Abstract
Diagnosis of post-kala-azar dermal leishmaniasis (PKDL), caused by Leishmania donovani, is difficult, as the dermal lesions are of several types and resemble those caused by other skin diseases, especially leprosy. Since the disease generally appears very late after the clinical cure of kala-azar in India, it is also difficult to correlate PKDL with a previous exposure to L. donovani. Very few attempts have been made so far to diagnose PKDL serologically, and the diagnostic methods vary in their sensitivities and specificities. Diagnosis of PKDL through sophisticated PCR methods, although highly sensitive, has limited practical use. We have developed a serodiagnostic method using an enzyme-linked immunosorbent assay to detect specific immunoglobulin (Ig) isotypes and IgG subclass antibodies in the sera of Indian PKDL patients. Our assay, which uses L. donovani promastigote membrane antigens, was 100% sensitive for the detection of IgG and 96.7% specific for the detection of IgG and IgG1. Optical density values for individual patients, however, demonstrated wide variations. Western blot analysis based on IgG reactivity could differentiate patients with PKDL from control subjects, which included patients with leprosy, patients from areas where kala-azar is endemic, and healthy subjects, by the detection of polypeptides of 67, 72, and 120 kDa. The recognition patterns of the majority of serum samples from patients with PKDL were also distinct from those of the serum samples from patients with visceral leishmaniasis (VL), at least for a 31-kDa polypeptide. To further differentiate patients with PKDL from those with active and cured VL, we analyzed the specific titers of the Ig isotypes and IgG subclasses. High levels of IgG, IgG1, IgG2, and IgG3 antibodies significantly differentiated patients with PKDL from patients cured of VL. The absence of antileishmanial IgE and IgG4 in patients with PKDL differentiated these patients from those with active VL. These results imply intrinsic differences in the antibodies generated in the sera from patients with PKDL and VL.
Human visceral leishmaniasis (VL), or kala-azar, is a systemic fatal disease caused by an intracellular protozoan parasite belonging to the Leishmania donovani complex. The parasite infects and multiplies preferentially in the macrophages of the spleen, liver, bone marrow, and lymph nodes of the host. Post-kala-azar dermal leishmaniasis (PKDL) appears as a dermatotropic form of the infection of this parasite as a sequel to kala-azar in >50% of the cases in Sudan and 10 to 20% of the cases in India (37, 57). In Sudan and other East African countries, patients develop PKDL during or within 6 months after treatment for VL (9, 31, 57). In India the disease occurs between 1 and 7 years after the cure of kala-azar, although longer periods of 20 to 30 years have also been reported (39, 55). In 8 to 20% of patients the disease may appear without an instance of previous symptomatic kala-azar (37, 55). The clinical manifestations seen in patients with PKDL are an erythematous rash, predominantly on the face; hypopigmented patches or macules which may spread over the whole body; or formations of papules, nodules, or plaques or all possible combinations of these (19, 32, 37). Prolonged treatment regimens with sodium antimony gluconate (SAG) are generally necessary, compounding the problems of cost, toxicity, and resistance associated with this first-line drug used for the treatment of VL (11, 50). Since kala-azar is anthroponotic in India, patients with PKDL are considered a reservoir of parasites, which plays an important role in interepidemic periods of VL (52, 56). Thus, for the control of VL, reliable diagnostic tests for the detection of PKDL deserve urgent attention.
Diagnostic methods based on the detection of parasites in skin smears are invasive and have low sensitivities, especially for macular lesions, where parasites are scanty (14, 56). PCR has a better sensitivity than microscopy for the detection of parasites in skin lesions (34, 46), but its utility is restricted to well-equipped laboratories. The diagnosis of PKDL is therefore made clinically by the distribution and the appearance of the lesions, along with the temporal association with VL and a history of VL (38, 57). PKDL, however, may occur after a long gap of recovery from VL (30, 39) and even without a prior case of symptomatic kala-azar (12, 37), compounding the problems of diagnosis. Moreover, since the lesions of PKDL resemble those that occur with a large number of skin conditions that occur in the area, differentiation of PKDL from other conditions, especially leprosy, has proved difficult (8, 40, 57).
