulectomy is a viable surgical alternative that may show a similar outcome to other surgical techniques.
Keywords: Temporomandibular joint, TMJ, ankylosis, mandibulectomy
Introduction
Temporomandibular joint ankylosis (TMJA) is an uncommon unilateral or bilateral condition that may affect both dogs and cats.1–3 Unilateral TMJA (Figure 1) is characterized by the partial or complete inability to open the mouth due to intra- or extra-articular fibrous, bony or fibro-osseous tissue proliferation from traumatic, developmental, inflammatory or neoplastic origin.2,4–6 Other conditions that can cause progressive inability to open the mouth are masticatory muscle myositis and severe ear disease. 2 Although it is known that fractures affecting the joint are the most common cause of TMJA in veterinary patients, there is no consensus regarding the pathophysiology of TMJA.2,3,7,8 In humans, TMJA may be related to a postoperative infection of the joint or calcification of hemarthrosis from an extra-articular hematoma. 7
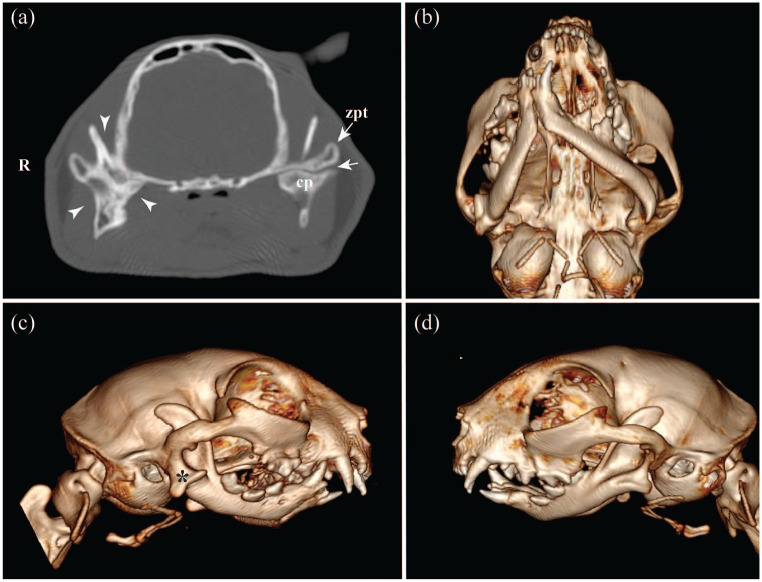
Figure 1.
CT transversal multiplanar rendering using (a) bone algorithm and (b–d) three-dimensional volumetric reconstructions of a 10-month-old domestic shorthair cat affected by right unilateral temporomandibular joint ankylosis. (a) Ankylotic bony tissue and obliteration of the joint space of the right temporomandibular joint (TMJ; arrowheads). At the contralateral joint, the articular space (arrow) is preserved between the condylar process (cp) and at the zygomatic process of the temporal bone (zpt). (b) Ventral view of the skull showing mandibular asymmetry and malocclusion. (c,d) Lateral views of the skull showing the bony proliferation at the ventrolateral aspect of the right TMJ (asterisk) and non-affected left TMJ (d)
TMJA has been classified into two types based on the anatomical area that is affected: intra-articular (true TMJA) if the abnormal tissue proliferation directly affects the head of the condylar process of the mandible, the mandibular fossa or retroarticular process of the temporal bone; and extra-articular (false TMJA or pseudoankylosis) if extracapsular structures are involved, such as the base of the skull, ramus of the mandible, zygomatic arch, masticatory muscles or the bony structures of the ear.6,9–11
TMJA affects the patient’s quality of life and may become life-threatening if not diagnosed and treated appropriately. Its diagnosis must be suspected during the awake physical examination and confirmed by a diagnostic imaging test.5,9,12 Veterinary patients affected by TMJA demonstrate a partial or complete inability to open the mouth, malocclusion, skull malformation, weight loss, vocalizing or yowling while trying to eat, halitosis and unkempt haircoat because of reduced self-grooming.2,5,11
Radiographic projections of the head have been routinely used for the initial evaluation of temporomandibular joint (TMJ) disorders in dogs and cats. Cross-sectional imaging techniques such as CT and, more recently, cone beam CT (CBCT) have been considered the gold standard diagnostic imaging techniques for evaluating the TMJ bony structures.6,13–16 MRI has been used for assessment of the TMJ soft tissue structures in the veterinary field but much less frequently than for humans, where it is widely used for assessment of the extra- and intra-articular soft tissue structures, in particular, for evaluation of the morphology and positioning of the articular disc.17,18
Although surgical excision of the abnormal tissue by gap arthroplasty, condylectomy or wide extra-articular osteotomy are currently the treatment of choice for TMJA, these procedures may be time-consuming, technically challenging and carry a potential risk of iatrogenic trauma and death owing to the complex anatomy surrounding the TMJ. Among these complications are excessive or uncontrollable intraoperative bleeding, facial paralysis and TMJA recurrence.2,6,11,12,19–21 The postoperative recurrence is associated with the inability to remove extensive areas of affected tissue owing to its proximity to vital structures such as the base of the skull and the maxillary neurovascular bundle.2,11,19–21
While the segmental mandibulectomy between the fourth premolar and first molar teeth had previously been proposed as a possible treatment for unilateral TMJA,22,23 veterinary practitioners have raised questions regarding which area of the mandible should be addressed and how much bony tissue must be removed. To the best of our knowledge, the specific area of the mandible and amount of bony tissue that must be excised during a segmental mandibulectomy in cats affected by unilateral TMJA has not been established. Thus, the purpose of the present study was to validate the area of the mandibular body (rostral, middle or caudal) and suggest the extent of bone tissue that must be osteotomized during a segmental mandibulectomy for treatment for unilateral TMJA in cats.
Materials and methods
Cadaver heads of adult domestic shorthair cats obtained from shelter animals and euthanized for reasons not related to this study were divided into three groups according to the mandibular segment that would be osteotomized (rostral, middle or caudal). The sex and weight of the cats were not available. Power calculations were performed to determine that a sample size of 30 specimens divided into three groups of 10 each was needed for 90% power, assuming a 5% significance level.
