Abstract
Objectives
There is a paucity of information on feline discospondylitis. This study aimed to describe the signalment, clinical and laboratory findings, aetiological agents, treatment and outcome in cats affected by discospondylitis.
Methods
This was a retrospective review of the medical records of cats diagnosed with discospondylitis at four referral institutions.
Results
A total of 17 cats were identified. Most were domestic shorthair cats (76.5%) and male (58.8%), with a median age of 9 years (range 0.9–14) and a median duration of clinical signs of 3 weeks (range 0.3–16). All cats presented with spinal hyperaesthesia; 3/17 had pyrexia. Neurological dysfunction was found in 64.7% of cats, which was indicative of a T3–L3 or L4–S2 spinal segment, associated nerve root or associated nerve neurolocalisation. Haematology, serum biochemistry and urinalysis revealed occasional inconsistent non-specific changes. All cats underwent urine culture; 9/17 cats also had a distinct tissue cultured. Positive bacterial cultures were obtained in two cats (11.8%) for Staphylococcus species (urine, blood and intradiscal fine-needle aspirate) and Escherichia coli (urine); both presented with multifocal discospondylitis. Treatment was non-surgical in all cats, with sustained antibiotic therapy for a median of 3 months (range 1–9). Analgesia provided included non-steroidal anti-inflammatory drugs, alone or in combination with gabapentin. Restricted exercise was advised for a minimum of 4 weeks. Outcome information available in 12 cats was excellent in terms of pain control and neurological function in 10 cats (83.3%) at the time of stopping antibiotics. Recurrence occurred in one case, which had received a single antibiotic for 6 weeks, and relapsed 4 months after presentation. One other case failed to improve and was euthanased during the course of hospitalisation.
Conclusions and relevance
Feline discospondylitis is uncommon and no obvious signalment predisposition was found in this study. Spinal hyperaesthesia was universally present, with neurological dysfunction also highly prevalent. Bacterial culture was unrewarding in most cases. Amoxicillin–clavulanic acid or cephalosporins are reasonable choices for first-line antibiotics. Prognosis was favourable, with no long-term evidence of recurrence in cats on sustained antibiotic therapy, for a mean duration of 3 months.
Keywords: Bacteria, spinal, infection, discitis, myelopathy, pyogenic, abscess
Introduction
Discospondylitis describes the infection of an intervertebral disc and its adjacent cartilaginous end plates and vertebral bodies, and is commonly bacterial or fungal in origin in both dogs and cats.1–4
Numerous reports of discospondylitis in dogs are available, describing associated signalment, clinical signs, typical imaging features, aetiological agents, treatment options and outcome.2–9 In contrast, the literature regarding feline discospondylitis is sparse, with only eight individual case reports.10–17 Despite recent advances in terms of diagnostic imaging features able to guide diagnosis of this condition in cats, 18 there is little information available on signalment, clinical features, neurological findings, treatment options and outcome.
The aim of this study was to describe, retrospectively, a population of cats with a diagnosis of discospondylitis, and report the signalment, clinical and neurological signs, possible predisposing factors, comorbidities, laboratory findings, aetiological agents, treatment and outcome.
Materials and methods
Animals
The medical records of cats diagnosed with discospondylitis at four referral institutions in the UK between February 2009 and January 2021 were reviewed. Cases were included when MRI evidence of discospondylitis was found. MRI evidence of discospondylitis included the involvement of one or more intervertebral discs, alone or in conjunction with adjacent end plates, according to previous reports. 18 Characteristic MRI features indicative of discospondylitis included a combination of (1) a T2-weighted (T2W) and short tau inversion recovery (STIR) hyperintense signal nucleus pulposus; (2) involvement of the adjacent vertebral endplates, and T2W and STIR hyperintense signal neighbouring soft tissue; and (3) contrast enhancement of at least one the following structures: nucleus pulposus, adjacent endplates or neighbouring soft tissue. 18
Information retrieved from the medical records included signalment, duration and type of clinical signs, and abnormalities on neurological examination. Evidence of pyrexia at the referring veterinary practice or at presentation to the referral centre was sought, and defined as an intrarectal temperature ⩾39.2°C. 19 The individually affected intervertebral disc sites were described. In searching for other sites of infection or possible predisposing factors (eg, cat bite abscess), as well as evidence of attempted antibiotic therapy previous to referral, clinical histories from referring veterinarians were sought up to 6 months before presentation, when available. Results of further diagnostic testing, including haematology and serum biochemistry, urinalysis and cerebrospinal fluid (CSF) analysis, were retrieved. Comorbidities identified at the time of presentation were also described. Feline leukaemia virus (FeLV) antigen, feline immunodeficiency virus (FIV) antibody and feline coronavirus (FCoV) antibody titres testing results were sought from the medical histories. In order to establish comparisons with available limited literature on feline discospondylitis, data from previous case report findings were also collected and are summarised in Table 1.
Table 1.
