A comparative approach spanning human, veterinary and wildlife medicine may allow us to more fully draw the broad comparisons necessary to solve the public health crisis of COVID-19 and prepare for future pandemics.
In the context of the ongoing COVID-19 pandemic, comparative medicine can be a powerful approach to help refine our understanding of coronavirus pathophysiology, vaccine efficacy and pharmaceutical interventions. With feline infectious peritonitis (FIP), in particular, coronaviruses have been recognized to be important causes of disease in animal species for many decades, in some cases being associated with the enigmatic outcomes now recognized for COVID-19 in humans. Cats and other animals have also risen to our attention as hosts for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Endotheliitis and vasculitis are becoming established as a component of systemic disease caused by SARS-CoV-2, including in children who develop multisystem inflammatory syndrome (MIS-C). 1 A case series has also described an equivalent multisystem inflammatory syndrome in adults (MIS-A), which has included a variety of extrapulmonary disease manifestations. 2 By analogy, feline coronavirus (FCoV) is well known for its ability to cause FIP, which is also a multisystem inflammatory syndrome of cats – although classically defined as either effusive (fluid accumulation in any body cavity) or non-effusive (development of granulomatous to pyogranulomatous lesions across body systems). 3 The multisystem signs are only more recently becoming recognized as connected to FCoV infection. 4 FIP most frequently occurs in younger cats, though all age groups are susceptible. A defining feature of FIP is the invasion of the macrophage, where mutations in the spike protein contribute to the cellular tropism of the virus. Antibody-dependent enhancement (ADE) is considered a possible mechanism underlying the development of FIP. ADE has not been considered a main mechanism driving COVID-19 pathology and SARS-CoV-2 replication in macrophages appears limited;5,6 however, these do remain open and unresolved questions.
Cats are also susceptible to SARS-CoV-2, both experimentally and in the community.7,8 Feline infections may be quite common, but clinical signs appear to consist of only mild respiratory signs. Cats seroconvert and can transmit to other animals, but the role of cats in spreading infection in the community appears to remain minor. Another set of susceptible animals are mustelids, including ferrets and mink. In particular, the spread of the virus between mink and humans has been observed – termed ‘spill-back’ – causing a public health crisis in Denmark. 9 Exposure of mink to SARS-CoV-2 from handlers in farms led to widespread infection. In many cases, the mink developed severe interstitial pneumonia and diffuse alveolar damage with systemic signs, including ‘hepatic lipidosis, chronic nephritis, sepsis, dystocia and urolithiasis’. 10 Experimental challenge of the closely related domestic ferret species has failed to yield severe signs, with ferrets typically responding like cats. As with cats, both mink and ferrets harbor their own coronaviruses, with ferrets showing a notable systemic outcome consisting of an FIP-like disease, but with less vasculitis.11 The impact of coinfection with human and animal coronaviruses in this set of susceptible species, including clinical presentation or viral recombination, is presently unclear.
The term ‘One Medicine’ was coined by Rudolf Virchow in the late 19th century 12 and this remains a cornerstone of 21st century pathology. Virchow is perhaps better associated with the eponymous ‘triad’ comprising the components of venous thrombosis, including venous stasis, activation of blood coagulation and endothelial damage. This triad is a contributor to the multisystem signs and symptoms in both COVID-19 and FIP. One Medicine also forms the foundation of the widely publicized ‘One Health’ triad of people, animals and the environment. The environment is key to SARS-CoV-2 spillover events, whether this is local (eg, in mink farms) or global (eg, in bats and their intermediate host species).
As with Virchow in the 19th century, we should today examine coronaviruses of both humans and animals using a comparative approach spanning human, veterinary and wildlife medicine – incorporating the two distinct triads that Virchow inspired (Figure 1). This may allow us to more fully draw the broad comparisons necessary to solve the public health crisis of COVID-19 and prepare for future pandemics.
Figure 1.