All specimens were submitted for physical and diagnostic imaging evaluation using CBCT. Specimens with skeletal malocclusion, gross bony alterations related to a possible trauma, neoplastic process or anatomical malformation, which could affect the range of mandibular motion, were not used in the present study.
The heads were scanned with the long axis of the mandibles parallel to the headstand using a portable CBCT unit (Xoran Technologies) at 0.3 mm voxel size, 24 × 14 cm field of view, 120 kVp, 57.6 mAs and 20 s of acquisition time. Bony structures and measurements were made using suitable bony window and level settings in a free DICOM viewer software (Horos, version 3-LGPL-3.0). The length of the skull was used as a head size reference. For this, the distance between the vestibular aspect of the maxillary incisor teeth and the caudal border of the occipital bone was measured on sagittal CBCT reformations (Figure 2).
Figure 2.
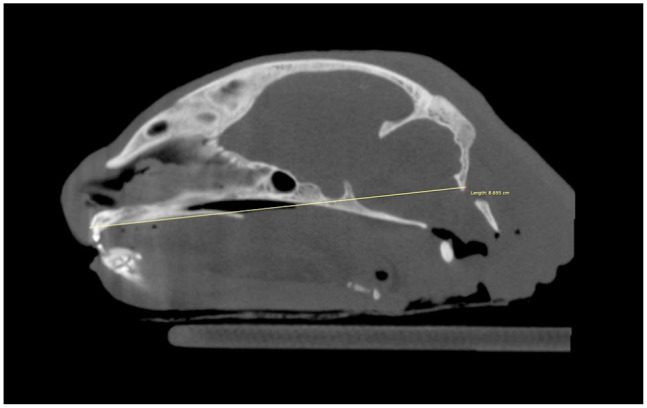
Sagittal multiplanar rendering of an adult cat skull using bone algorithm reconstruction. The skull’s length was obtained by measuring the distance between the vestibular aspect of the maxillary incisor teeth and the caudal border of the occipital bone
After the CT examination, the heads were frozen for preservation until the measurements of the pre- and postoperative vertical range of mandibular motion could be obtained. For the experimental phase, the heads were thawed for 24 h at room temperature (71°F [22°C]). During this stage of the study, the maximum vertical range of mandibular motion was measured twice: before creating the unilateral TMJA, and after performing the proposed segmental mandibulectomy.
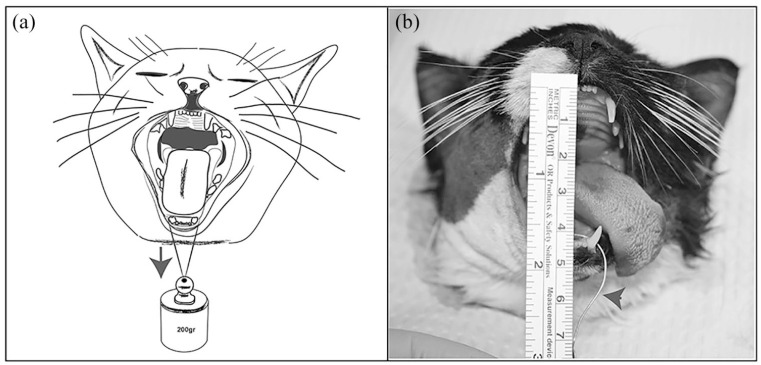
To consistently apply the same degree of force to open the mouth during the pre- and postoperative moments, a weight of 200 g was hung around the mandibular canine teeth using a 24 G wire (Figure 3a). The 200 g load was previously tested on all the heads of this study to recreate a similar opening to that which would be obtained by manually applying mild tension with the operator’s fingers. The vertical range of mandibular motion was obtained by measuring the distance between the incisal margins of the maxillary and mandibular incisor teeth, and their values were measured with a surgical ruler and recorded for statistical purposes (Figure 3b).
Figure 3.
(a) Illustration and (b) photograph showing how a constant weight of 200 g was hung around the mandibular canine teeth using a 24 G wire (arrows). The vertical range of mandibular motion corresponded to the (b) distance between the incisal margins of the maxillary and mandibular incisor teeth
The effect of a right unilateral TMJA was obtained by ligating the right coronoid process to the most caudal region of the zygomatic arch using a 24 G wire. For this, the temporalis and masseter muscles were exposed through a 20 mm skin incision over the caudal third of the right zygomatic arch. Sharp and blunt dissection of the muscle fibers was performed until the underlying coronoid process was exposed. Two orifices were performed in both the coronoid process and the most caudal region of the zygomatic arch. The orifices were then used to pass the wires that were twisted together, allowing for locking the mandibles in a closed mouth position (Figure 4).
Figure 4.
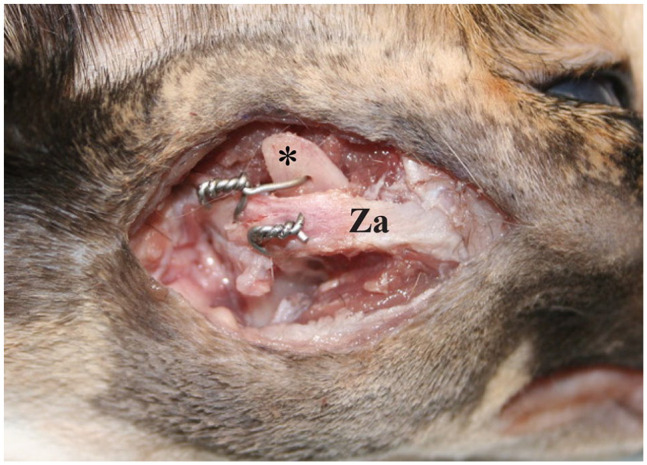
Lateral photograph of the cadaveric specimen showing how the zygomatic arch (Za) and coronoid process (asterisk) were fixed together to mimic a unilateral temporomandibular joint ankylosis. Two 24 G wires were used to lock the lower jaw in a closed mouth position
The 30 cadaver heads were randomly divided into three groups of 10 specimens according to the mandibular region that would undergo a segmental mandibulectomy (Figure 5). Group 1 had rostral segmental mandibulectomy, performed between the mandibular symphysis and the furcation of the third premolar tooth. Group 2 had middle segmental mandibulectomy, performed between the furcation of the third premolar and first molar teeth. Group 3 had caudal segmental mandibulectomy, performed between the furcation of the first molar tooth and the rostral aspect of the mandibular foramen. Each segmental mandibulectomy was planned on CBCT parasagittal images, and their rostrocaudal length values were recorded along with the skull size for statistical purposes.