Summary of the main findings of previous case reports describing feline discospondylitis
| Case report | Signalment (age, sex, breed) | Duration, clinical and neurological findings, haematology (Hm) and serum biochemistry (Sb) findings | Microbial culture results (Cultures), concurrent comorbidities (CCM) and associated findings (AF) | Antibiotic treatment (Tx) and outcome |
|---|---|---|---|---|
| Norsworthy (1979) 10 | 2-year-old ME Persian | Duration: 5 days Clinical findings: lethargy, anorexiaNeurological findings: progressive paraparesis |
Cultures: none | Tx: none |
| CCM: none describedAF: none described | Outcome: euthanasia | |||
| Malik et al (1990) 11 | 15-year-old ME DSH | Duration: 5 weeksClinical findings: inappetence, respiratory signs (ocular and nasal discharge), pyrexiaNeurological findings: pelvic limb ataxia progressing into paraplegiaHm: segmented neutrophilia, monocytosis, lymphocytopenia and eosinopenia; Döhle bodies were present in many of the neutrophils | Culture (at necropsy) – from paravertebral abscess: Streptococcus canis in large numbers, Actinomyces viscosus in moderate numbers and a small number of Escherichia coli | Tx: previous treatment with amoxicillin–clavulanic acid by referring veterinarian for 1 weekOutcome: spontaneous death |
| CCM: suspected concomitant respiratory tract infection, paravertebral abscess, chronic meningomyelitis of the overlying spinal cord | ||||
| Watson and Roberts (1993) 12 | 13-year-old FN DSH | Duration: 2 weeksClinical findings: anorexia, dehydration, emaciationNeurological findings: hyperaesthesia of the lumbar spine | Cultures – urine: E coliCCM: concurrent chronic pyelonephritisAF: none described | Tx: ampicillinOutcome: initial improvement but euthanasia 2 months later |
| Sb: elevated urea and creatinine | ||||
| Aroch et al (1999) 13 | 1-year-old MN DSH | Duration: 1 month Clinical findings: back pain, pyrexia, anorexia, lethargy and dehydrationNeurological findings: paraparesis, proprioceptive deficit, upper motor neuron bladderHm: leukocytosis with neutrophiliaSb: hyperglobulinaemia |
Cultures – blood, urine, CSF: all negative; E coli (at necropsy)CCM: meningomyelitis of the overlying spinal cord, haemorrhages on the paravertebral musclesAF: none described | Tx: initially amoxicillin (short courses), then amoxicillin–clavulanic acid (2 weeks) and enrofloxacin; worsened while hospitalised |
| Outcome: progressive despite antibiotic therapy and euthanasia in 6 days | ||||
| Packer et al (2005) 14 | 8-month-old MN Scottish Fold | Duration: 2 weeksClinical findings: reluctance to ambulateNeurological findings: pelvic limb lameness, severe lumbosacral hyperaesthesiaSb: hypercalcaemia |
Cultures – urine: Enterococcus species (aerobic conditions) and Clostridium perfringens (anaerobic conditions); blood, CSF negativeCCM: sublumbar abscessAF: none described | Tx: enrofloxacin and ampicillin for 8 weeksOutcome: good at 10 weeks |
| Hill et al (2015) 15 | 6.5-year-old MN Siamese | Duration: 6 monthsClinical findings: gallop rhythm; otherwise unremarkableNeurological findings: lumbar hyperaesthesia, pelvic limb ataxia, tail paresis | Cultures: FNA and urine negativeCCM: none describedAF: none described | Tx: amoxicillin–clavulanic acid for 2 months |
| Outcome: no further painful episodes at 11 months | ||||
| Kemelman et al (2019) 16 | 20-month-old male Maine Coon |
Duration: 1 monthClinical findings: increased level of aggression, decreased appetite and activity, obstipation, heart gallop rhythm; otherwise unremarkable | Cultures: surgical biopsy negativeCCM: none describedAF: none described | Tx: amoxicillin–clavulanic acid 20 mg/kg for 6 weeks |
| Outcome: complete resolution at 12 months | ||||
| Neurological findings: lumbar hyperaesthesia | ||||
| Liatis et al (2020) 17 | 8-year-old MN DSH | Duration: 1 monthClinical findings: low tail carriage and tail painNeurological findings: tail paresis with proximal movement and marked pain on palpation of the proximal third of the tailSb: hyperproteinaemia (88 g/l [RI 60–85]) with moderate hyperglobulinaemia | Cultures: intervertebral space aspirates and urine negativeCCM: none describedAF: none described | Tx: 6-week course of antibiotics (12.5 mg/kg clindamycin PO q24h, 20 mg/kg cefalexin PO q12h) |
| Outcome: complete resolution at 3 weeks | ||||
ME = male entire; DSH = domestic shorthair; FN, female neutered; CSF = cerebrospinal fluid; FNA = fine-needle aspirate; MN = male neutered; RI = reference interval
Bacterial cultures, treatment and outcome
Bacterial cultures, including urine, blood or percutaneous fine-needle aspiration (FNA) of affected intervertebral discs or altered paraspinal structures, or associated abscesses and CSF, were described, as well as antibiotic sensitivity results. Treatment protocols were collected, including antibiotic dosage regimen and length of treatment, as well as analgesic therapy. Surgical treatment, if any, was described, as well as advised exercise restriction measures. Outcome was obtained from the clinical histories when available describing the response to treatment in terms of pain management, neurological dysfunction and possible recurrences. Outcome was considered (1) excellent if complete resolution of clinical signs was present at follow-up consultations or the owner considered the cat to be clinically normal and pain-free; (2) incomplete recovery if there was substantial but incomplete improvement in clinical signs or the owner considered the cat to have some recurrent episodes of pain; or (3) poor if the cat did not improve or deteriorated further. Long-term outcome (>6 months) describing recurrences was collected via telephone interviews with the owners or the referring veterinarians.