In recent years major advances have been made in the development of diagnostic methodologies that focus on evaluation of the patient's antibody response to determine whether infection or exposure has taken place. Serological tests, including direct agglutination tests and enzyme-linked immunosorbent assays (ELISAs) based on crude or recombinant antigens, are highly sensitive (>90%) for the diagnosis of VL (27, 43, 53). On the other hand, serological tests for the diagnosis of PKDL demonstrate various sensitivities and specificities (10, 45). These tests are based on the detection of antileishmanial immunoglobulin G (IgG) antibodies, which may persist for years after recovery from VL (25, 54). It is therefore difficult to differentiate a positive test result for suspected PKDL from a previous case of VL, limiting the value of these methods in areas of endemicity. Recent studies investigating the pathogenic significance of leishmanial antigen (LAg)-specific Ig isotypes and IgG subclasses have revealed variations in the diagnostic sensitivities and specificities of these antibodies in the sera of patients with kala-azar (2, 4, 6, 43). The levels of these isotypes are significantly elevated during disease and undergo a differential decline following resolution of the infection (3, 4, 6). Furthermore, the antigen-specific pattern of isotype reactivity of sera from patients with kala-azar (41, 48) indicates the potential utility of these isotypes not only for the diagnosis of kala-azar but also for monitoring of the effectiveness of treatment for kala-azar. The purpose of the present study was to explore the extension of this concept for the specific diagnosis of PKDL in comparison to other skin diseases and to differentiate PKDL from active and cured VL.
MATERIALS AND METHODS
Study subjects.
Twenty-three Indian patients, mostly from Bihar, who had suspected PKDL and who were admitted to the School of Tropical Medicine, Calcutta, India, between 1999 and 2003 were included in this study (Table 1). All except one of the patients had a history of kala-azar. The time that had elapsed after cure of kala-azar ranged from 1 to 20 years. Diagnosis of PKDL was done by clinical examination of the lesions, determination of a history of kala-azar and residence in an area of endemicity, and other clinical parameters. Three of these patients had hypopigmented macular lesions, while the rest showed a generalized presentation of papules, nodules, and plaques, indicating a polymorphic distribution. Except for the patients with macular lesions, the infection was confirmed by positive slit smears of the lesions and node biopsy specimens. Blood samples were collected before the initiation of chemotherapy with SAG. For comparison, 10 kala-azar patients who came from Bihar and who were admitted to the same hospital were also included in this study. Blood from these patients was obtained before the initiation of chemotherapy and after successful treatment with SAG (20 mg/kg of body weight). Thirty individuals were included as controls. Of these individuals, 25 lived in Calcutta and had no history of kala-azar. They consisted of 10 patients with clinically confirmed leprosy; 5 patients with different skin diseases, like ringworm, sarcoidosis, skin patches, petyriasis rosea, and erythema multiforme; and 10 healthy controls from the Indian Institute of Chemical Biology (IICB). In addition, sera from five healthy controls from Bihar, an area where VL is endemic, were sampled with the kind help of Shyam Sundar. Sera were collected from these patients before chemotherapy. This study was approved by the Ethical Committee on Human Subjects at IICB. Consent for blood sampling was obtained from all patients and donors.
TABLE I.
Study subjects
| Subject | No. of subjects | Age (yr [mean ± SD]) | History of kala-azara | Lesion, lymph node, or splenic aspirate biopsy specimen available |
|---|---|---|---|---|
| PKDL patients | ||||
| Polymorphic | 20 | 37.52 ± 7.50 | 20/20 | 20/20 |
| Macular | 3 | 31.00 ± 18.08 | 2/3 | 0/3 |
| Kala-azar patients | ||||
| Pretreatment | 10 | 28.40 ± 23.80 | 10/10 | |
| Posttreatment | 10 | |||
| Leprosy patients | 10 | 45.20 ± 3.49 | 0/10 | |
| Patients with other skin diseases | 5 | 36.60 ± 13.05 | 0/5 | |
| Healthy controls | 10 | 26.60 ± 1.07 | 0/10 | |
| Controls from areas of endemicity | 5 | 43.00 ± 5.24 | 0/5 |
Data represent the number of subjects with the indicated characteristic/total number of subjects in the group.
Antigen preparation.
