Abstract
Practical relevance:
An infertile tom used to be a relatively rare presentation in feline practice. However, as a result of breeding strategies targeting specific morphological/behavioural traits, and the influence of inbreeding (especially practised in rare breeds), among other factors, feline infertility has become a genuine clinical problem. Furthermore, increased interest among cat breeders and pet owners in diagnosing and treating an ‘unsuccessful’ tom (including using assisted reproductive techniques [ARTs]), has made such patients more commonplace in veterinary practice.
Clinical challenges:
A definitive cause for male infertility is often difficult to identify. One of the biggest challenges is the lack of fertility-based reference values for semen quality and hormone levels. Moreover, the literature in this field is scant and many reproductive tract conditions described in other species have not been reported in cats. The establishment of diagnostic tools and algorithms is essential to facilitate a reliable assessment of fertility potential.
Global importance:
There is a growing demand for appropriate veterinary care relating to feline reproduction. There is an expectation among breeders, particularly of pedigree cats of high breeding value, that the same options widely used for dogs (including ARTs) should also be available for cats.
Equipment and technical skills:
Equipment required for investigating male infertility includes a microscope and ultrasound machine; a blood analyser may also be useful, but samples can instead be sent to a laboratory. The skills required are often already performed by veterinarians; for example, catheterising the urethra, performing ultrasonography and blood sampling.
Evidence base:
There are some reports of fertility problems in male cats in the literature, but this area of feline reproduction is still relatively undeveloped. This review draws on the comprehensive knowledge developed and shared by specialists in this field, and is supported by the authors’ own clinical experience.
Keywords: Male infertility, mating disorders, libido problems, disorders of sexual development, poor semen quality, semen assessment, hormonal treatment, pharmacological treatment
Introduction
Until recently, very little attention had been paid to the role of the tom cat in reproductive failure. There is only one original article on this subject available in the scientific literature, 1 and in reviews and veterinary textbooks, the female cat or male dog are the main focus of attention, with typically only a few paragraphs devoted to the torn.2–5 It is likely that diagnostic challenges (trouble with semen collection in cats) and limited treatment options have contributed to this situation; however, since the introduction of urethral semen collection, which has partially addressed the first issue, 6 the authors have observed that infertile toms have become increasingly frequent patients in their clinic. In this review, the authors share their experience of how they deal with fertility problems in the male cat, and highlight the challenges that remain to be addressed.

Classification of infertility
There are several different classifications of infertility in the tom, which are listed in the box below, and are crucial in defining the problem.
Defining the problem
In order to identify and characterise a fertility problem, clinicians should try to answer the following three key questions.
Does the problem genuinely concern the male?
First, in the case of reproductive failure, it is important to establish whether the problem does, in fact, concern the male. Reproduction is an interaction between individuals of two sexes, and lack of success may lay with the male, female or both. Therefore, if possible, the female should also be examined or female infertility should be ruled out (eg, by breeding the female with another male). Infertility in the queen is the focus of an accompanying review in this series. 7
Is infertility primary or acquired?
Second, an important aspect when dealing with male reproductive problems is to determine whether infertility is primary (an adult tom that has never sired offspring despite several attempts) or secondary (loss of fertility in a previously fertile tom).
Is the problem with copulation or fertilisation?
Third, another important step is to identify whether male infertility is a result of an inability to inseminate a female cat (to mate and to introduce spermatozoa into the female genital tract), that is, a mating disorder; or an inability to fertilise a female cat in the case of inadequate sperm production and/or poor sperm quality.
Problems of sperm delivery vs sperm production/quality require different treatment approaches and identification of the problem at the outset of the diagnostic process is therefore critical. In some cases of testicular dysfunction, both types of infertility may be present – low testosterone levels may lead to simultaneous lack of libido and spermatogenic arrest.
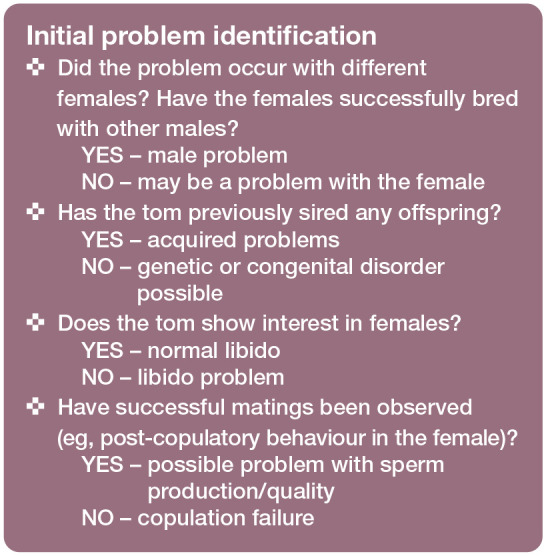
The ‘Initial problem identification’ box lists questions that should not be overlooked during history-taking to help define the problem.
Causes of male infertility
Problems with mating; failure to copulate
According to Fontaine, 8 mating disorders, whether male or female, account for the majority of cases of feline infertility, and this is confirmed by the current authors’ own observations.
Problems with mating can be subdivided into two categories: (1) the tom shows no interest in copulation (libido problem); or (2) the tom tries to copulate, but fails (anatomical/physical problem or inexperience).