Results
Signalment, clinical signs and predisposing factors
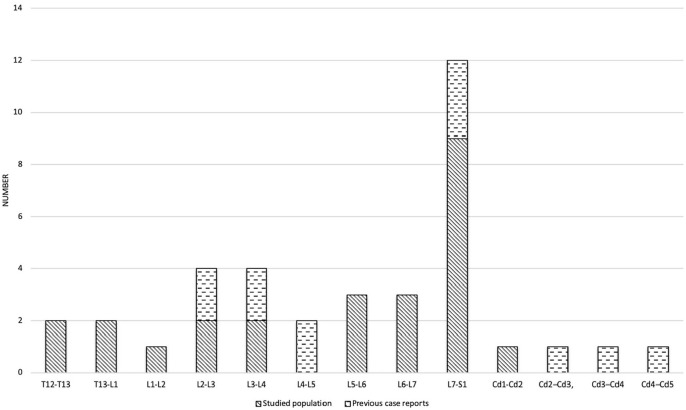
Seventeen cats with a diagnosis of discospondylitis were identified. Thirteen had previously been included in a study describing the imaging features of feline discospondylitis. 18 Breed distribution was domestic shorthair (DSH; n = 13), Maine Coon (n = 3) and Siamese (n = 1). There were seven female cats and 10 male cats; all the cats were neutered and had a median age of 9 years (range 0.9–14). The median duration of clinical signs before presentation was of 3 weeks (range 0.3–16), and all cats presented with hyperaesthesia on spinal palpation. Pyrexia was identified in 3/17 (17.6%) cats, with temperatures of 39.3°C, 39.4°C and 39.5°C, respectively. Further details on clinical and neurological findings are detailed in Table 2. Twenty-five foci of discospondylitis were identified in the 17 cats, with four presenting a multifocal discospondylitis: two cats with two affected sites, and two cases of three and five affected sites, respectively. Discospondylitis was identified most commonly at L7–S1 in 9/25 (36%) cats; further information on the distribution of affected sites in this study and previous case reports is available for Figure 1.
Table 2.
Clinical and neurological signs, and haematological and serum biochemistry findings for the cats in the present study
| n = 17 | |
|---|---|
| Clinical signs | |
| Reluctance to jump | 9 (52.9) |
| Lethargy | 7 (41.2) |
| Inappetence/anorexia | 4 (23.5) |
| Pyrexia | 3 (17.6) |
| Dysuria | 2 (11.8) |
| Obstipation | 1 (5.9) |
| Neurological signs | |
| Spinal hyperaesthesia | 17 (100) |
| Spinal hyperaesthesia alone | 6 (35.3) |
| Neurological dysfunction | 11 (64.7) |
| – Paraparesis/pelvic limb ataxia | 7 (41.2) |
| – Pelvic limb lameness | 6 (35.3) |
| – Abnormal pelvic withdrawal/patellar reflexes | 5 (29.4) |
| – Abnormal tail carriage/tail paresis | 5 (29.4) |
| – Muscle wastage | 2 (11.8) |
| – Biting extremities/paraesthesia | 1 (5.9) |
| Haematological findings | |
| Unremarkable | 14 (82.4) |
| Leukocytosis with neutrophilia | 1 (5.9) |
| Leukocytosis with neutrophilia and monocytosis | 1 (5.9) |
| Mild non-regenerative anaemia | 1 (5.9) |
| Reticulocytosis without anaemia | 1 (5.9) |
| Serum biochemistry findings | |
| Unremarkable | 10 (58.8) |
| Hyperglobulinaemia | 4 (23.5) |
| Increased urea | 1 (5.9) |
| Increased urea/creatinine | 1 (5.9) |
| Increased creatinine kinase | 1 (5.9) |
| Hypoalbuminaemia | 1 (5.9) |
Data are n (%)
Figure 1.
Chart presenting distribution of discospondylitis sites in this study and previously published case reports10–17
Previous referring veterinarian histories, available for nine cats, revealed other sites of infection or possible predisposing factors in two cats: a cat bite at the tail base 3 months before presentation and an ongoing chronic gingivostomatitis complex, respectively. Evidence of attempted antibiotic therapy prior to referral was found in 3/9 available clinical histories. Haematology and serum biochemistry results were available in all 17 cases; identified abnormalities are described in Table 2. Urinalysis was unremarkable in all 17 cases. CSF analysis was within normal limits in three cases. Comorbidities diagnosed at the time of presentation included a presumed adjacent empyema or meningeal involvement (n = 7), suspected abscess in the adjacent paraspinal tissues (n = 4), adjacent T2W hyperintense iliopsoas muscle with femoral nerve involvement (n = 2), megacolon (n = 2), chronic renal disease with hypertension and cachexia (n = 1), endocarditis (n = 1), adjacent intraparenchymal oedema and syringomyelia (n = 1) and retroperitoneal lymphadenopathy (n = 1). Three cases had results for FeLV and FIV available, which were negative; one of these cases also had FCoV antibody titres measured – the obtained value of 1/640 was interpreted as compatible with exposure.