Figure 5.
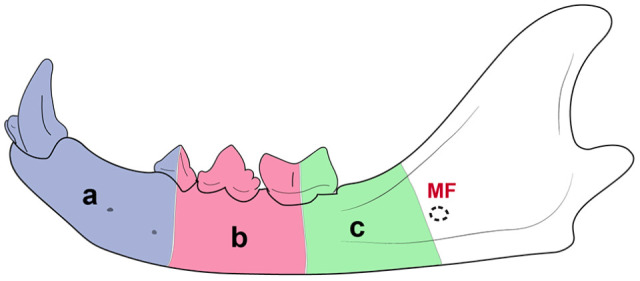
Illustration of an adult cat’s mandible depicting the regions that underwent segmental mandibulectomy. (a) Rostral segmental mandibulectomy, performed between the mandibular symphysis and the third premolar tooth’s furcation. (b) Middle segmental mandibulectomy, performed between the furcations of the third premolar and first molar teeth. (c) Caudal segmental mandibulectomy, performed between the furcation of the first molar tooth and the rostral aspect of the mandibular foramen (MF)
For the rostral segmental mandibulectomy, an intraoral full-thickness mucoperiosteal incision was made along the gingival sulcus between the right mandibular first incisor tooth and the distal region of the third premolar tooth, followed by a vertical incision at the third premolar tooth′s distal aspect. This allowed the vestibular gingiva and oral mucosa to be raised from the underlying mandibular body using a periosteal elevator. A number 15 scalpel blade and sharp-blunt were used for the separation of the mandibular symphysis.
Sectioning of the third premolar tooth and osteotomy of the mandible were performed with a long #700 carbide bur in a high-speed dental handpiece. The mandibular segment was separated in a rostrocaudal direction from the symphysis to the third premolar tooth by breaking any remaining bony attachments with a dental elevator, thus exposing the inferior alveolar neurovascular bundle.
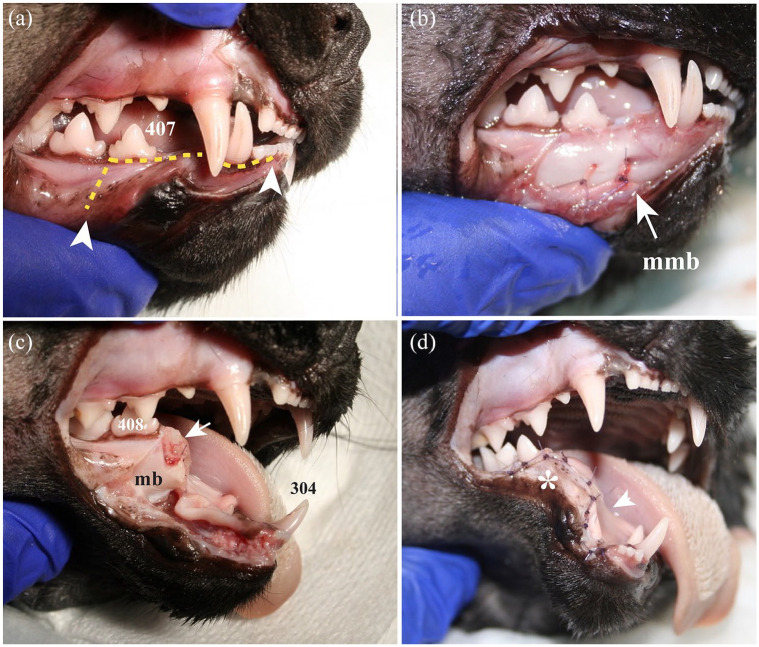
The mouth was opened at this point, and the remaining distal root tip of the third premolar tooth was extracted using a winged dental elevator. Sharp alveolar and mandibular bone edges were smoothed before soft tissue apposition. The intraoral surgical incision’s apposition was performed using absorbable monofilament suture material. Figure 6 shows the most relevant features for the rostral segmental mandibulectomy.
Figure 6.
Lateral view of the rostral aspect of the right mandible of a cat head specimen showing the main features of the rostral segmental mandibulectomy. (a) A surgical marker pen was used to outline the planned incisions along the gingival sulcus of the mandibular incisor teeth and labial/buccal mucosa (arrowheads). (b) A full-thickness mucoperiosteal incision made with a number 15 scalpel blade and sharp dissection with a periosteal elevator allowed exposure of the mandibular body and placement of a ligature around the middle mental neurovascular bundle with 4-0 absorbable monofilament suture material (mmb). (c) After removing the rostral aspect of the mandible and placing the ligature around the inferior alveolar neurovascular bundle, the distal root of the right mandibular third premolar tooth was extracted. Sharp alveolar bone edges (arrow) and the remaining portion of the mandibular body (mb) were smoothed with a #22 round diamond bur. (d) The labial/buccal flap was sutured over the wound, using a 5-0 absorbable monofilament suture material in a simple interrupted pattern. The remaining mandibular body (asterisk) provoked tension on the mucoperiosteal and flap (arrowhead), resulting in a decreased vertical range of motion of the contralateral mandible postoperatively.
407 = right mandibular third premolar; 408 = right mandibular fourth premolar; 304 = left mandibular canine teeth
The middle segmental mandibulectomy was made through an extraoral approach. A 2–3 cm length full-thickness skin incision performed on the ventral aspect of the mandible allowed exposure of the underlying mandibular body between the third premolar and first molar teeth. An osteotomy was made through the furcations of the third premolar and first molar teeth, avoiding damage to the inferior alveolar neurovascular bundle. The mandibular segment removal, root remnant extraction, smoothing of bone edges and placement of the ligature around the inferior alveolar bundle were performed as described for the rostral segmental mandibulectomy. Figure 7 depicts the soft tissue apposition and the most relevant features for the middle segmental mandibulectomy.
Figure 7.