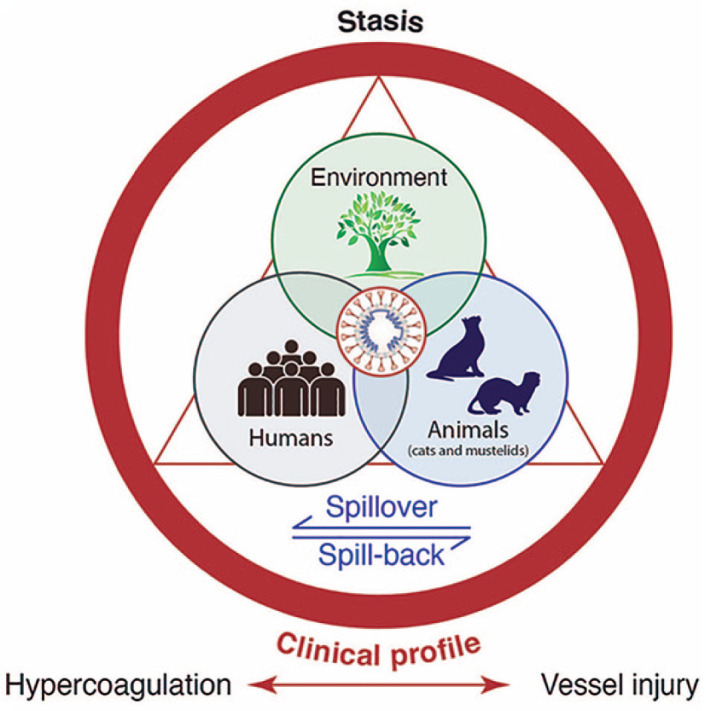
The intersection of the One Health triad with Virchow’s triad for coronavirus disease
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: AES is supported by NIH Comparative Medicine Training Program T32OD011000. Work in the authors’ laboratory is funded in part by the Cornell Feline Health Center and the EveryCat Health Foundation (formerly Winn Feline Foundation).
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not specifically required for publication in JFMS.
Informed consent: This work did not involve the use of animals and therefore informed consent was not required. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
References
- 1. Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol 2020; 20: 453–454. DOI: 10.1038/s41577-020-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection – United Kingdom and United States. MMWR Morb Mortal Wkly Rep 2020; 69: 1450–1456. DOI: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pedersen NC. A review of feline infectious peritonitis virus infection: 1963–2008. J Feline Med Surg 2009; 11: 225–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kipar A, Meli ML. Feline infectious peritonitis: still an enigma? Vet Pathol 2014; 51: 505–526. [DOI] [PubMed] [Google Scholar]
- 5. Hui KPY, Cheung M-C, Perera RAPM, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med 2020; 8: 687–695. DOI: 10.1016/S2213-2600(20)30193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li D, Edwards RJ, Manne K, et al. The functions of SARS-CoV-2 neutralizing and infection-enhancing antibodies in vitro and in mice and nonhuman primates [preprint]. bioRxiv. Published online 2 January 2021. DOI: 10.1101/2020.12.31.424729. [DOI] [Google Scholar]
- 7. Stout AE, André NM, Jaimes JA, et al. Coronaviruses in cats and other companion animals: where does SARS-CoV-2/COVID-19 fit? Vet Microbiol 2020; 247: 108777. DOI: 10.1016/j.vetmic.2020.108777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patterson EI, Elia G, Grassi A, et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nat Commun 2020; 11: 6231. DOI: 10.1038/s41467-020-20097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koopmans M. SARS-CoV-2 and the human-animal interface: outbreaks on mink farms. Lancet Infect Dis 2021; 21: 18–19. DOI: 10.1016/S1473-3099(20)30912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molenaar RJ, Vreman S, Hakze-van der Honing RW, et al. Clinical and pathological findings in SARS-CoV-2 disease outbreaks in farmed mink (Neovison vison). Vet Pathol 2020; 57: 653–657. [DOI] [PubMed] [Google Scholar]
- 11. Stout AE, Guo Q, Millet JK, et al. Coronaviruses associated with the superfamily Musteloidea. MBio 2021; 12: e02873–20. DOI: 10.1128/mBio.02873-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schultz M. Rudolf Virchow. Emerg Infect Dis 2008; 14: 1480–1481. DOI: 10.3201/eid1409.086672. [DOI] [Google Scholar]