L. donovani AG83, originally isolated from an Indian kala-azar patient, was cultured in vitro for antigen preparation, as described earlier (1). Briefly, stationary-phase promastigotes, harvested after the third or fourth passage, were washed four times in ice-cold 20 mM phosphate-buffered saline (PBS; pH 7.2) and suspended at a concentration of 1.0 g of cell pellet (2.5 × 1010 stationary-phase promastigotes) in 50 ml of cold 5 mM Tris-HCl buffer (pH 7.6). The suspension was vortexed six times for 2 min each time, with a 10-min interval on ice between each vortexing, and centrifuged at 2,310 × g for 10 min. The crude ghost membrane pellet thus obtained was resuspended in 10 ml of the same Tris buffer and sonicated three times for 1 min each time on ice in an ultrasonicator. The suspension was finally centrifuged at 5,190 × g for 30 min, and the supernatant containing the LAgs was harvested and stored in aliquots at −70°C until use. The amount of protein obtained from 1.0 g of cell pellet, as assayed by the method of Lowry et al. (28), was 16 mg.
ELISA for parasite-specific Igs.
The LAg-specific ELISA for the detection of IgG, IgM, IgA, IgE, and IgG subclass antibodies was carried out on polystyrene round-bottom microtiter plates (Tarsons, Calcutta, India), as described earlier (2). The plates were coated with 100 μl of 20 μg of LAg per ml in 20 mM phosphate buffer (pH 7.5) at 4°C overnight. The plates were then washed three times with PBS supplemented with 0.05% Tween 20. Excess reactive sites were blocked with 1% bovine serum albumin (BSA) for 3 h at room temperature; and the plates were washed as described above and subsequently incubated overnight at 4°C with sera from patients with PKDL, patients with active and cured VL, and the other controls that had been serially diluted in PBS containing 1% BSA. After the plates were washed, peroxidase-conjugated goat polyclonal antibody directed against human IgG, IgM, IgA, and IgE (Sigma Immunochemicals, St. Louis, Mo.) was applied at a 1:5,000 dilution with PBS for 3 h at room temperature. After four washes, o-phenylenediamine dihydrochloride was applied as the enzyme substrate and the optical density (OD) was read at 450 nm in an ELISA reader.
For the determination of human IgG subclass antibodies, the LAg-coated wells, incubated with serially diluted sera, were treated with mouse anti-human IgG subclass-restricted monoclonal antibodies (Sigma Immunochemicals) at a 1:2,500 dilution overnight at 4°C. After three washes, peroxidase-conjugated goat anti-mouse IgG (Sigma Immunochemicals) was applied at a 1:5,000 dilution for 3 h at room temperature. The color reaction was carried out as described above, and the OD at 450 nm was measured. Each serum sample was titrated at the dilution required to reach half-maximal absorbance (A450 = 1.0).
SDS-PAGE and Western blotting.
The LAgs (6 μg/lane) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% polyacrylamide gel in a Mini-Protean II apparatus (Bio-Rad Laboratories, Richmond, Calif.). The resolved proteins were electrophoretically transferred to nitrocellulose membranes (Bio-Rad) in 20 mM Tris-HCl-190 mM glycine-20% (vol/vol) methanol buffer at 85 V/cm for 90 min in a Transblot apparatus (Bio-Rad). The nitrocellulose strips were then blocked overnight at room temperature in 100 mM Tris-buffered saline (TBS; pH 7.6) containing 0.1% Tween 20 (electrophoresis reagent; Sigma).
Immunoblotting.
A minor modification of the immunoblotting procedure outlined by Rolland-Burger et al. (42) was used to detect LAg-specific antibodies in the patient sera. The strips were washed once in washing buffer (100 mM TBS, 0.05% Tween 20) and then incubated with diluted serum samples (1:100 in washing buffer) for 1 h at room temperature with constant stirring. The samples included sera from patients with PKDL and leprosy, healthy individuals from IICB who were never exposed to an area of endemicity, and the individuals from regions of endemicity. After incubation with the primary antibody, the strips were washed three times for 20 min each time with washing buffer. After the last wash, goat anti-human IgG conjugated with peroxidase (Genei, Bangalore, India) diluted in washing buffer (1:1,000) was added and the mixture was incubated for 1 h. The strips were then washed thrice, as described above. The last wash was done without Tween 20. The membranes were developed for 2 to 3 min with a 3,3′-diaminobenzidine tetrahydrochloride (DAB) substrate (Sigma) dissolved in 100 mM TBS (0.05% DAB, 0.015% H2O2). With the onset of color development, the reaction was stopped by rinsing with double-distilled H2O several times.