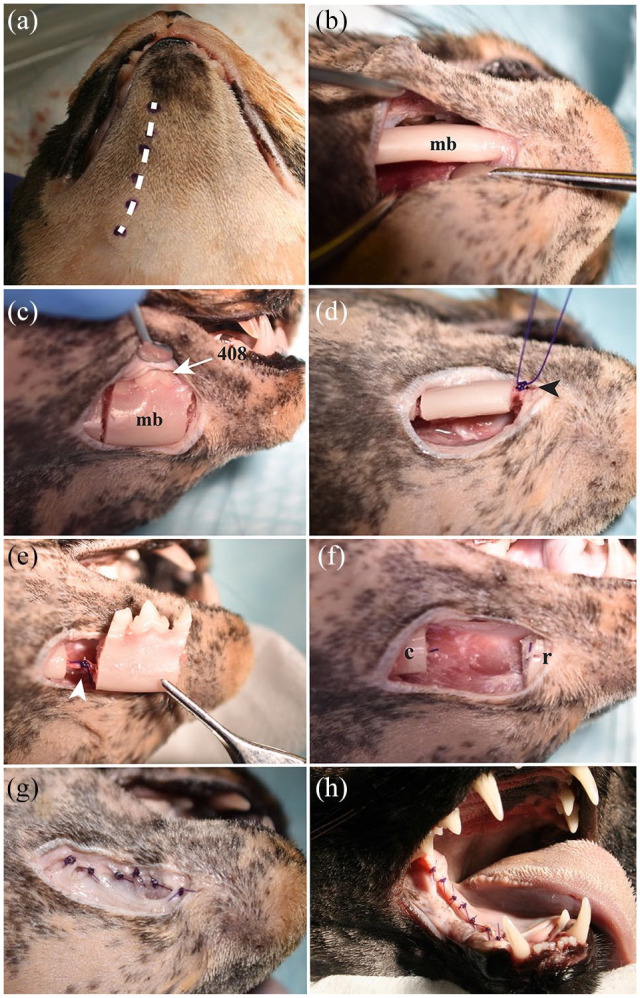
(a,b,d,g) Ventral, (c,e,f) lateral and (h) intraoral views of a cat head specimen showing the main features of the middle segmental mandibulectomy. (a) Planned full-thickness skin incision over the ventral margin of the mandible (dotted line). (b) The mandibular body (mb) was exposed between the third premolar and first molar teeth using a periosteal elevator. (c) Osteotomy of the mandibular body (mb) performed between the furcations of the third premolar and first molar teeth using a #700L carbide bur in a highspeed dental handpiece. Notice the right mandibular fourth premolar tooth (408) after removing the alveolar mucosa and gingival tissue. (d) Inferior alveolar neurovascular bundle ligature at the rostral aspect of the bone fragment, using a 4-0 monofilament suture (arrowhead). (e) Inferior alveolar neurovascular bundle ligature at the caudal aspect of the bone fragment (arrowhead). (f) Gap created between the rostral (r) and caudal (c) mandibular body segments. (g) Subcutaneous tissue apposition using an absorbable 4-0 monofilament suture in a simple interrupted pattern. (h) Intraoral mucosa apposition using an absorbable 5-0 monofilament suture in a simple interrupted pattern. Mild tension of the mucoperiosteal flap was observed along the caudal portion of the ‘ankylosed’ mandible
For the caudal segmental mandibulectomy, a 2–3 cm length full-thickness skin incision along the ventrocaudal aspect of the mandibular body was made. This incision allowed the lift of the lingual and vestibular soft tissues between the first molar tooth and the rostral margin of the mandibular foramen. The mandibular osteotomy was made between the first molar tooth′s furcation and the rostral aspect of the mandibular foramen. The remaining root tip of the first molar tooth was extracted using a winged dental elevator. Figure 8 depicts the most relevant features for the caudal segmental mandibulectomy.
Figure 8.
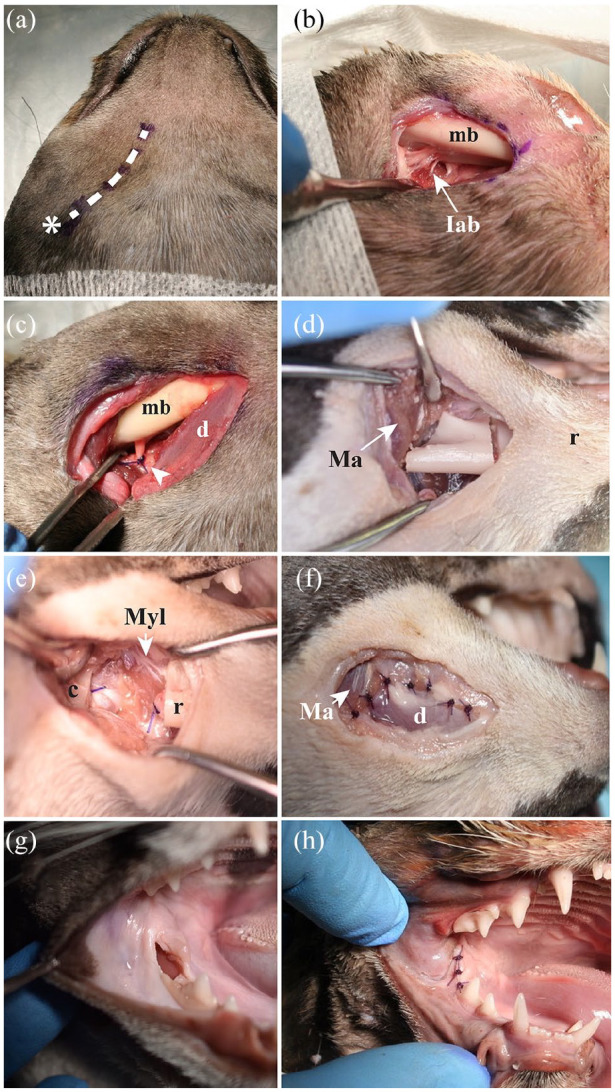
(a–c,f) Ventral, (d,e) lateral and (g,h) intraoral views of a cat head specimen showing the main features of the caudal segmental mandibulectomy. (a) Planned full-thickness skin incision over the ventral margin of the mandible, extending from the molar area to the angular process region (asterisk). (b) The mandibular body (mb) was exposed between the first molar tooth and the mandibular foramen using a periosteal elevator. The inferior alveolar bundle (Iab) was isolated before its entrance into the mandibular canal through the mandibular foramen. (c) Ligature of the inferior alveolar neurovascular bundle using a 3-0 monofilament absorbable suture material (arrowhead). The digastricus muscle (d) is separated and detached from the mandibular body. (d) Osteotomy of the mandibular body performed between the furcation of the first molar tooth and the rostral border of the mandibular foramen using a #700L carbide bur in a high-speed dental handpiece. The masseter muscle (Ma) is detached from the lateral aspect of the mandibular body and retracted caudally. r = rostral region of the mandible. (e) Gap created on the mandibular body. r = rostral; c = caudal; Myl = mylohyoid muscle. (f) Subcutaneous tissue apposition using an absorbable 4-0 monofilament suture in a simple interrupted pattern. (g) Minimal intraoral surgical wound. (h) Intraoral mucosa apposition using an absorbable 5-0 monofilament suture in a simple interrupted pattern. No tension of the mucoperiosteal flap was observed along the caudal portion of the ‘ankylosed’ mandible
As mentioned earlier, following the rostral, middle and caudal segmental mandibulectomies, the postoperative vertical range of mandibular motion was measured in all specimens as performed during the preoperative stage. The postoperative vertical range of mandibular motion values and the presence of any developing malocclusion were recorded for statistical purposes.