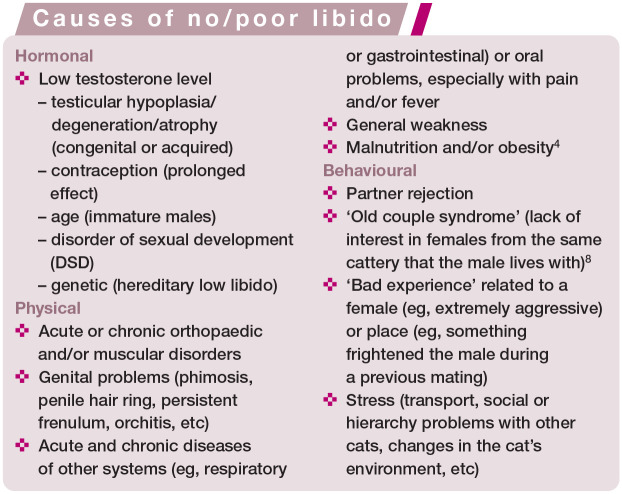
Libido problems
A tom may show a complete lack of interest in females (loss of libido), or libido may be only partially diminished (low/poor libido). Problems with libido may have a hormonal (low testosterone level), physical (diseases of any systems or part of the body, causing pain, discomfort and/or fever) or behavioural origin (see the box below). The male may also have hereditary low libido, with normal testosterone levels in the blood; 1 such males should be excluded from breeding. 4
Anatomical/physical problems
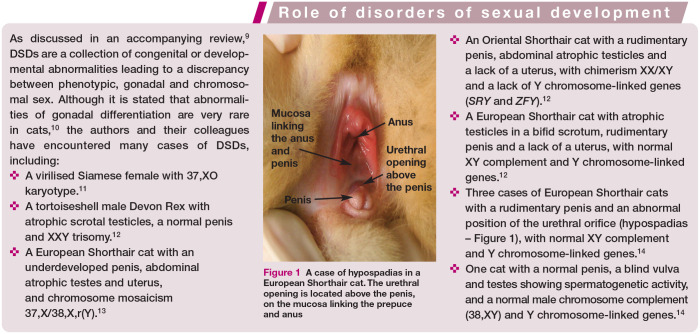
Anatomical causes of problems with mating may be congenital or acquired. Congenital problems include abnormalities of the external genitalia connected with DSDs (see box above) such as hypospadias (Figure 1), persistent penile frenulum, abnormal prepuce (Figure 2) or double penis. Acquired causes include hair rings, trauma, inflammation, neoplasia and accumulation of smegma (Figure 3).1,10 Diseases affecting the penis and prepuce are rare in toms. 5 The most common problem is twisting of hair around the prepuce in longhaired breeds.
Figure 1.
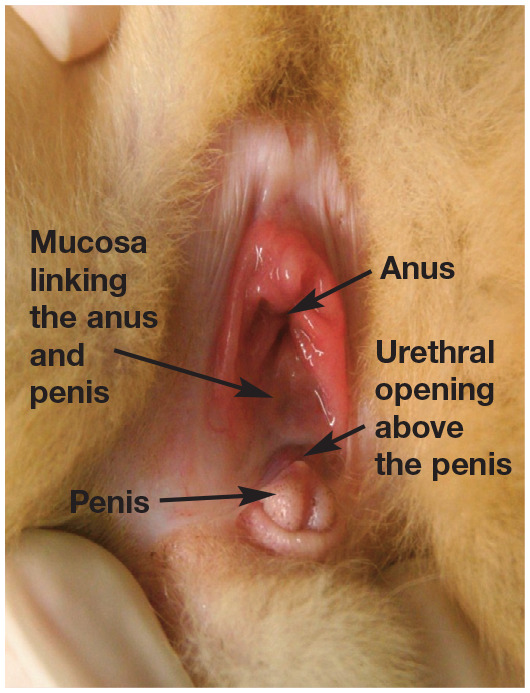
A case of hypospadias in a European Shorthair cat. The urethral opening is located above the penis, on the mucosa linking the prepuce and anus
Figure 2.
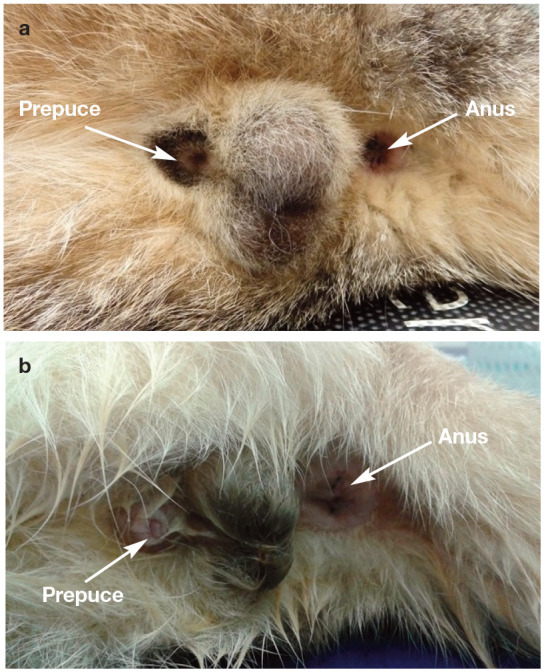
(a) Normal prepuce covering the whole penis. (b) Abnormal prepuce, not covering the whole penis, predisposing to drying of the penile mucosa and inflammation
Figure 3.
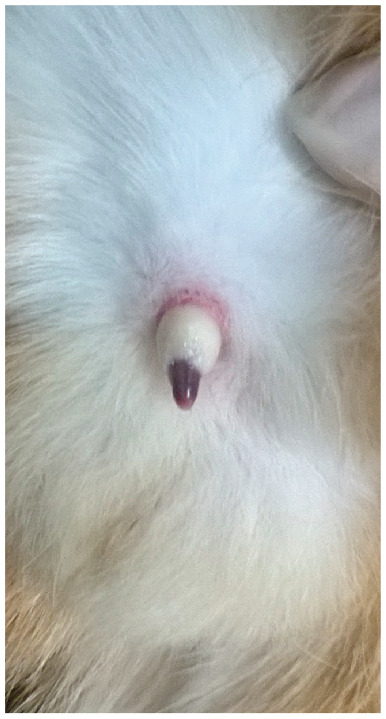
Accumulation of smegma around the penis. Exteriorisation of the penis could only be achieved under anaesthesia
Veterinarians need to keep in mind that anatomical obstacles preventing proper copulation may be related to other parts of the body, notably the musculoskeletal system (especially the hindlimbs and spine) and oral cavity (during mating, the male cat grabs the skin on the female’s neck). 8 Severe obesity may also make mating physically impossible, as the male may be unable to mount the female and flex appropriately to insert the penis, or may be too heavy for a small female.
Inexperience
Mating problems may also be due to a lack of reproductive experience of the male. A male may try to mount the female, but does so ineptly and does not achieve intromission. This affects mostly young males, but in some breeds (eg, Persian, Exotic Shorthair, British Shorthair) sexual maturity may only be achieved at the age of 3 years or older. 4
Failure to fertilise the egg
If mating and/or a queen’s ‘after reaction’ are observed, thus confirming intromission, then the fertility problem may be due to the sperm failing to fertilise the egg. There are two possible explanations for this condition: (1) absent or low quality sperm production; or (2) sperm is produced but cannot be expelled (ejaculatory disorders).