Bacterial cultures, treatment and outcome
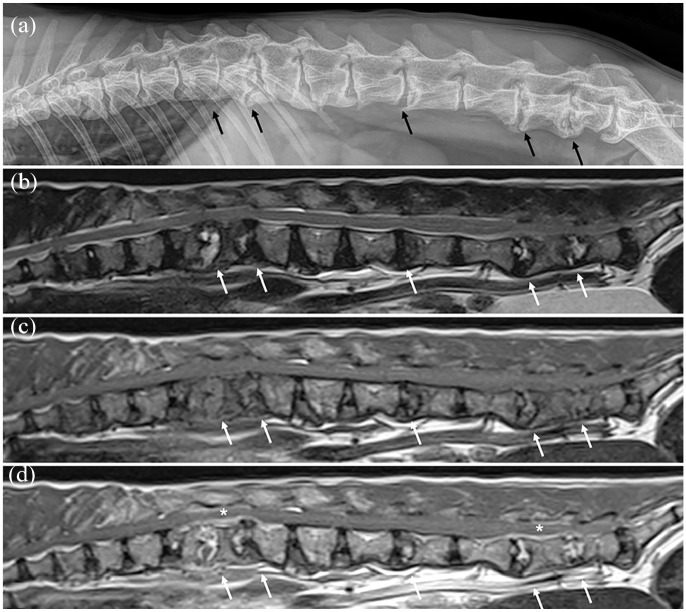
Urine bacterial cultures were performed in all cats. Nine cats also had a distinct tissue cultured: blood cultures in eight cats; FNA sampling in four cats; and lumbar CSF in one case (Table 3). Positive bacterial cultures were achieved in two cats, including Staphylococcus species (n = 1) and Escherichia coli (n = 1). Staphylococcus species were found in urine, blood and intradiscal FNA in a single case, and were sensitive to all tested antibiotics. Escherichia coli was found in urine (only bacterial culture performed in this case) and was resistant to clindamycin alone. Both of these cats presented with multifocal discospondylitis, in two sites (L1–L2 and L5–L6) and in five sites (T12–T13, T13–L1, L3–L4, L5–L6 and L6–L7), respectively, depicted in Figure 2. In another case, cytology of a fluid-filled cavity adjacent to an affected L7–S1 disc identified ‘intracellular rods’ within neutrophils; however, bacterial culture of the fluid was negative. None of the cases had fungal cultures performed.
Table 3.
Bacterial cultures, antibiotics and outcome for the cats in the present study
| Urine culture | n = 17 | |
| Negative | 15 (88.2) | |
| Staphylococcus species | 1 (5.9) | |
| Escherichia coli | 1 (5.9) | |
| Blood culture | n = 8 | |
| Negative | 7 (87.5) | |
| Staphylococcus species | 1 (12.5) | |
| FNA cultures | n = 4 | |
| Intradiscal | n = 3 | |
| – Negative | 2 (66.7) | |
| – Staphylococcus species | 1 (33.3) | |
| Paraspinal musculature | n = 1 | |
| – Unidentified ‘intracellular rods’ | 1 (100) | |
| CSF culture | n = 1 | |
| – Negative | 1 (100) | |
| Antibiotics, dosage regimen | Number of cases | Used as monotherapy |
| Amoxicillin–clavulanic acid, 15–20 mg/kg q12h | 8 (47.1) | 3 (17.6) |
| Metronidazole, 10 mg/kg q12h | 6 (35.3) | 0 (0) |
| Cefalexin, 20 mg/kg q12h | 5 (29.4) | 2 (11.8) |
| Marbofloxacin, 2 mg/kg q24h | 4 (23.5) | 1 (5.9) |
| Clindamycin, 5.5 mg/kg q12h | 2 (11.8) | 2 (11.8) |
| Median (range) time on antibiotics for cases surviving hospitalisation (months) | 3 (1–9) | |
| Outcome | Available in 12 cases | |
| Full neurological improvement | 10 (83.3) | |
| Euthanasia during hospitalisation | 1 (8.3) | |
| Early recurrence (<6 months) | 1 (8.3) | |
Data are n (%)
FNA = fine-needle aspirate; CSF = cerebrospinal fluid
Figure 2.
Example of multifocal feline discospondylitis on (a) left lateral vertebral column radiograph and (b–d) mid-sagittal plane MRI: (b) T2-weighted, (c) T1-weighted pre-contrast and (d) T1-weighted post-contrast images. Evidence of discospondylitis was found at the level of the T12–T13, T13–L1, L3–L4, L5–L6 and L6–L7 intervertebral discs (affected sites indicated by arrows); areas with epidural/meningeal enhancement are indicated by asterisks. This cat had positive bacterial culture results on blood, urine and L6–L7 intervertebral disc fine-needle aspiration for Staphylococcus species; cerebrospinal fluid culture was negative
Treatment was non-surgical in all cats, with antibiotic drugs being started at the individual clinician’s discretion and maintained for a median of 3 months (range 1–9) for cats having survived the initial hospitalisation, with one cat having been euthanased after 3 days of treatment (see below). A single antibiotic was used as the first-line treatment in eight cats; two antibiotics in seven cats; and three antibiotics in a single case with multifocal discospondylitis (Figure 2). Details on the antibiotics used and dosage regimens are given in Table 3. Pain relief, which included non-steroidal anti-inflammatory drugs (NSAIDs; most typically meloxicam, alone or in combination with gabapentin), was also provided to all patients. Exercise restriction was advised in all cats, either by means of strict cage or room rest, or avoiding jumping and the stairs, for a minimum period of 4 weeks.