Statistical analysis
Power calculations were based on a normal assumption and performed using the observed standard deviations of the three groups in a pilot study performed in 12 specimens (data available on request). All statistical analyses were conducted in R version 3.6.2 (The R Statistical Computing). Treatments were compared pairwise via a Mann–Whitney–Wilcoxon test to determine if a statistically significant difference of 5% existed between the groups. Determination of differences in the head size, preoperative range of mandibular motion and size of the removed bone fragment was performed via a Kruskal–Wallis test. Response values were transformed to be the proportion of recovery of the presurgical vertical range of motion of the mandibles (postoperative vertical range of motion measurement divided by preoperative vertical range of motion measurement).
Results
The skull size and the preoperative vertical range of mandibular motion for the rostral, middle and caudal segmental mandibulectomies are given in Table 1. A significant statistical difference was observed for the skull size between the heads that underwent rostral and middle segmental mandibulectomy, and between the heads that underwent middle and caudal segmental mandibulectomy (P = 0.035). Although the vertical range of mandibular motion was associated with the head′s size, where the most prominent heads displayed the broadest range of motion values (Figure 9), no significant statistical difference was observed between the three proposed groups for the preoperative vertical range of mandibular motion.
Table 1.
Skull size and the preoperative vertical range of mandibular motion for the rostral, middle and caudal segmental mandibulectomies
| Rostral segmental mandibulectomy (n = 10) |
Middle segmental mandibulectomy (n = 10) |
Caudal segmental mandibulectomy (n = 10) |
P value | |
|---|---|---|---|---|
| Skull size (mm) | 8.62 ± 0.82 (7.5–10) | 8.00 ± 0.48 (7.1–9.1) | 8.52 ± 0.52 (7.9–9.5) | 0.035 |
| Preoperative vertical range of mandibular motion (mm) | 4.67 ± 0.85 (3.6–6.4) | 4.33 ± 0.46 (3.4–4.9) | 4.66 ± 0.61 (3.8–5.9) | 0.612 |
Data are mean ± SD (range). P <0.05 shows a significant statistical difference between the skull size
Figure 9.
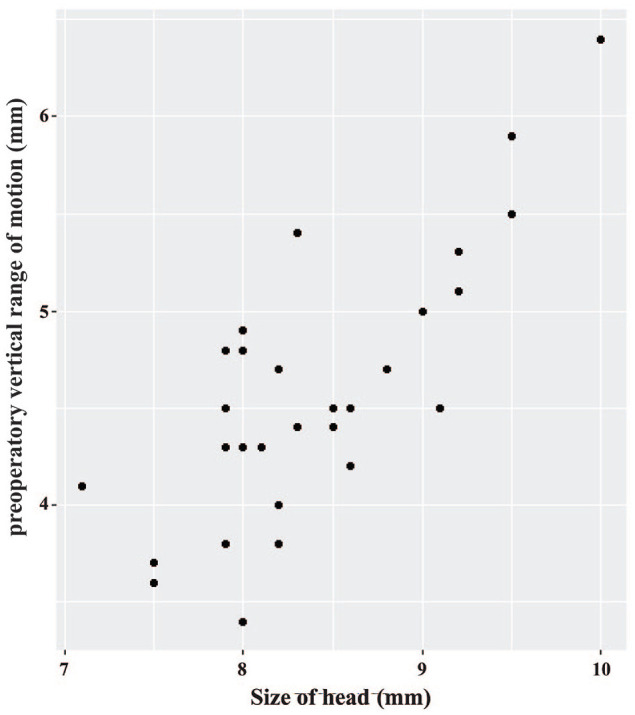
Relationship between the preoperative vertical range of mandibular motion and the size of the head. Larger heads showed a wider preoperative mouth opening; however, this was not statistically significant
Comparison of the pre- and postoperative vertical range of mandibular motion for the rostral, middle and caudal segmental mandibulectomies are summarized in Table 2. A significant statistical difference was observed between the pre- and postoperative vertical range of mandibular motion between the proposed mandibulectomies (P <0.001), where the caudal segmental mandibulectomy showed the highest vertical range of mandibular motion values.
Table 2.