Impaired sperm production/quality
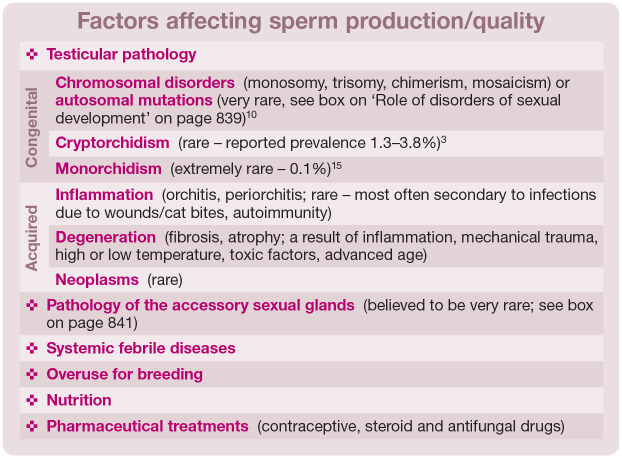
Problems with semen quality may be due to impaired spermatogenesis or the result of factors acting on sperm cells outside the testicle (eg, during epididymal maturation or even during ejaculation); may be congenital or acquired; and can be caused by multiple factors (see box above). Depending on the nature, severity and duration of the factor(s) involved, the loss of fertility may be permanent (congenital disorders, severe or chronic damage) or temporary (mild changes). For the latter, return to fertility can be observed about 2 months after the resolution of the underlying factor. This is explained by the duration of spermatogenesis in cats, which is approximately 47 days. 15
✜ Congenital problems Male infertility may have a genetic basis. Cases of DSDs with chromosomal disorders such as monosomy, trisomy, chimerism and mosaicism, or autosomal mutations, have been described in the literature, most often leading to inhibition of spermatogenesis and aspermia.1,10–14 Individuals with DSDs are often easily identifiable due to gonadal hypoplasia or aplasia and/or ambiguous external genitalia (see box on page 839 and Figure 1). Sometimes the only sign of an abnormal karyotype (XXY or XX/XY) is a male with a tortoiseshell/calico coat. Developmental defects related to the reproductive system include cryptorchidism (ie, failure of one or both testes to reach the scrotum by 7-8 months of age). In cats, this phenomenon is described less frequently than in dogs, with a prevalence of about 1.3-3.8%, 3 and it is more common in purebred individuals, especially Persians. 16 Unilateral inguinal cryptorchidism is most commonly observed. 3 Depending on the position of the retained testicle(s), either complete infertility (bilateral abdominal cryptorchidism) or reduced semen quality with low fertility (unilateral or testicles under the skin of the groin) can be observed. 3 Such cats should be excluded from breeding. The presence of only one testicle (monorchidism) is extremely rare (prevalence 0.1% 15 ).
✜ Acquired problems The most common causes of poor sperm quality are acquired conditions of the reproductive system – trauma, inflammation or degeneration – often occurring simultaneously or sequentially. Inflammation of the testicles (orchitis) is relatively rare in cats and the aetiology is poorly understood. It can develop after trauma and scrotal wounds (eg, bites) penetrating deep into the tissue. 5 Necrotic orchitis owing to feline infectious peritonitis has been reported. 17 Based on what is known in other species, bacterial infections can also be considered a potential cause, albeit no reports of infertility due to specific infections (eg, Mycoplasma or Chlamydia species) is available for toms. In addition to diseases directly affecting testicles, disturbed spermatogenesis and reduced sperm quality can also be sequelae of diseases of other systems where there is accompanying fever.
In terms of non-infectious factors, damage to testicular tissue may occur due to mechanical trauma, high/low temperature or toxic insults. Autoimmune inflammation is often reported in humans; 18 however, there are no confirmed cases of this pathology in cats. Testicular degeneration (atrophy and/or fibrosis) is more common, and may be the result of chronic inflammation (often unnoticed by owners) or age-related changes. 19 Inflammation of the tissues surrounding the testicle (periorchitis) can also lead to necrosis, infarction or atrophy of the testicle. 5 Testicular tumours in cats are occasionally observed, tending to occur in undescended testes. 20
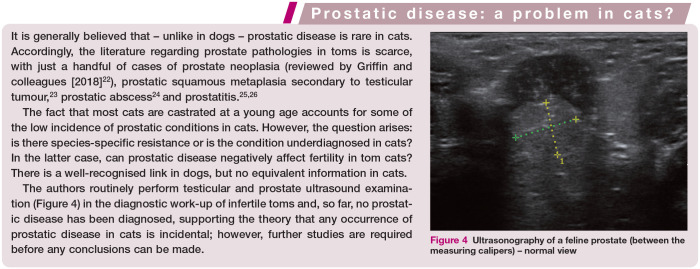
In other species, such as the dog, pathology of the accessory sex glands is an important factor that negatively affects the quality of sperm. Cats have two types of accessory sex glands – the prostate and bulbourethral glands (see accompanying review on normal reproduction in the tom 21 ) – but pathology of these organs has only been reported in isolated cases, 5 and any impact on fertility in affected cats is unknown (see box on page 841).
There is little information regarding the relationship between nutrition and sperm quality in male cats. Linoleic acid deficiency in the diet leads to degenerative changes in the testes and reduced sperm count in the epididymis. 27 Moreover, hypervitaminosis A can lead to permanent degeneration of the testicles. 20 Unlike queens, a diet low in arachidonic acid does not impair male cat fertility. 27
Figure 4.
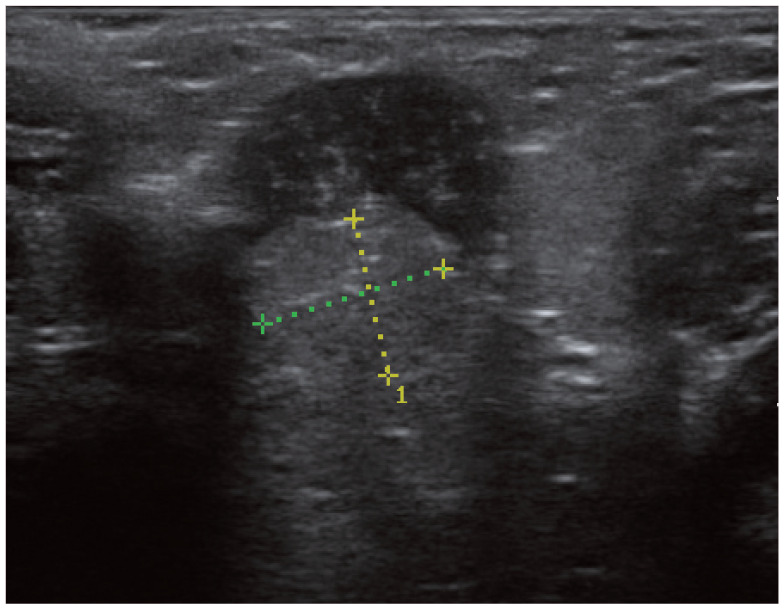
Ultrasonography of a feline prostate (between the measuring calipers) – normal view
Toms that receive a slow-release 4.7 mg deslorelin implant to inhibit spermatogenesis can sometimes take longer than expected to recover fertility (up to 25 months),28,29 and the owner should be informed about this possibility. Among the non-reproductive drugs that negatively affect spermatogenesis, the most frequently mentioned are antifungal preparations and steroids.