Statistical analysis.
Data were compared for statistical significance by the two-tailed unpaired Student's t test and nonparametric Mann-Whitney test with Graphpad Prism software. P values of <0.05 were considered significant. The lower limit of positivity (the cutoff) was determined from the mean of the healthy controls + 2 standard deviations at a dilution of 1:900 (13).
RESULTS
Specific antibody responses of Indian PKDL patients.
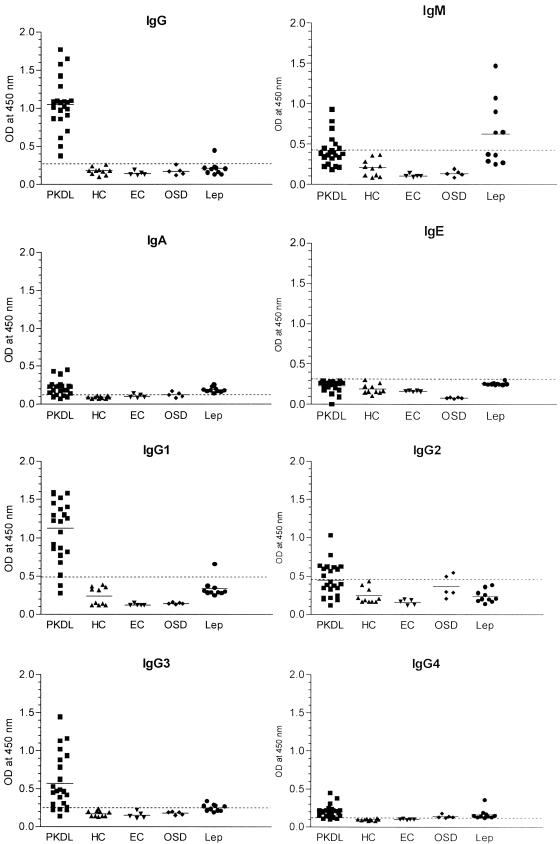
In this study, 53 serum samples, including 23 serum samples from patients with PKDL, 10 serum samples from healthy controls, 5 serum samples from controls from areas of endemicity, 10 serum samples from patients with leprosy, and 5 serum samples from patients with other skin diseases (Table 1), were tested for their antileishmanial antibody responses to the membrane antigens prepared from L. donovani promastigotes (LAgs). Figure 1 and Table 2 show the performances of the sensitivities and the specificities of the ELISAs with sera from patients with PKDL through the responses of the IgG, IgM, IgA, IgE, and IgG subclasses. The reactivities of the IgG, IgA, and IgG subclass antibodies from the sera of patients with PKDL with LAg were significantly higher than those of the sera from the controls, including healthy controls, controls from areas of endemicity, and patients with leprosy and other skin diseases (P < 0.0003). The sera were applied at a 1:900 dilution, and the results are represented in an x-y scatterplot (Fig. 1). Among all the Ig isotypes, the IgG response was the best, with the IgG ELISA showing a 100% sensitivity, followed by the IgG1 ELISA (sensitivity, 90.90%). ELISAs for these two antibodies also showed the highest specificity (96.7%) (Table 2), with a cross-reaction to both antibodies by only one serum sample from a patient with leprosy (Fig. 1). ELISAs for the LAg-specific IgA, IgG3, and IgG4 responses of PKDL patients were moderately sensitive (86.4, 81.8, and 86.4%, respectively); but their specificities were comparatively lower (50, 83.3, and 53.4%, respectively). The highest cross-reactivity was observed with sera from patients with leprosy. All of the serum samples from patients with leprosy had cross-reactive IgA and IgG4 responses to LAg, and 50% of the serum samples from patients with leprosy had cross-reactive IgG3 responses with LAg, with OD values just above the cutoff. ELISAs for IgM and IgG2 had lower sensitivities than those for the other isotypes, and the sera from patients with leprosy had considerable cross-reactive IgM responses to LAg. In comparison to the other isotypes, IgE in sera from patients with PKDL showed an absolute absence of an LAg-specific response and no cross-reaction with the other control sera.
FIG. 1.
LAg-specific antibody reactivities (absorbances) of sera from patients with PKDL, healthy controls (HC), controls from areas of endemicity (EC), patients with other skin diseases (OSD), and patients with leprosy (Lep). The horizontal bars represent the means for the different groups. The dotted lines indicate the cutoff value (the mean for the healthy controls + 2 standard deviations).