Comparison of the pre- and postoperative vertical range of mandibular motion for the rostral, middle and caudal segmental mandibulectomies
| Segmental mandibulectomy | n | Preoperative vertical range of mandibular motion (mm) |
Postoperative vertical range of mandibular motion (mm) |
P value |
|---|---|---|---|---|
| Rostral | 10 | 4.67 ± 0.85 (3.6–6.4) | 2.29 ± 0.21 (2.1–2.8) | <0.001 |
| Middle | 10 | 4.33 ± 0.46 (3.4–4.9) | 3.55 ± 0.63 (2.5–4.5) | <0.001 |
| Caudal | 10 | 4.66 ± 0.61 (3.8–5.9) | 4.2 ± 0.61 (3.5–5.5) | <0.001 |
Data are mean ± SD (range). A significant statistical difference was observed between the pre- and postoperative vertical range of mandibular motion between the proposed mandibulectomies
A Wilcoxon rank-sum test showed that the caudal and middle mandibulectomies had a statistically significant better vertical range of mandibular motion than their rostral counterpart (P <0.001). This test also demonstrated that the caudal segemental mandibulectomy had a statistically significant better vertical range of mandibular motion than the middle segmental mandibulectomy (P = 0.034). Table 3 shows the recovery percentage of the vertical range of mandibular motion regarding the preoperative measurement in all groups. Of the proposed segmental mandibulectomies, the specimens that underwent caudal segmental mandibulectomy presented the highest postoperative percentage of recovery of range of mandibular motion (90.4%).
Table 3.
Percentage recovery of normal mouth opening when comparing pre- and postoperative vertical range of mandibular motion values in all groups
| Segmental mandibulectomy | Recovery of normal mouth opening (%) |
|---|---|
| Rostral | 50.4 |
| Middle | 81.9 |
| Caudal | 90.4 |
Although the present study demonstrated that the excised mandibular segments were associated with skull size (Figure 10), no significant statistical difference (P = 0.415) was evident between the sizes of the osteotomized rostral, middle and caudal mandibular segments (Table 4). On manual manipulation, all the specimens in this study presented postoperative lateral instability of the remaining non-ankylosed (contralateral) mandible.
Figure 10.
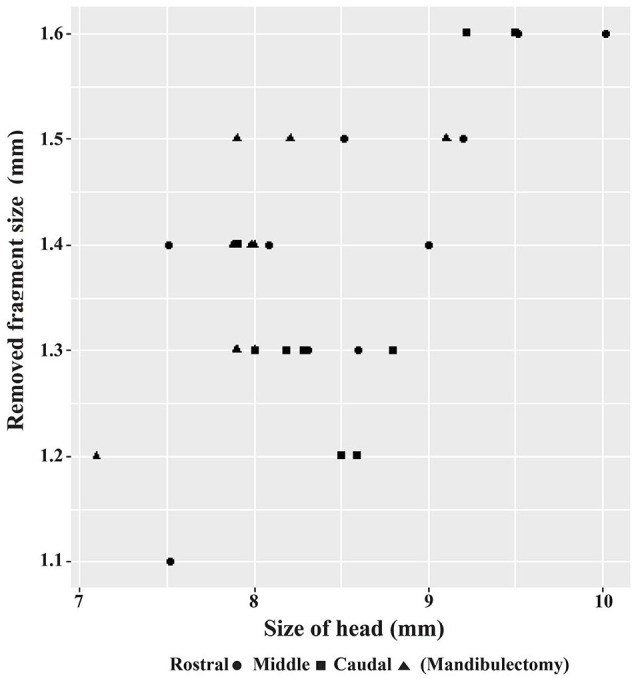
Relationship between the size of the removed segment of the mandible and the size of the head. Larger heads were associated with larger osteotomized bony segments; however, this was not statistically significant
Table 4.
Maximum and minimum sizes with respective means and standard deviations for the rostral, middle and caudal osteotomized segments
| Rostral segmental mandibulectomy (n = 10) |
Middle segmental mandibulectomy (n = 10) |
Caudal segmental mandibulectomy (n = 10) |
P value | |
|---|---|---|---|---|
| Fragment size (mm) |
1.41 ± 0.15 (1.1–1.6) | 1.38 ± 0.10 (1.2–1.5) | 1.35 ± 0.14 (1.2–1.65) | 0.415 |
Data are mean ± SD (range). P >0.05 shows no significant statistical difference between the segment sizes of the proposed mandibulectomies
Discussion
In this study, we compared three different regions of the mandibular body that underwent segmental mandibulectomy, as an alternative to surgical excision of the ankylosed tissue, for the treatment of unilateral TMJA. Although the segmental mandibulectomy has been recommended as an alternative treatment for both extensive and less invasive TMJA disorders,22,23 to our knowledge, no studies have been performed to establish the region of the mandibular body and the amount of bony tissue that has to be removed. Our research describes and compares the segmental mandibulectomy performed on three different regions of the mandibular body as an alternative for conventional surgical techniques to treat unilateral TMJA in cats.
We demonstrated that caudal segmental mandibulectomy was the procedure that allowed us to open the mouth closest to the preoperative vertical range of mandibular motion, with 90.4% recovery of normal mouth opening. The second best procedure was middle segmental mandibulectomy (81.9% recovery). Mandibulectomies performed rostral to the third premolar tooth showed a significantly decreased vertical range of mandibular motion (50.4% recovery) compared with middle and caudal segmental mandibulectomies.
The fact that the rostral segmental mandibulectomy showed a decreased vertical range of mandibular motion was associated with tension over the mucoperiosteal flap. While opening the mouth, most of the lingual alveolar mucosa remained attached to the remaining ‘ankylosed’ mandible, decreasing the flap′s elasticity, as seen in Figure 6. In a clinical setting, the tension generated while opening the mouth would cause poor wound healing, dehiscence and failure of the surgical procedure. Our findings show that the extent of mouth opening varies significantly depending on which portion of the mandibular body is resected.
Our study showed a relationship between head size and the preoperative vertical range of mandibular motion, with larger heads presenting the highest vertical range of mandibular motion values. A possible limitation of this study is that our research used cadaver heads, and thus the findings reported here could be different from those obtained from clinical patients where the masticatory muscles would show different physical characteristics. However, the vertical range of mandibular motion values may be used as a reference in the clinical setting in cats.
In line with previous literature,22,23 the segmental mandibulectomies proposed in this study allowed us to open the mouth at different levels. All three techniques showed a significant statistical difference when comparing the preoperative and postoperative vertical range of mandibular motion values. The preoperative range of mandibular motion was not fully recovered after performing the suggested mandibulectomies.