Ejaculation disorders
In rare cases, sperm is produced normally but cannot be expelled due to obstruction or aplasia of the ductus deferens, 5 or retroejaculation (instead of being ejaculated, sperm cells pass into the bladder and are found in large quantities in the urine). Retroejaculation has been reported in dogs, and incidental cases have been described in cats.1,4 It is difficult to estimate the incidence of this phenomenon in males, as the passage of some sperm into the bladder is observed both with natural mating and with sperm collection under anaesthesia. True retroejaculation can be suspected when no sperm cells are present in the ejaculate but are found in significant amounts in the urine. 1
Diagnosis
Identifying the problem in a case of suspected male infertility is not an easy process. Crucially, as discussed earlier, three key facts need first to be established: (1) does the problem really concern the male? (2) Is infertility primary or acquired? (3) Is it a problem of sperm delivery (copulation) or sperm production/quality (fertilisation)? The veterinarian can then start a more detailed work-up using several diagnostic methods, as described below.
Thorough general and reproductive history
It is important, as part of the general history-taking, to ask about the cat’s feeding regimen and living environment, past and present diseases, and medications taken previously and currently (including permanently). Information about relationships with other cats in the cattery, as well as any behavioural problems or changes in the cat’s environment, can be helpful. Attention then turns to collecting a detailed reproductive history: when did the infertility problem first arise? What are the breeding protocols within the cattery? Are matings observed? A common mistake is for breeders not to observe copulation, and in particular post-coital behaviour in the queen (which confirms intromission), so supervised mating should be recommended.
Clinical examination: general and reproductive
During the clinical examination, attention focuses first on the general condition of the male, nutritional status (body condition score) and condition of the coat and teeth. An orthopaedic examination will determine if there is any pain in the limbs or spine. Abdominal palpation, auscultation of the chest and temperature measurement will allow other conditions to be ruled out.
Examination of the male reproductive system begins by looking at the scrotum and palpating the testicles, paying attention to size, symmetry and consistency. There are no reference values for testicular size in tom cats, so this is assessed subjectively. Measurements can be taken using ultrasonography to monitor testicular volume (and changes) over time. 21 Normal consistency of the testicles is described as firm, but elastic; a hard or flaccid consistency may indicate fibrosis and atrophy, respectively. The testicles should be moveable within the scrotum – if they are not, it may suggest that an inflammatory process has led to adhesions. When assessing the prepuce, attention should be paid to possible hair curling around the base of the foreskin in longhaired breeds. The penis of an intact male should have visible well-developed spines (Figure 5). There should be no petechiae or ecchymosis on the prepuce and penile mucosa, and the prepuce should completely cover the penis (Figure 2a).
Figure 5.
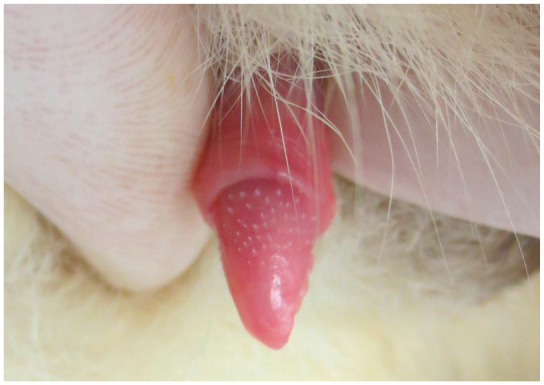
Normal feline penis with characteristic penile spines
Hormone assessment
Results of blood testosterone assessment can be difficult to interpret. While a high level (>1-9 ng/ml 30 ) would be considered normal, a low baseline result (<1 ng/ml) does not exclude proper testicular function. Many mature, intact males show testosterone levels below the detectability threshold; 31 this is due to pulsatile, episodic testosterone release. 31 For this reason, it is suggested that stimulation tests are performed using 250 IU human chorionic gonadotropin (hCG) or 25-50 μg gonadotropin-releasing hormone (GnRH).3,30,31
A good indicator of testosterone levels is the presence of penile spines. These structures appear under the influence of testosterone and disappear when levels decrease (eg, after castration), though this process of disappearance takes about 6 weeks. 3 Alternatively, anti-Mullerian hormone (AMH) levels can be measured in serum. AMH is produced in males by Sertoli cells and therefore intact cats show high levels (4.8-81.3 ng/ml 32 ). Thus, AMH may be used to distinguish castrated or anorchid males from those with cryptorchidism. Moreover, extrapolating from human medicine, AMH might serve as a marker of Sertoli cell function, although its clinical use in this context has not been evaluated in cats.
Semen assessment
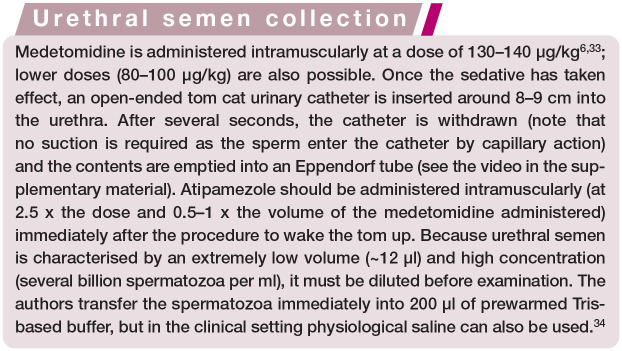
In the past, semen collection in cats under clinical conditions was problematic. Currently, the method of choice is catheterisation of the urethra under medetomidine sedation. 6 For a brief outline of the technique, see the box and the accompanying video in the supplementary material. Although obtaining semen from the male is no longer a technical challenge, interpretation of the results may be difficult for a number of reasons, as outlined below.