TABLE 2.
Sensitivities and specificities of IgG, IgM, IgA, IgE, and IgG subclass ELISAs for PKDL patients
| Antibody (cutoff value) | Sensitivitya (%) | Specificitya (%) |
|---|---|---|
| IgG (0.275) | 100.0 | 96.7 |
| IgM (0.420) | 36.4 | 83.3 |
| IgA (0.122) | 86.4 | 50.0 |
| IgE (0.315) | 0.0 | 100.0 |
| IgG1 (0.479) | 90.9 | 96.7 |
| IgG2 (0.448) | 45.5 | 93.3 |
| IgG3 (0.254) | 81.8 | 83.3 |
| IgG4 (0.120) | 86.4 | 53.4 |
Calculations were done with respect to cutoff OD values for the respective Ig ELISA.
Western blot profiles in response to LAg.
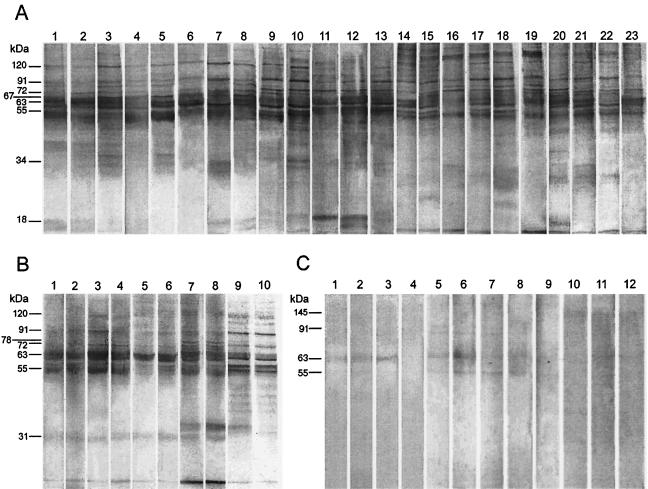
Even though our ELISA was 100% sensitive, it showed low ODs for some PKDL patients, which might cause diagnostic confusion, especially confusion with leprosy. To improve the differentiation of patients with PKDL serologically from patients with leprosy, individuals who live in an area of endemicity, and other healthy controls, we selected 4 samples each from patients with leprosy, controls who lived in an area of endemicity, and healthy controls and 23 serum samples from patients and tested them for their IgG reactivities to SDS-PAGE-separated polypeptides of LAg transferred onto nitrocellulose. The comparative IgG reactivity patterns of these samples are shown in Fig. 2. The LAg-specific pattern of recognition varied for the sera from the PKDL patients, depending on the reactivity of each serum sample, but these patterns were clearly distinct from those for the sera of patients with leprosy, healthy controls, and controls who lived in areas of endemicity. Despite the heterogeneous response to LAg, eight polypeptides with relative molecular masses of 51, 55, 63, 67, 72, 78, 91, and 120 kDa were reactive with 95.7 to 100% of the serum samples from patients with PKDL. In addition, major bands of 34 kDa (78%), 155 kDa (74%), 36 kDa (69.6%), 47 and 145 kDa (65%), 43 and 57 kDa (52%), and 39, 97, and 190 kDa (48%) were frequently recognized. While most of these frequently recognized bands were also reactive with sera from patients with kala-azar and patients who had been cured of kala-azar (Fig. 2B) (41), differences in reactivity were observed for the 31- and 67-kDa polypeptides. Although the 31-kDa polypeptide was not recognized by 82.6% of the serum samples from patients with PKDL, it was recognized by 100% of the serum samples from patients with VL and 73 to 100% of the serum samples from patients cured of VL (Fig. 2B) (41). Conversely, the 67-kDa polypeptide, which was recognized by 100% of the serum samples from patients with PKDL, was recognized by only 6.7% (41) to 20% (Fig. 2B) of the serum samples from patients with VL before and after treatment. Among the controls, very faint cross-reactions were observed, mostly to the 63-kDa (100%) and 145-kDa (75%) peptides for the sera from patients with leprosy, the 63-kDa peptide (75%) for the sera from healthy individuals, and the 91- and 78-kDa peptides (75%) and the 63- and 55-kDa peptides (100%) for the sera from the controls from areas of endemicity (Fig. 2C).