Excision of a minimum of 1.2 cm in length of the mandible was necessary to achieve a nearly full recovery of the preoperatory vertical range of mandibular motion during the caudal segmental mandibulectomy. Although it is possible that removing bony fragments larger than 1.2 cm in length may be unnecessary, this assumption must be validated in a clinical setting as bone regeneration during the healing process could promote reconsolidation of the remnant segments at the osteotomized area. Bone proliferation along the surgical gap could reduce the range of motion or cause the inability to open the mouth during the postoperative period.
As proposed previously, 23 an extraoral ventral approach was performed to expose the mandibular body for the middle and caudal segmental mandibulectomies. During the caudal mandibulectomy, this approach easily allowed exposure and isolation of the inferior alveolar neurovascular bundle before its entrance into the mandibular canal through the mandibular foramen. Preemptive ligation of this bundle can prevent intraoperative hemorrhage during the mandibular body osteotomy.
If abnormal tissue from the TMJA extends into the caudal aspect of the mandibular body and the extraoral ventral approach is not feasible, an extraoral lateral approach through a lateral buccotomy could be performed. For this approach, a full-thickness incision through the skin, subcutaneous tissue and masseter muscle is performed to expose the mandibular body′s most caudal aspect. Special care should be taken to avoid iatrogenic injury to the parotid duct, dorsal and ventral branches of the facial nerve, and vasculature.
While it has previously been recommended to perform osteotomy between teeth during mandibulectomies,24,25 we elected to perform it through the furcation of teeth to avoid accidental damage of adjacent teeth.26,27 After extracting the root remnants, the sectioned tooth′s alveolar margin served as support for the sutured mucoperiosteal flap, thus reducing the occurrence of dehiscence and improving the healing process in the clinical patient. Although the caudal mandibulectomy proposed in this study could be performed caudally to the first molar tooth, the bony defect created might not be sufficient, and a bone bridge could form between the osteotomized margins owing to the healing process. New studies are needed in patients to test whether the segment removed caudally from the first molar tooth would be sufficient to prevent postoperative bone consolidation.
Osteotomy of the mandibular segments was efficiently achieved at the proposed sites of the mandibular body using a carbide bur sited in a high-speed dental handpiece. In concordance with previous literature, this technique allowed precise cuts in a timely manner without causing major iatrogenic damage to adjacent soft tissue.28–30 Recently, piezoelectric bone surgery technology has been recommended in maxillofacial surgery in veterinary patients due to its ability to selectively cut mineralized tissue in delicate places, significantly reducing the trauma to underlying soft tissue (eg, neurovascular bundle, muscles, etc).21,31
As previously reported in patients that underwent gap arthroplasty or condylectomy,9,12,21 all specimens in the present study showed mandibular drifting after performing segmental mandibulectomy. This postoperative complication is considered acceptable in clinical cases; as the patient can eat, drink and groom itself, selective dental extractions may be required to avoid further oral soft tissue trauma.
Our study focused on cats affected by unilateral TMJA, where the proposed mandibulectomies allowed opening of the mouth without presenting a hanging effect of the rostral portion of the remaining mandibular body. In this study, the contralateral mandible (not osteotomized) supported the remaining contralateral fragment while opening and closing the mouth.
The bilateral segmental mandibulectomy may not be a suitable surgical procedure for cats affected by bilateral TMJA owing to a hanging effect of the mandibles′ remaining rostral portion. In cats affected by bilateral TMJA, in which gap arthroplasty or condylectomy may not be a safe procedure because of the risk of iatrogenic damage of neurovascular structures, 23 the hanging effect of the rostral portion of the mandibles caused by a caudal segmental bilateral mandibulectomy could be avoided or decreased by performing a bilateral commissurorraphy; however, this assumption must be tested in a future studies.
A limitation of our research is the use of cadaver heads instead of clinical patients. Although we believe that our findings would reflect the surgical outcome in clinical patients affected by unilateral TMJA, the postoperative extent of mouth opening may be less in feline patients with TMJA due to atrophy of the masticatory muscles that may have developed as a consequence of the chronic effect of the ankylosis.
From the results of this study, it is clear that the more caudal the segmental mandibulectomy is performed, the more vertical range of mandibular motion is observed. New studies are necessary to compare the proposed caudal segmental mandibulectomy with gap arthroplasty or condylectomy as these surgical procedures are performed more caudally than the caudal segmental mandibulectomy and they may offer a better outcome in terms of recovery or improvement of the vertical range of mandibular motion.
Caudal segmental mandibulectomy is a viable surgical technique that may eliminate the risk of iatrogenic periarticular neurovascular damage inherent to more invasive instrumentation at the TMJ area. Since more bony tissue can be excised from the mandibular body during a caudal segmental mandibulectomy, this surgical technique may decrease TMJA recurrence associated with exuberant bony proliferation at the articular region during the healing process; however, this assumption needs to be proved in clinical cases.
Conclusions
We described and compared unilaterally performed segmental mandibulectomies for the treatment of unilateral TMJA in cat cadavers. From the three proposed mandibular body regions to be excised, the caudal segmental mandibulectomy was the procedure that showed the highest postoperative vertical range of mandibular motion. The removal of a minimum of 1.2 cm of the mandibular body was required to achieve nearly full recovery of the presurgical mouth opening.
Footnotes
Accepted: 5 November 2020
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not necessarily required.