No sperm collected
If no sperm cells are obtained, this may be due to impaired sperm production or failure of collection. In such cases, alkaline phosphatase (ALP) analysis can be undertaken using a haematological analyser. ALP is an enzyme produced in the testes and epi-didymides, 31 and thus its activity in normal feline ejaculate is high (>20,000 IU/l31,35). A low result may mean that only fluid from the accessory glands was collected and no spermatozoa were expelled (eg, due to obstruction of the duc-tus deferens or retroejaculation), or it may simply reflect a failure in semen collection; approximately 10% of cases may be unsuccessful. 36 In such cases, another semen collection after 1 week is recommended. To increase semen output and the chance of successful collection, transscrotal testicular massage can be performed. 37 High ALP activity in an azoospermic ejaculate sample indicates true azoospermia.
Lack of reference values for semen parameters in fertile toms
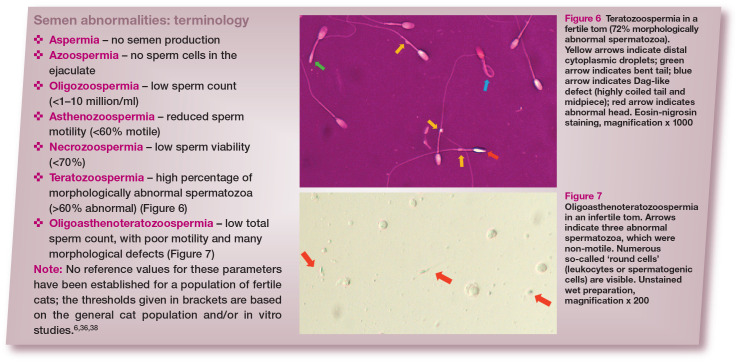
Research into feline semen quality has been performed on the general population of cats, and for the majority of animals, fertility was not known. Therefore, although some reference values for semen parameters are available (thresholds for several basic parameters are given in the box), it has been reported in the literature, and also observed in the authors’ clinical practice, that some toms proven to be fertile have semen quality below these values, especially in terms of morphology (Figure 6). 34 Information about semen quality in fertile toms would be indispensable and the collection of a large amount of data, ideally from many centres worldwide, should be a priority for the research community.
Figure 6.
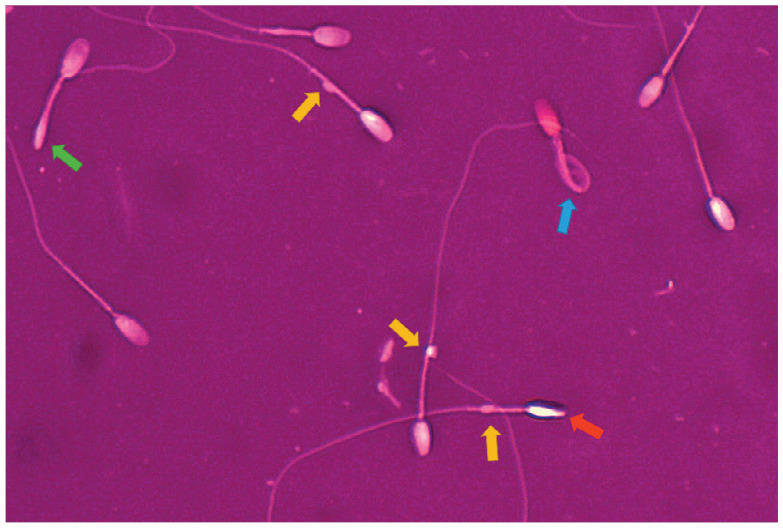
Teratozoospermia in a fertile tom (72% morphologically abnormal spermatozoa). Yellow arrows indicate distal cytoplasmic droplets; green arrow indicates bent tail; blue arrow indicates Dag-like defect (highly coiled tail and midpiece); red arrow indicates abnormal head. Eosin-nigrosin staining, magnification x 1000
Figure 7.
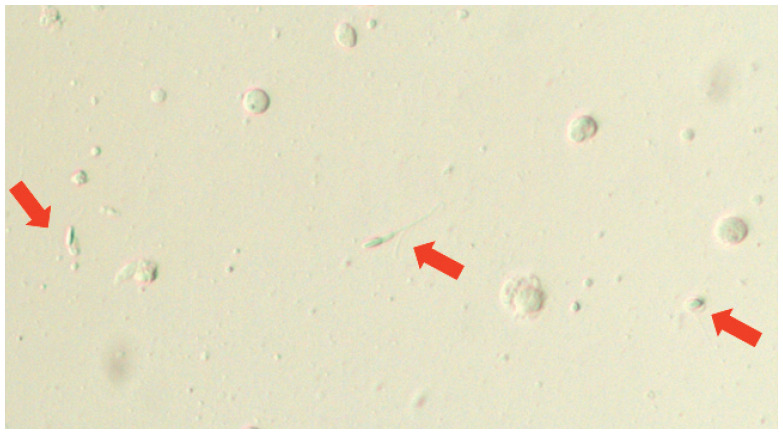
Oligoasthenoteratozoospermia in an infertile tom. Arrows indicate three abnormal spermatozoa, which were non-motile. Numerous so-called ‘round cells’ (leukocytes or spermatogenic cells) are visible. Unstained wet preparation, magnification x 200
A single sample is not representative
As reported in other species, especially in humans, 39 semen quality for a given donor may vary significantly between collections. The authors have also reported high intra-individual variation in cats (Figure 8). 40 Therefore, repeat semen collections (at least two or three) are required before semen quality is implicated in male infertility.
Figure 8.
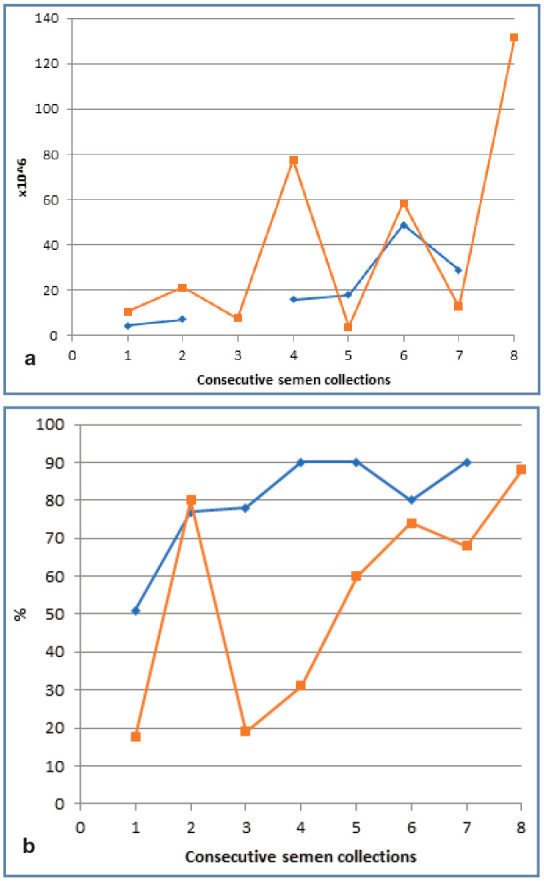
Variation in (a) sperm count and (b) motility in two cats undergoing repeat semen collection and assessment; cat 1 (blue line) for scientific purposes, cat 2 (orange line) for artificial insemination
‘Hidden’ spermatozoan defects
Sometimes basic semen analysis reveals no abnormalities, but spermatozoa may still possess defects at the molecular level, such as DNA fragmentation or lack of crucial receptors or binding proteins. In these instances more advanced methods of semen assessment (eg, flow cytometry or functional in vitro tests) are required. The authors have reported three cases of males with reduced fertility (low number of kittens in the litter, some with congenital defects), in which an increased percentage of spermatozoa with abnormal chromatin structure was demonstrated. 41 However, some defects (eg, de novo gene mutation, resulting in early embryo death) cannot be diagnosed and can only be hypothesised in cases of idiopathic infertility.