Outcome information was available in 12 cats (70.6%). In 10/12 cats (83.3%) the outcome was excellent in terms of pain control and neurological function at the time of stopping the antibiotic protocol (Table 3). In one of the improved cats with megacolon, despite resolution of pain and neurological deficits without recurrence, a subtotal colectomy was performed 11 months following diagnosis, with the cat developing chronic diarrhoea after surgery. One case had an incomplete recovery having received a single antibiotic for 6 weeks with initial considerable improvement, with clinical signs relapsing 4 months after presentation; this case was subsequently lost to follow-up. A single case had a poor outcome, having failed to respond to intravenous antibiotic medication and pain relief while hospitalised, and the owner opted for euthanasia 3 days after presentation. Long-term follow-up information was obtained in four cats, all with an excellent outcome, at a median of 31 months (range 12–36) from presentation, without evidence of recurrence. These cats had been treated with two antibiotics (n = 3; amoxicillin–clavulanic acid and metronidazole, cefalexin and marbofloxacin, and cefalexin and metronidazole) or with a single antibiotic (n = 1; clindamycin) for 2, 3, 4 and 9 months, and had been off antibiotics for 34, 27, 8 and 23 months, respectively.
Discussion
This is the largest reported population of cats diagnosed with discospondylitis, describing the typical signalment, clinical and neurological findings, treatment and outcome.
In accordance with previous reports, the most affected breed was the DSH, the most common intervertebral disc affected was L7–S1 (Table 1) and cats were mainly mature adult cats with a median age of 9 years.10–17 Male cats were found to be marginally over-represented (58%) in the study population, which is in agreement with 7/8 previously reported cats.10–17 Discospondylitis in cats has only been found in the thoracolumbar, lumbosacral and coccygeal vertebral column, with no reported instances of cervical discospondylitis.
Discospondylitis can be challenging to diagnose in dogs, as signs are variable and sometimes vague, including pain of unknown origin, lethargy, reluctance to move, fever, anorexia, weight loss and neurological deficits usually occurring secondarily to either concurrent empyema or pathological spinal fracture.1,2 Systemic illness with fever or weight loss are present in only 30% of affected dogs, and hyperaesthesia and neurological dysfunction is not always present.1,2 This inherent difficulty in diagnosis seems to be evident in feline discospondylitis. The most consistent clinical sign was spinal hyperaesthesia, which was found in all cats, and was the sole neurological sign in 6/17 of cats. However, signs of neurological dysfunction seemed to be highly prevalent (present in 11/17 of the study cats). These signs were related to T3–L3 spinal segment neurolocalisation, characterised by paraparesis/pelvic limb ataxia; or a L4–S2 spinal segment, associated nerve root or associated named nerve (eg, sciatic) neuro-localisation, characterised by pelvic limb lameness/nerve root signature or tail paresis. In previously reported cats, the described clinical signs included spinal hyperaesthesia in all cats and neurological deficits, including proprioceptive ataxia, paraparesis or paraplegia, in 5/8 cats.10–17 Non-specific clinical signs such as a reluctance to jump, lethargy or inappetence/anorexia were present in a smaller percentage of cats (Table 2). Pyrexia was identified in a minority of the present cats (n = 3/17), having also only been reported in 2/8 of previously reported cats.11,13 The higher prevalence of neurological dysfunction in cats (n = 11/17 [65%]) vs dogs (reported as being 48% in a large case series), 3 could be interpreted as an indication that cats affected with discospondylitis might be more prone to an adjacent meningomyelitis/neuritis than dogs, a clinical suspicion that has been raised previously. 11 However, this remains speculative considering the small number of cases in the reported population, the limited CSF samples analysed and the fact that compressive lesions such as epidural abscesses could also be contributing to this higher prevalence.
Adjunctive investigations, including haematology, serum biochemistry and urinalysis, did not reveal consistent abnormalities in the current population, with hyperglobulinaemia (23.5%) and leukocytosis (11.8%) being the most prevalent non-specific findings in this study (Table 2). This is in agreement with previous reports, where changes in haematology, including neutrophilia, lymphocytopenia and eosinopenia (n = 1/8); leukocytosis with neutrophilia (n = 1/8);11,13 abnormalities in serum biochemistry, including hyperglobulinaemia (n = 2/8); increased urea and creatinine (n = 1/8) and hypercalcaemia, were reported inconsistently.12,14,17 CSF analysis, despite only having been performed in a minority of cats, did not reveal any abnormalities, which is in agreement with findings in dogs, where analysis is typically unremarkable or reveals only non-specific changes.2,6 Acute-phase proteins, namely C-reactive protein (CRP), have recently been studied in canine discospondylitis in two case series, with 61–87.5% of dogs presenting with increased serum CRP, which was a more prevalent finding than pyrexia/hyperthermia and leukocytosis in both studies, alongside hyperglobulinaemia in one of the studies.20,21 Acute-phase proteins were not assessed in this population. In cats, the serum concentration of CRP is not reported to be significantly increased after an inflammatory stimulus, with early increases in serum amyloid A protein, followed by alpha 1-acid glycoprotein and haptoglobin, appearing to be more consistently found in feline inflammatory processes. 22 Future studies on feline discospondylitis could explore changes in these latter proteins as possible relevant markers of inflammation.