Informed consent: This work did not involve the use of animals and therefore informed consent was not required. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Lenin A Villamizar-Martinez  https://orcid.org/0000-0002-3604-6807
https://orcid.org/0000-0002-3604-6807
References
- 1. Lantz G. Temporomandibular joint ankylosis: surgical correction of three cases. J Am Anim Hosp Assoc 1985; 21: 173–177. [Google Scholar]
- 2. Gatineau M, El-Warrak AO, Marretta SM, et al. Locked jaw syndrome in dogs and cats: 37 cases (1998–2005). J Vet Dent 2008; 25: 16–22. [DOI] [PubMed] [Google Scholar]
- 3. Sullivan M. Temporomandibular ankylosis in the cat. J Small Anim Pract 1989; 30: 401–405. [Google Scholar]
- 4. Tomlinson J, Presnell K. Mandibular condylectomy effects in normal dogs. Vet Surg 1983; 12: 148–154. [Google Scholar]
- 5. Maas CP, Theyse LF. Temporomandibular joint ankylosis in cats and dogs: a report of 10 cases. Vet Comp Orthop Traumatol 2007; 20: 192–197. [PubMed] [Google Scholar]
- 6. Gemmill T. Conditions of the temporomandibular joint in dogs and cats. In Pract 2008; 30: 36–43. [Google Scholar]
- 7. Ferretti C, Bryant R, Becker P, et al. Temporomandibular joint morphology following post-traumatic ankylosis in 26 patients. Int J Oral Maxillofac Surg 2005; 34: 376–381. [DOI] [PubMed] [Google Scholar]
- 8. Yan YB, Liang SX, Shen J, et al. Current concepts in the pathogenesis of traumatic temporomandibular joint ankylosis. Head Face Med 2014; 10: 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meomartino L, Fatone G, Brunetti A, et al. Temporomandibular ankylosis in the cat: a review of seven cases. J Small Anim Pract 1999; 40: 7–10. [DOI] [PubMed] [Google Scholar]
- 10. Larguier L, Jamet N. False ankylosis of the temporomandibular joint in a cat. Correction by partial zygomatic arch resection. Vet Comp Orthop Traumatol 2015; 6: 455–458. [DOI] [PubMed] [Google Scholar]
- 11. Strøm PC, Arzi B, Cissell DD, et al. Ankylosis and pseudoankylosis of the temporomandibular joint in 10 dogs (1993–2015). Vet Comp Orthop Traumatol 2016; 29: 409–415. [DOI] [PubMed] [Google Scholar]
- 12. Villamizar LA, Kowalesky J, Fugita M, et al. Anquilosis temporomandibular en un gato Persa. Sel Vet 2013; 21: 51–59. [Google Scholar]
- 13. Villamizar LA, Villegas CM, Gioso MA, et al. Morphologic and morphometric description of the temporomandibular joint in the domestic dog using computed tomography. J Vet Dent 2016; 33: 75–82. [DOI] [PubMed] [Google Scholar]
- 14. Arzi B, Cissell DD, Verstraete FJ, et al. Computed tomographic findings in dogs and cats with temporomandibular joint disorders: 58 cases (2006–2011). J Am Vet Med Assoc 2013; 242: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heney CM, Arzi B, Kass PH, et al. Diagnostic yield of dental radiography and cone-beam computed tomography for the identification of anatomic structures in cats. Front Vet Sci 2019; 6. DOI: 10.3389/fvets.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zavodovskaya R, Vapniarsky N, Garcia T, et al. Intra- and extra-articular features of temporomandibular joint ankylosis in the cat (Felis catus). J Comp Pathol 2020; 175: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Talmaceanu D, Lenghel LM, Bolog N, et al. Imaging modalities for temporomandibular joint disorders: an update. Clujul Med 2018; 91: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Macready DM, Hecht S, Craig LE, et al. Magnetic resonance imaging features of the temporomandibular joint in normal dog. Vet Radiol Ultrasound 2010; 51: 436–440. [DOI] [PubMed] [Google Scholar]
- 19. Rajan R, Reddy NV, Potturi A, et al. Gap arthroplasty of temporomandibular joint ankylosis by transoral access: a case series. Int J Oral Maxillofac Surg 2014; 43: 1468–1472. [DOI] [PubMed] [Google Scholar]
- 20. Anderson MA, Orsini PG, Harvey CE. Temporomandibular ankylosis: treatment by unilateral condylectomy in two dogs and two cats. J Vet Dent 1996; 13: 23–25. [Google Scholar]
- 21. Aghashani A, Verstraete FJM, Arzi B. Temporomandibular joint gap arthroplasty in cats. Front Vet Sci 2020; 7: 482. DOI: 10.3389/fvets.2020.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Villamizar LA, Reiter A. Caudal segmental mandibulectomy as alternative treatment for unilateral temporomandibular joint ankylosis in two cats and one dog. Proceedings of the 31st Veterinary Dental Forum; 2017 Sept 14–17; Nashville TN, USA. Meridian, ID: Foundation for Veterinary Dentistry, 2017, p 11. [Google Scholar]
- 23. Arzi B. Temporomandibular joint ankylosis and pseudoankylosis. In: Verstraete F, Lommer MJ, Arzi B. (eds). Oral and maxillofacial surgery in dogs and cats. 2nd ed. St Louis, MO: Elsevier, 2020, pp 377–382. [Google Scholar]
- 24. Verstraete JM, Arzi B, Lantz C. Mandibulectomy techniques. In: Verstraete F, Lommer MJ, Arzi B. (eds). Oral and maxillofacial surgery in dogs and cats. 2nd ed. St Louis, MO: Elsevier, 2020, pp 515–528. [Google Scholar]
- 25. Arzi B, Verstraete JM. Mandibular rim excision in seven dogs. Vet Surg 2010; 39: 226–231. [DOI] [PubMed] [Google Scholar]
- 26. Romanelli G, Lewis J. Management of oral and maxillofacial neoplasia. In: Reiter A, Gracis M. (eds). BSAVA manual of canine and feline dentistry and oral surgery. 4th ed. Gloucester: British Small Animal Veterinary Association, 2018, pp 290–300. [Google Scholar]
- 27. Walker K, Reiter AM, Lewis J. Marginal mandibulectomy in the dog. J Vet Dent 2009; 26: 194–198. [DOI] [PubMed] [Google Scholar]
- 28. Villamizar-Martinez LA, Reiter AM, Sánchez MD, et al. Benign cementoblastoma (true cementoma) in a cat. JFMS Open Rep 2016; 2. DOI: 10.1177/2055116915626847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walker KS, Reiter AM, Tzt D, et al. Marginal mandibulectomy in the dog. J Vet Dent 2009; 26: 194–198. [DOI] [PubMed] [Google Scholar]
- 30. Lobprise HB, Soukup J. Oral surgery – oral and maxillofacial tumors. In: Lobprise H, Dodd J. (eds). Wiggs’s veterinary dentistry principles and practice. Hoboken, NJ: John Wiley & Sons, 2019, pp 289–309. [Google Scholar]
- 31. Hennet P. Piezoelectric bone surgery: a review of the literature and potential applications in veterinary oromaxillofacial surgery. Front Vet Sci 2020; 2: 8. DOI: 10.3389/fvets.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]