Microbiological examination
Little is known about the natural bacterial flora of the male cat genitourinary system. There are two reports describing the presence of different species of bacteria in preputial swabs and in semen.31,42 Since bacteria were isolated mostly from clinically healthy individuals in these studies, the results of bacteriological testing should be interpreted with caution.
Testicular biopsy
In many cases, definitive diagnosis of male infertility can only be made by testicular biopsy, which allows for assessment of the seminal tubules and the process of spermatogenesis. Although this procedure is described in cats, 43 it is performed extremely rarely in veterinary practice.
Treatment
Depending on the cause, treatment of male infertility may range from very simple and straightforward (eg, shaving hair around the penis to avoid recurrence of hair rings), to not possible (eg, congenital disorders, severe degenerative changes in the testicles), in which case the male is excluded from reproduction. It is, therefore, crucial to recognise the underlying problem to enable specific treatment, where available.
Proper organisation of mating
Because copulatory problems are the main cause of reproductive failure, ensuring optimal conditions for mating is a fundamental step. The general rule is to bring the female cat to the male, not the other way round. An inexperienced tom should be mated with an experienced, placid queen. Matings should take place in a calm environment known to the tom; any changes are undesirable. An adequate amount of space should be provided (eg, to facilitate escape of the male from any post-copulation aggression by the female).
Management of general and reproductive diseases
Before starting any hormonal therapy, general and reproductive diseases that may affect libido, mating ability and spermatogenesis should be ruled out or treated. In the case of longhaired cats, where the problem is curling of the hair around the prepuce, regular clipping of the perineal area is recommended. Persistent frenulum should be surgically corrected. Infectious diseases should be treated according to general principles. It should be remembered that owing to the duration of spermatogenesis, the effects of therapy may sometimes not be apparent for a month or two.
Acute diseases usually cause temporary disorders of spermatogenesis and, after the recovery period, the quality of the sperm returns to normal. Chronic processes often lead to irreversible degeneration of the testicular tissue and permanent loss of fertility; in the case of testicular atrophy and azoosper-mia, there is often no effective treatment and the prognosis is unfavourable. A male with genetic or congenital abnormalities should be excluded from breeding.
Hormonal therapy
Hormonal therapy can be used both for the stimulation of libido and to improve semen quality. Although it is quite common for breeders to request this form of treatment, it should be used with caution.
Treatment with testosterone or its analogues is not recommended. 3 The most common way to increase testosterone is by stimulation using GnRH at a dose of 1-3 mg/kg 20 or hCG at a dose of 50-100 IU/cat. 44 However, these preparations should not be used in the long term (owing to negative feedback on the hypothala-mic-pituitary-testicular axis and receptor desensitisation). Although there are no data about the use of GnRH or hCG as a treatment for the above-mentioned conditions, the GnRH analogue deslorelin (Suprelorin; Virbac) can be used to improve spermatogenesis. Romagnoli and colleagues 45 treated healthy cats with a 9.4 mg deslorelin implant and observed a slight increase in semen quality during the first month. The treatment had originally been given to suppress testicular function, which started to occur as early as the second month post-treatment. The initial improvement in semen quality following administration of deslorelin has also been reported in dogs, 46 and this effect is likely due to deslorelin causing a temporarily increased release of LH that then stimulates testicular testosterone secretion (flare-up effect). Because testosterone starts to drop after around a week, 45 the implant must be removed soon after achieving the flare-up effect. Implantation near the umbilicus facilitates its removal.
In human medicine, hormonal therapy is much more advanced than in animals. 47 Although there are no data about the use of human protocols for the treatment of feline infertility, some approaches may potentially be useful. In humans, an abnormally low testosterone to estradiol (T:E) ratio has been linked to infertility. 48 In cats, a reduced testicular T:E ratio was associated with terato-zoospermia, 49 suggesting that medications affecting oestrogen production and/or activity can be used to improve semen quality. Possible drugs include aromatase inhibitors (eg, anastrozole, testolactone, letrozole) and oestrogen receptor modulators (eg, clomiphene citrate, tamoxifen). Moreover, some human studies have shown that abnormally high levels of prolactin negatively affect male fertility. 47 Therefore, the use of antipro-lactin drugs (eg, bromocriptine, cabergoline) may be a solution in some cases. The authors have used such therapy in dogs (data not published), but their use for infertile toms needs further investigation.
Diet and nutritional supplements
Nutritional errors should be corrected, which in the case of obesity in cats is not easily achieved. In human medicine 50 and in dogs, 51 dietary supplements containing antioxidants (zinc, selenium, omega-3 fatty acids, co-enzyme Q10, carnitine) are sometimes used successfully to treat infertility/subfertility, but there are no reports on their use in cats.
Pharmacological enhancement of ejaculation
In bulls 52 and dogs, 53 the ability of pharmacological agents such as oxytocin and prostaglandin F2alpha to increase semen output during collection has been tested, with variable results. No such data exist for cats, but the idea of administering these agents before mating seems to be worthy of further investigation.
Assisted reproductive techniques
Where a specific cause cannot be found or implementation of treatment is impossible, assisted reproductive techniques (ARTs) might offer a solution. Mating problems can be solved using artificial insemination. For a male with obstruction of the vas deferens, the last chance for breeding might be post-castration sperm collection from the epididymis followed by artificial insemination (see accompanying review 54 ). Moreover, when semen quality is poor, classical in vitro fertilisation can be used and, if very poor, in vitro fertilisation with intracytoplasmic sperm injection (ICSI), both followed by embryo transfer. Currently, these techniques are rarely used in cats and no clinical data are available on their role in the treatment of infertility.