The aetiology of discospondylitis is typically undetermined; however, haematogenous dissemination, migrant foreign bodies or direct extension of infected paravertebral structures have been implicated.1,2 Although not detected in many cases, the source of infection has been suspected to be related to urogenital tract infections, dermatitis, periodontitis, endocarditis or other sites of infection in dogs.1,2,5 It seems likely that the possible aetiology of discospondylitis in cats is similar to that of dogs. In the population reported here, the cats with a previous history of a cat bite abscess and chronic gingivostomatitis, as well as the concomitant identification of endocarditis, were considered compatible with haematogenous spread. The presence of paravertebral abscesses and concomitant empyema/meningeal involvement in these cats could either have been the origin of the discospondylitis or have occurred secondarily by direct extension of the infection. Other potential predisposing factors and infectious comorbidities in previous reports in cats included pyelonephritis, paraspinal abscess and respiratory tract infections.11,13,14 Possible co-infection with FeLV, FIV or FCoV were not investigated in detail in the reported population. Considering that both FeLV and FIV infection have the potential to induce immunosuppression, 23 exploring a possible relation with discospondylitis might be of interest in future studies.
In previously reported cases of feline discospondylitis, positive bacterial cultures were achieved in two cats ante-mortem, through urine cultures,12,14 and post-mortem cultures were positive in another two cats.11,13 E coli was found as a single agent in two cats, alongside Streptococcus canis and Actinomyces viscosus in another case; a single case presented mixed Enterococcus species and Clostridium perfringens infection.11–14 In the current population, bacterial cultures identified the causative agents in only 2/17 (11.8%) cats, E coli and Staphylococcus species, with another case of unidentified ‘intracellular rods’ having been described on cytology of FNAs of affected paraspinal musculature. The prevalence distribution of bacterial species in cases of feline discospondylitis seems to correlate with the most commonly identified pathogens reported in cats with bacterial urinary tract infections.24,25 Both cats with positive bacterial cultures in the current population presented with multifocal discospondylitis. The presence of several affected foci, which were distributed over non-adjacent areas of the vertebral column in both of these cats, likely indicated a more disseminated infectious process, increasing the likelihood of a positive bacterial culture. However, the small number of cats available make this analysis a mere clinical conjecture. Negative bacterial blood or urine cultures are also highly prevalent in canine discospondylitis, representing about 40–75% of cases, with a higher yield of 75% positive results for percutaneous disc aspiration in a single study of 10 dogs.2,3,7 Concomitant sampling of multiple tissues is recommended in cases of discospondylitis in order to maximise the chances of obtaining a positive culture. 21 Despite all current cats having undergone urine cultures, almost half of cats did not have a distinct tissue cultured. The isolation of the causative microorganism may also have been complicated by treatment with antibiotics prior to referral; however, this was only seen in a minority of cases (n = 3/9). Of the cases with a positive culture, one had not received antibiotics previous to referral and, for the other cat, this information was not available from the medical history.
All of the cases of discospondylitis in this study were managed medically with antibiotics provided for a variable period of time, which was also the most commonly reported treatment modality in previous case reports (n = 5/8).12–15,17 In previous cases, two cats were euthanased or had died before treatment was initiated,10,11 and surgical management was performed in a single case alongside broad-spectrum antibiotics. 16 Medical treatment of discospondylitis is based on broad-spectrum antibiotics, ideally based on bacterial culture and sensitivity results, and pain relief. Fungal or algal discospondylitis has not yet been reported in cats, being a less described but possible aetiology of discospondylitis in dogs.3,26 Conservative measures such as cage rest or room rest, and general recommendations such as avoiding jumping and stairs are typically established, for a minimum period of 4 weeks. Nonetheless, guidelines and the length of time of restricted exercise in cats with discospondylitis are still subjective, and have not been studied in detail in either cats or dogs.2,3,27
Previously reported antibiotic treatment protocols in feline discospondylitis cases that improved were based on a combination of enrofloxacin and ampicillin for 8 weeks in one case, 14 and amoxicillin–clavulanic acid for 2 months in another case, 15 with medication having been discontinued at that stage. In the current population, antibiotic monotherapy or dual therapy was initiated in the majority of cats, with a single case being initiated on three antibiotics following diagnosis of a multifocal discospondylitis. As medication is typically initiated before bacterial culture results are available, and bacterial culture results were mostly negative, in the majority of cases antibiotics were initiated empirically based on the individual clinician’s preference. However, considering that the most commonly identified bacteria in feline discospondylitis appear to be E coli, followed by Staphylococcus species, Streptococcus species, Enterococcus species, C perfringens and A viscosus,11–14 antibiotic choice should be based on presumptive activity against these organisms and, in particular, against E coli. This considered, amoxicillin–clavulanic acid, trimethoprim–sulfamethoxazole (not used in our population and considered particularly difficult to use in cats) or cephalosporins would be advisable first-line choices unless otherwise contraindicated by comorbidities. 28 Second-line antibiotics, including metronidazole, fluoroquinolones (such as marbofloxacin, which was used in the current cohort of patients) or clindamycin, should typically only be initiated when supported by bacterial sensitivity results or in case of a poor response to first-line antibiotics. Despite clindamycin having been used in two of the current cats as monotherapy, presenting a broad anaerobic coverage, it is not thought to possess activity against aerobic Gram-negative bacteria such as the typical case of E coli, 29 and therefore should not be used as a first-line monotherapy, unless indicated by culture and sensitivity results.