FIG. 2.
Western blot analysis of the IgG reactivities with SDS-PAGE-separated (6 μg/lane) membrane proteins of LAg of sera from patients with PKDL (A); kala-azar (VL) (B) before (odd-numbered lanes) and after (even-numbered lanes) treatment; and controls (C), including healthy controls (lanes 1 to 4), controls from areas of endemicity (lanes 5 to 8), and patients with leprosy (lanes 9 to 12). Each lane represents one serum sample. The molecular masses of the prominent bands are marked on the left.
Differential diagnosis of PKDL from active and cured VL.
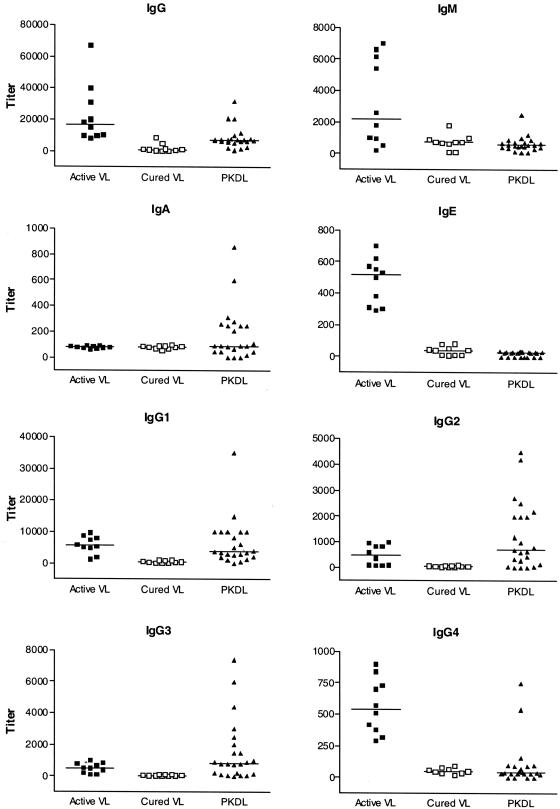
To differentiate PKDL serologically from untreated and cured VL, a clinical study was undertaken with Indian kala-azar patients by using the titers of LAg-specific Ig isotype and IgG subclass antibodies (Fig. 3). The intergroup differences were analyzed by the nonparametric Mann-Whitney test. Although high levels of anti-LAg IgG, IgM, IgA, IgG1, IgG2, and IgG3 antibodies were observed in the sera of PKDL patients, the titers of the IgG, IgM, IgE, and IgG4 antibodies in patients with the active stage of kala-azar were significantly higher (P = 0.0011, 0.0029, <0.0001, and <0.0001, respectively) than those in PKDL patients. In contrast to the sera from patients with active VL, none of the serum samples from patients with PKDL showed an antigen-specific IgE response, and the IgG4 response was very low in 21 of the 23 serum samples from patients with PKDL tested. This lack of LAg-specific IgE and IgG4 responses in PKDL patients may help to discriminate patients with PKDL from those with VL. The titers of anti-LAg IgG, IgG1, IgG2, and IgG3 antibodies in the sera of PKDL patients were significantly higher (P = 0.0005, <0.0001, 0.0043, and 0.0005 respectively) than those of the antibodies that persisted in the patients cured of VL in the past. The level of IgE, which was low in individuals cured of VL, was significantly higher (P = 0.0328) in the sera of patients with PKDL. The specific patterns of the Ig isotype and IgG subclass responses of the sera from patients with PKDL in comparison to those of the sera from patients with active and cured VL suggest that this response is due to the occurrence of PKDL rather than the persistence of antibodies to VL.
FIG. 3.
Comparison of LAg-specific IgG, IgM, IgA, IgE, and IgG subclass antibody titers in sera from patients with PKDL with those in sera from patients with VL before and after treatment. Symbols represent individual patients, and the horizontal bars indicate the median isotype levels in 10 samples from patients with VL and 23 samples from patients with PKDL. Groups were compared for statistical significance by the nonparametric Mann-Whitney test.