Key Points.
✜ Male cat infertility is a challenge for veterinary practitioners, in terms of determining both the aetiology and treatment options.
✜ A thorough, step-by-step diagnostic work-up, including semen collection and evaluation, is a crucial element for identification of the underlying cause, which may enable specific treatment.
✜ Both breeders and veterinarians have to accept that sometimes the cause cannot be identified and/or there is no adequate treatment.
✜ It remains for the existing knowledge gaps (eg, reference values for semen parameters in fertile males) to be filled, and ARTs to be developed to a level where they could become commercially available for cats, as they are in human medicine.
Footnotes
Supplementary material: The following file is available online:
✜ Video showing urethral semen collection in a cat.
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not specifically required for publication in JFMS.
Informed consent: This work did not involve the use of animals (including cadavers) and therefore informed consent was not required. No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
References
- 1. Axnér E, Ström B, Linde-Forsberg C, et al. Reproductive disorders in 10 domestic male cats. J Small Anim Pract 1996; 37: 394–401. [DOI] [PubMed] [Google Scholar]
- 2. Fontbonne A, Prochowska S, Niewiadomska Z. Infertility in purebred cats – a review of the potential causes. Theriogenology 2020; 158: 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Little S. Feline reproduction: problems and clinical challenges. J Feline Med Surg 2011; 13: 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keenan LRJ. The infertile male. In: Simpson GM. (ed). Manual of small animal reproduction and neonatology. Gloucester: British Small Animal Veterinary Association, 1998, pp 83–94. [Google Scholar]
- 5. Foster RA. Common lesions in the male reproductive tract of cats and dogs. Vet Clin North Am Small Anim Pract 2012; 42: 527–545. [DOI] [PubMed] [Google Scholar]
- 6. Zambelli D, Prati F, Cunto M, et al. Quality and in vitro fertilizing ability of cryopreserved cat spermatozoa obtained by urethral catheterization after medetomidine administration. Theriogenology 2008; 69: 485–490. [DOI] [PubMed] [Google Scholar]
- 7. Fontbonne A. Infertility in queens: clinical approach, experiences and challenges. J Feline Med Surg 2022; 24: 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fontaine E. The #1 cause of infertility in the feline species. https://breeders.royalcanin.co.nz/cat/articles/reproduction/the-1-cause-of-infertility-in-the-feline-species (2017, accessed 10 May 2021). [Google Scholar]
- 9. Foster RA. Disorders of sexual development in the cat: current state of knowledge and diagnostic approach. J Feline Med Surg 2022; 24: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verstegen JP. Conditions of the male. In: Simpson GM. (ed). Manual of small animal reproduction and neonatology. Gloucester: British Small Animal Veterinary Association, 1998, pp 71–82. [Google Scholar]
- 11. Szczerbal I, Nizanski W, Dzimira S, et al. X monosomy in a virilized female cat. Reprod Domest Anim 2015; 50: 344–348. [DOI] [PubMed] [Google Scholar]
- 12. Szczerbal I, Krzeminska P, Dzimira S, et al. Disorders of sex development in cats with different complements of sex chromosomes. Reprod Domest Anim 2018; 53: 1317–1322. [DOI] [PubMed] [Google Scholar]
- 13. Szczerbal I, Stachowiak M, Nowacka-Woszuk J, et al. Disorder of sex development in a cat with chromosome mosaicism 37,X/38,X,r(Y). Reprod Domest Anim 2017; 52: 914–917. [DOI] [PubMed] [Google Scholar]
- 14. Nowacka-Woszuk J, Szczerbal I, Salamon S, et al. Testicular disorder of sex development in four cats with a male kary-otype (38,XY; SRY-positive). Anim Reprod Sci 2014; 151: 42–48. [DOI] [PubMed] [Google Scholar]
- 15. França LR, Godinho CL. Testis morphometry, seminiferous epithelium cycle length, and daily sperm production in domestic cats (Felis catus). Biol Reprod 2003; 68: 1554–1561. [DOI] [PubMed] [Google Scholar]
- 16. Millis DL, Hauptman JG, Johnson CA. Cryptorchidism and monorchism in cats: 25 cases (1980-1989). J Am Vet Med Assoc 1992; 200: 1128–1130. [PubMed] [Google Scholar]
- 17. Foster RA, Caswell JL, Rinkardt N. Chronic fibrinous and necrotic orchitis in a cat. Can Vet J 1996; 37: 681–682. [PMC free article] [PubMed] [Google Scholar]
- 18. Silva CA, Cocuzza M, Borba EF, et al. Cutting-edge issues in autoimmune orchitis. Clin Rev Allergy Immunol 2012; 42: 256–263. [DOI] [PubMed] [Google Scholar]
- 19. Elcock LH, Schoning P. Age-related changes in the cat testis and epididymis. Am J Vet Res 1984; 45: 2380–2384. [PubMed] [Google Scholar]
- 20. Max A. Cats. Obstetrics and reproduction [article in Polish]. Galaktyka, Łódź, 2010. [Google Scholar]
- 21. Johnson AK. Normal feline reproduction: the tom. J Feline Med Surg 2022; 24: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Griffin MA, Culp WTN, Rebhun RB. Lower urinary tract neoplasia. Vet Sci 2018; 5: 96. DOI: 10.3390/vetsci5040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tucker AR, Smith JR. Prostatic squamous metaplasia in a cat with interstitial cell neoplasia in a retained testis. Vet Pathol 2008; 45: 905–909. [DOI] [PubMed] [Google Scholar]
- 24. Mordecai A, Liptak JM, Hofstede T, et al. Prostatic abscess in a neutered cat. J Am Anim Hosp Assoc 2008; 44: 90–94. [DOI] [PubMed] [Google Scholar]
- 25. Roura X, Camps-Palau MA, Lloret A, et al. Bacterial prostatitis in a cat. J Vet Intern Med 2002; 16: 593–597. [DOI] [PubMed] [Google Scholar]
- 26. Pointer E, Murray L. Chronic prostatitis, cystitis, pyelonephritis, and balanoposthitis in a cat. J Am Anim Hosp Assoc 2011; 47: 258–261. [DOI] [PubMed] [Google Scholar]
- 27. MacDonald ML, Rogers QR, Morris JG, et al. Effects of linoleate and arachidonate deficiencies on reproduction and spermatogenesis in the cat. J Nutr 1984; 114: 719–726. [DOI] [PubMed] [Google Scholar]