The duration of antibiotic therapy in successful cases was of a mean of 3 months in this study, with the only recurrence of clinical signs occurring in a cat treated for 6 weeks with a single antibiotic. Although this study’s population sample was small, it appears reasonable that cats suffering from discospondylitis should receive a minimum of 3 months of continuous antibiotic therapy, which could be extended further according to clinical response, or – potentially – based on repeat imaging findings. In feline discospondylitis, repeat radiographs have been reported in one instance following 9 months of treatment, where complete radiological resolution was not achieved. 18
The outcome in the current population was excellent in terms of pain control and neurological function in the majority of analysed cases (83.3%), with a single cat being euthanased shortly after diagnosis. This is in contradiction to previous reports, where half of the cats were either euthanased or died: one case was euthanased at the time of diagnosis; 10 one case died spontaneously shortly after diagnosis; 11 and two cats responded initially to broad-spectrum antibiotics only to be euthanased 6 days or 2 months later, respectively.12,13 In more recent reports, three cats were managed with broad-spectrum antibiotics alone, with a good outcome reported at 3 weeks, 10 weeks and 11 months, respectively,14,15,17 and one case was surgically managed alongside broad-spectrum antibiotics, with a good outcome reported at the 12 month follow-up. 16 In the current study population only one instance of recurrence was recorded, in a cat that received a shorter, 6-week course of antibiotics. Although this was identified in a single cat, it seems to reinforce the need for longer periods of antibiotic therapy in cases of feline discospondylitis. Outcomes following treatment in the current population appear to agree with reports in dogs, where improvement has been reported to range from 69% to 100%.3,8,9,30
Disease prevalence was not investigated in this study. However, based on the paucity of reports of discospondylitis in cats, this appears to be a rare presentation in this species. Our findings of a low yield on positive cultures from these cats supports the notion that this may be a more common condition than the literature suggests. Before consistent diagnostic imaging features, including cross-sectional imaging, as well as recognisable clinical features, were described in dogs, discospondylitis was considered to be a rare condition.3,4,6,9 The imaging features of discospondylitis in cats have been reported elsewhere, aiding in the recognition and diagnosis of this condition. 18
This study was limited by its retrospective and multicentre nature, which meant that treatment protocols was not standardised and information gathered might have been incomplete. Previous medical history from the referring veterinarians was not always available and were not standardised; therefore, detailed predisposing factors information and previous therapy details might have been missed. Long-term follow-up information was obtained in a small number of cats, through inherently subjective telephone interviews, meaning that recurrences could have gone unreported.
Conclusions
The results of this study confirm that feline discospondylitis is an uncommon condition. Spinal hyperaesthesia appears to be the hallmark of this condition, being present in all cats, and being the sole clinical sign in 35.3% of cats. Neurological dysfunction seemed to be highly prevalent, considerably more so than pyrexia, and might be more prevalent in cats than in dogs. Adjunct investigations, including haematology, serum biochemistry and urinalysis, only revealed non-specific changes in a small percentage of cats. Considering the variable and non-specific clinical presentation, imaging is critical in establishing a diagnosis of feline discospondylitis. 18
Bacterial cultures were positive in 11.8% of cats, identifying the presence of E coli and Staphylococcus species. Considering the high prevalence of negative bacterial culture results, empirical antimicrobial therapy was necessary in most cats.
Amoxicillin–clavulanic acid or cephalosporins were considered reasonable standalone first-line antibiotics, with metronidazole, fluoroquinolones (such as marbofloxacin) and clindamycin being possible second-line antibiotics, according to the studied population. Ideally, antibiotics should be supported by bacterial sensitivity results; however, empirical treatment should take into consideration the most prevalent bacteria found in this condition, namely E coli. Analgesia should be included in the management of this disease in all cats, with evidence that NSAIDs and gabapentin can be of help. Restricted exercise for a minimum of 4 weeks was advised in all cats. Overall, the outcomes appeared to be positive, with no long-term evidence of recurrence in cats following sustained antibiotic therapy for a median duration of 3 months.
Footnotes
Accepted: 2 May 2021
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognised high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Sergio A Gomes  https://orcid.org/0000-0002-9452-7262
https://orcid.org/0000-0002-9452-7262
Cristina Toni  https://orcid.org/0000-0002-8003-1784
https://orcid.org/0000-0002-8003-1784
Clare Rusbridge  https://orcid.org/0000-0002-3366-2110
https://orcid.org/0000-0002-3366-2110