DISCUSSION
Parasitological methods have been considered the first choice for the diagnosis of leishmaniases, including PKDL. However, demonstration of parasites in skin smears or biopsy specimens from PKDL patients is hampered by the fact that lesions occur mainly on the face. Besides, parasites are scanty and are found in only one-third of the patients (23, 34). Parasites are extremely difficult to demonstrate in the hypopigmented macuoles, which may be the initial manifestation of PKDL (6, 28, 46). The disease is therefore often misdiagnosed as leprosy, a coendemic disease that resembles PKDL both clinically and pathologically (8, 12, 40). PCR is a sensitive and specific assay for the diagnosis of PKDL (34, 46). However, PCR is expensive and requires sophisticated facilities and trained personnel, restricting its use. Thus, a more economical and practical assay is required for the sensitive and specific diagnosis of PKDL.
Given the strong humoral response that accompanies leishmanial infections, it is not surprising that tests based on serological techniques have been developed for the diagnosis of VL (49). In comparison, attempts at the serodiagnosis of PKDL have been few, and these lack sensitivity and specificity. Antileishmanial antibodies of the IgG, IgM, and IgG subclasses have been demonstrated in the sera of PKDL patients (18, 21). However, to our knowledge, determination of the subclass distribution of the antibody response for the diagnosis of PKDL has not been investigated. In this study we have evaluated the utility of leishmanial membrane antigen (LAg)-specific Ig isotypes and IgG subclasses for the specific diagnosis of PKDL. The sera of a high proportion of the PKDL patients had elevated levels of anti-LAg IgG, IgM, IgA, and IgG subclass (IgG1, IgG2, and IgG3) antibodies. In contrast, the sera of the patients with PKDL demonstrated an absence of IgE and low levels of IgG4. The high sensitivities and specificities of IgG and IgG1 in the LAg-specific ELISA demonstrate the potential of this assay for the diagnosis of PKDL.
Among the Ig isotype and IgG subclasses studied, IgG showed the highest sensitivity and specificity for LAg in PKDL patients. The IgG ELISA conducted with LAg appeared to be more sensitive than serodiagnosis with whole cells by a direct agglutination test (35, 57), an IgG ELISA with total parasite antigen extract (45), as well as a strip test and an IgG ELISA with recombinant K39 (rK39) antigen (47, 54) and other specific antigens (13). The sensitivity of the IgG ELISA for LAg observed in the present study for the diagnosis of PKDL (100%) was even higher than that reported for PCR in Sudan (82.7%) (34) and India (93.8%) (46). The specificity of the assay (96.7%), although lower than those of PCR and the strip test with rK39 (100%) (46, 47), was higher than the specificities of ELISAs with promastigote and amastigote antigen extracts and rK39 (45).
Increases in the sensitivity and specificity of the serodiagnosis of PKDL by use of a combination of specific antigens has been observed earlier (13), indicating the strong potential of the components of LAg for use in the diagnosis of PKDL. However, a false-positive reaction with LAg was observed with a serum sample from a leprosy patient, a disease often confused with PKDL (8, 12, 40, 57). Moreover, the IgG antibody reactivities of sera from patients with PKDL with LAg, although significantly higher than those of sera from the controls, demonstrated wide variations in IgG absorbance just above the cutoff value for some specimens. The Western blot technique is among the most sensitive and specific serological methods; in addition, it provides information about the parasite's antigenic profile (42). We therefore investigated by immunoblotting the activities of sera from patients with PKDL in comparison to those of sera from patients with leprosy and negative controls. The band patterns of the PKDL patient sera in this study were consistent, with at least eight immunodominant bands, regardless of the level of IgG reactivity with LAg in the ELISA. Apart from the 63-kDa polypeptide, none of these polypeptides showed cross-reactivity with antibodies from healthy individuals or leprosy patients. A mild cross-reaction, however, with the 55-, 78-, and 91-kDa polypeptides was observed with sera from controls from areas of endemicity, restricting the achievement of 100% sensitivity and specificity of recognition by PKDL patient sera to reactivities with polypeptides of 67, 72, and 120 kDa. Of the polypeptides most frequently recognized by sera from patients with PKDL, all except the 67-kDa polypeptide were also recognized by the majority of the serum samples from patients with kala-azar before and after treatment. Again, a 31-kDa band of LAg recognized by the majority of the VL patient sera both before and after treatment showed a lack of reactivity with the majority of PKDL patient serum samples, demonstrating the potential for the use of these polypeptides for the differentiation of PKDL from past and ongoing VL infections. Previous investigations (18, 26, 44) on the host immune response to L. donovani antigens in PKDL patients report specific reactions to antigens different from those observed in the present study. A similar lack of reactivity to an antigen fraction (200 kDa) by PKDL patient sera but the reactivity of VL patient sera to this antigen fraction (26) demonstrates that adaptations of these antigens may be helpful in differentiating PKDL from active and past VL.