- 28. Fontaine C. Long-term contraception in a small implant: a review of Suprelorin (deslorelin) studies in cats. J Feline Med Surg 2015; 17: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nuñex Favre R, García MF, Garci Mitacek MC, et al. Reestablishment of sperm quality after long-term deslorelin suppression in tomcats. Anim Reprod Sci 2018; 195: 302–308. [DOI] [PubMed] [Google Scholar]
- 30. Romagnoli S, Baldan A, Righetti C, et al. Use of the gonadotropin-releasing hormone (GnRH) stimulation test to monitor gonadal function in intact adult male cats. Reprod Domest Anim 2017; 52: 24–27. [DOI] [PubMed] [Google Scholar]
- 31. Johnston SD, Root MV, Olson PNS. Ovarian and testicular function in the domestic cat: clinical management of spontaneous reproductive disease. Anim Reprod Sci 1996; 42: 261–274. [Google Scholar]
- 32. Axnér E, Ström Holst B. Concentrations of anti-Mullerian hormone in the domestic cat. Relation with spay or neuter status and serum estradiol. Theriogenology 2015; 83: 817–821. [DOI] [PubMed] [Google Scholar]
- 33. Zambelli D, Raccagni R, Cunto M, et al. Sperm evaluation and biochemical characterization of cat seminal plasma collected by electroejaculation and urethral catheterization. Theriogenology 2010; 74: 1396–1402. [DOI] [PubMed] [Google Scholar]
- 34. Axnér E, Linde Forsberg C. Sperm morphology in the domestic cat, and its relation with fertility: a retrospective study. Reprod Domest Anim 2007; 42: 282–291. [DOI] [PubMed] [Google Scholar]
- 35. Valiente C, de la Sota PE, Arauz S, et al. Ejaculation training, seminal alkaline phosphatase and semen preservation through cooling in a milk-based extender in domestic cats. J Feline Med Surg 2014; 16: 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prochowska S, Nizanski W, Ochota M, et al. Characteristics of urethral and epididymal semen collected from domestic cats – a retrospective study of 214 cases. Theriogenology 2015; 84: 1565–1571. [DOI] [PubMed] [Google Scholar]
- 37. Prochowska S, Nizanski W. Transscrotal stimulation of the testes and epididymides improves urethral sperm collection in domestic cats. Reprod Fertil Dev 2021; 33: 437–440. [DOI] [PubMed] [Google Scholar]
- 38. Howard JG, Brown JL, Bush M, et al. Teratospermic and normo-spermic domestic cats: ejaculate traits, pituitary-gonadal hormones, and improvement of spermatozoa motility and morphology after swim up processing. J Andro 1990; 11: 204–215. [PubMed] [Google Scholar]
- 39. World Health Organisation. WHO laboratory manual for the examination and processing of human semen. 5th ed. Switzerland: World Health Organisation, 2010. [Google Scholar]
- 40. Prochowska S, Nizanski W, Ochota M. Multiple sperm collection from the same male by urethral catheterization after administration of medetomidine [article in Polish]. Proceedings of the XIV Congress, ‘Problems in small animal reproduction -fertility, pregnancy, neonate’, 2018 Oct 13-14; Wroclaw, Poland. [Google Scholar]
- 41. Prochowska S, Nizanski W. Clinical use of sperm chro-matin structure assay in feline subfertility diagnosis – case reports. Proceedings of the 12th EVSSAR Congress; 2019 June 28-29; Berlin, Germany. [Google Scholar]
- 42. Ström Holst B, Bergstrom A, Lagerstedt AS, et al. Characterization of the bacterial population of the genital tract of adult cats. Am J Vet Res 2003; 64: 963–968. [DOI] [PubMed] [Google Scholar]
- 43. Leme DP, Visacre E, Castro VB, et al. Testicular cytology by fine needle aspiration in domestic cats. Theriogenology 2018; 106: 46–52. [DOI] [PubMed] [Google Scholar]
- 44. Plumb DC. Plumb’s veterinary drug handbook: desk. 9th ed. Stockholm, WI: Pharma Vet, 2018. [Google Scholar]
- 45. Romagnoli S, Baldan A, Ferro S, et al. Length of efficacy and effect of implant location in adult tom cats treated with a 9.4 mg deslorelin subcutaneous implant. J Feline Med Surg 2019; 21: 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Romagnoli S, Siminica A, Sontas BH, et al. Semen quality and onset of sterility following administration of a 4.7-mg deslorelin implant in adult male dogs. Reprod Domest Anim 2012; 47: 389–392. [DOI] [PubMed] [Google Scholar]
- 47. Khourdaji I, Lee H, Smith RP. Frontiers in hormone therapy for male infertility. Transl Androl Urol 2018; 7 Suppl 3: 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pavlovich CP, King P, Goldstein M, et al. Evidence of a treatable endocrinopathy in infertile men. J Urol 2001; 165: 837–841. [PubMed] [Google Scholar]
- 49. Müller G, Martino-Andrade AJ, Santos AS, et al. Testicular testosterone: estradiol ratio in domestic cats and its relationship to spermatogenesis and epididymal sperm morphology. Theriogenology 2012; 78: 1224–1234. [DOI] [PubMed] [Google Scholar]
- 50. Torres-Arce E, Vizmanos B, Babio N, et al. Dietary antioxi-dants in the treatment of male infertility: counteracting oxidative stress. Biology (Basel) 2021; 10: 241. DOI: 10.3390/biology10030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Domoslawska A, Zdunczyk S, Nizanski W, et al. Effect of selenium and vitamin E supplementation on semen quality in dogs with lowered fertility. Bull Vet Inst Pulawy 2015; 59: 85–90. [Google Scholar]
- 52. Palmer CW, Amundson SD, Brito LF, et al. Use of oxytocin and cloprostenol to facilitate semen collection by electro-ejaculation or transrectal massage in bulls. Anim Reprod Sci 2004; 80: 213–223. [DOI] [PubMed] [Google Scholar]
- 53. Traas AM, Kustritz MV. Effect of administrating oxytocin or prostaglandin F2alpha on characteristics of the canine ejaculate. Can Vet J 2004; 45: 999–1002. [PMC free article] [PubMed] [Google Scholar]
- 54. Zambelli D, Cunto M. Artificial insemination in queens in the clinical practice setting: protocols and challenges. J Feline Med Surg 2022; 24: 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]