Mark Lowrie  https://orcid.org/0000-0002-4993-589X
https://orcid.org/0000-0002-4993-589X
References
- 1. Moore MP. Discospondylitis. Vet Clin North Am Small Anim Pract 1992; 22: 1027–1034. [DOI] [PubMed] [Google Scholar]
- 2. Thomas WB. Diskospondylitis and other vertebral infections. Vet Clin North Am Small Anim Pract 2000; 30: 169–182. [DOI] [PubMed] [Google Scholar]
- 3. Burkert AB, Kerwin SC, Hosgood GL, et al. Signalment and clinical features of diskospondylitis in dogs: 513 cases (1980–2001). J Am Vet Med Assoc 2005; 227: 268–275. [DOI] [PubMed] [Google Scholar]
- 4. Carrera I, Sullivan M, McConnell F, et al. Magnetic resonance imaging features of discospondylitis in dogs. Vet Radiol Ultrasound 2010; 52: 125–131. [DOI] [PubMed] [Google Scholar]
- 5. Kornegay JN, Barber DL. Diskospondylitis in dogs. J Am Vet Med Assoc 1980; 177: 337–341. [PubMed] [Google Scholar]
- 6. Harris JM, Chen AV, Tucker RL, et al. Clinical features and magnetic resonance imaging characteristics of diskospondylitis in dogs: 23 cases (1997–2010). J Am Vet Med Assoc 2013; 242: 359–365. [DOI] [PubMed] [Google Scholar]
- 7. Fischer A, Mahaffey MB, Oliver JE. Fluoroscopically guided percutaneous disk aspiration in 10 dogs with disko-spondylitis. J Vet Intern Med 1997; 11: 284–287. [DOI] [PubMed] [Google Scholar]
- 8. Hurov L, Troy G, Turnwald G. Diskospondylitis in the dog: 27 cases. J Am Vet Med Assoc 1978; 173: 275–281. [PubMed] [Google Scholar]
- 9. Bennett D, Carmichael S, Griffiths IR. Discospondylitis in the dog. J Small Anim Pract 1981; 22: 539–547. [DOI] [PubMed] [Google Scholar]
- 10. Norsworthy GD. Discospondylitis as a cause of posterior paresis. Feline Pract 1979; 9: 39–40, [Google Scholar]
- 11. Malik R, Latter M, Love DN. Bacterial discospondylitis in a cat. J Small Anim Pract 1990; 31: 404–406. [Google Scholar]
- 12. Watson E, Roberts RE. Discospondylitis in a cat. Vet Radiol Ultrasound 1993; 34: 397–398. [Google Scholar]
- 13. Aroch I, Shamir M, Harmelin A. Lumbar diskospondylitis and meningomyelitis caused by Escherichia coli in a cat. Feline Pract 1999; 27: 20–22. [Google Scholar]
- 14. Packer RA, Coates JR, Cook CR, et al. Sublumbar abscess and diskospondylitis in a cat. Vet Radiol Ultrasound 2005; 46: 396–399. [DOI] [PubMed] [Google Scholar]
- 15. Hill MF, Warren-Smith C, Granger N. What is your diagnosis? Diskospondylitis. J Am Vet Med Assoc 2015; 247: 743–745. [DOI] [PubMed] [Google Scholar]
- 16. Kemelman E, Koreshkov A, Lapshin M, et al. Discospondylitis in a cat. Russian Vet J 2019; 4: 22–26. [Google Scholar]
- 17. Liatis T, Hammond G, Miro AC, et al. Presumptive coccygeal diskospondylitis in a cat. Vet Rec Case Rep 2020; 8: e001262. DOI: 10.1136/vetreccr-2020-001262. [Google Scholar]
- 18. Gomes SA, Behr S, Garosi LS, et al. Imaging features of discospondylitis in cats. J Feline Med Surg 2020; 22: 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spencer SE, Knowles T, Ramsey IK, et al. Pyrexia in cats: retrospective analysis of signalment, clinical investigations, diagnosis and influence of prior treatment in 106 referred cases. J Feline Med Surg 2017; 19: 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nye G, Liebel FX, Harcourt-Brown T. C-reactive protein in dogs with suspected bacterial diskospondylitis: 16 cases (2010–2019). Vet Rec Open 2020; 7: e000386. DOI: 10.1136/vetreco-2019-000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trub SA, Bush WW, Paek M, et al. Use of C-reactive protein concentration in evaluation of diskospondylitis in dogs. J Vet Intern Med 2021; 35: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kajikawa T, Furuta A, Onishi T, et al. Changes in concentrations of serum amyloid A protein, α1-acid glycoprotein, haptoglobin, and C-reactive protein in feline sera due to induced inflammation and surgery. Vet Immunol Immunopathol 1999; 68: 91–98. [DOI] [PubMed] [Google Scholar]
- 23. Hartmann K. Clinical aspects of feline immunodeficiency and feline leukemia virus infection. Vet Immunol Immunopathol 2011; 143: 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dorsch R, von Vopelius-Feldt C, Wolf G, et al. Feline urinary tract pathogens: prevalence of bacterial species and antimicrobial resistance over a 10-year period. Vet Rec 2014; 176: 201. DOI: 10.1136/vr.102630. [DOI] [PubMed] [Google Scholar]
- 25. Teichmann-Knorrn S, Reese S, Wolf G, et al. Prevalence of feline urinary tract pathogens and antimicrobial resistance over five years. Vet Rec 2018; 183: 21–28. [DOI] [PubMed] [Google Scholar]
- 26. Manino PM, Oliveira F, Ficken M, et al. Disseminated protothecosis associated with diskospondylitis in a dog. J Am Vet Med Assoc 2014; 50: 429–435. [DOI] [PubMed] [Google Scholar]
- 27. Kerwin S. Discospondylitis and related spinal infections in the dog and cat. In: James Fingeroth J, Thomas WB. (eds). Advances in intervertebral disc disease in dogs and cats. Hoboken, NJ: Wiley-Blackwell, 2015, pp 161–167. [Google Scholar]
- 28. Papich MG. Antibacterial drug therapy. In: Ettinger SJ, Feldman EC, Cote E. (eds). Textbook of veterinary internal medicine. 8th ed. St Louis, MO: Elsevier Health Sciences, 2017, pp 1762–1769. [Google Scholar]
- 29. Smieja M. Current indications for the use of clindamycin: a critical review. Can J Infect Dis 1998; 9: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gage ED. Treatment of discospondylitis in the dog. J Am Vet Med Assoc 1975; 166: 1164–1169. [PubMed] [Google Scholar]