Most serological tests for the diagnosis of PKDL are of limited value in areas of endemicity, as they are also positive for kala-azar for patients with active and past cases of kala-azar (3, 18, 20, 41, 44, 45, 56). Even rK39 ELISA titers remain positive for up to 2 years after treatment, with no decline in OD values (54). As a result these tests cannot differentiate PKDL from active and past kala-azar. Moreover, tests that can be used to differentiate PKDL from VL are also important for the conclusive diagnosis of PKDL instead of leprosy in leprosy patients with a history of VL. Ig isotypes and IgG subclass antibodies in kala-azar patients demonstrated differential responses after successful chemotherapy (3, 6), and Ig isotype and IgG subclass specificities were observed in PKDL patient sera. Therefore, as an attempt to further differentiate PKDL from active kala-azar as well as past cases of kala-azar, we investigated the LAg-specific titers of these antibodies of PKDL patient sera and compared them with those of sera from patients with active and cured kala-azar. PKDL could be differentiated from active VL by an almost complete absence of IgE and IgG4. In addition, an elevation of IgG, IgG1, IgG2, and IgG3 titers in patients with PKDL in comparison to the negligible levels of these isotypes in individuals cured of VL could help differentiate PKDL from past VL. Although sera from leprosy patients with past VL were not evaluated in the present study, the ability to differentiate PKDL from both active and cured VL will also help with the specific diagnosis of PKDL instead of leprosy in leprosy patients with a history of VL. Our results reinstate the observation that differentiation of PKDL from active and past kala-azar cannot be performed through the analysis of specific IgG levels alone (54). Moreover, a differential Ig isotype profile in patients with PKDL in comparison to that in patients with active and cured VL, together with a lack of IgG recognition of the 31-kDa band, suggest that these antibodies in PKDL are not remnants of previous exposure to VL but are specific for the dermal disease.
Interestingly, the IgA responses in PKDL patients appeared to be reciprocal to the IgE responses in VL patients. While the significance of these observations has not been investigated in this study, recent advances suggest the involvement of interleukin-10 (IL-10) and transforming growth factor β (TGF-β) in enhancing IgA production in human cells (7, 29, 33). IL-4 and IL-13 are involved in the induction of IgE (15, 36, 58), and the presence of IL-10 appears to down regulate the IL-4-induced IgE levels (24). The IL-4 and IL-10 responses have been extensively documented in patients with VL (17, 51, 58) and PKDL. However, higher levels of IL-10 have been reported in patients with PKDL, and higher IL-10 levels are also predictive of the dermal disease (16, 22). Although TGF-β has not been reported so far in patients with PKDL, recent evidence indicates that regulatory T cells characterized by their ability to produce IL-10 and TGF-β may play an important role in the reactivation of persistent infections like PKDL (5). It is therefore tempting to speculate that the decreased levels of production of IgE and the enhanced production of IgA in patients with PKDL, reciprocal to the situation in patients with VL, is due to the activities of IL-10, probably with help from TGF-β; and this speculation needs to be investigated.
In conclusion, our results demonstrate the use of ELISA and immunoblotting for the reliable and sensitive diagnosis of PKDL. The data presented here reveal that differentiation of PKDL not only from leprosy but also from active and cured VL is possible. These observations are sufficiently promising to warrant further validation to determine the potentials of Ig isotype profiles as markers for the differential diagnosis of PKDL.
Acknowledgments
We gratefully acknowledge the patients of the School of Tropical Medicine, Calcutta, India, who participated in this study. We thank the volunteer blood donors in Calcutta and Bihar. We thank Samir Bhattacharya, IICB, for supporting this work.
This work was supported through grants from CSIR, DST, and UGC, Government of India. S.S. is a research fellow supported by UGC.
REFERENCES
- 1.Afrin, F., and N. Ali. 1997. Adjuvanticity and protective immunity elicited by Leishmania donovani antigens encapsulated in positively charged liposomes. Infect. Immun. 65:2371-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anam, K., F. Afrin, D. Banerjee, N. Pramanik, S. K. Guha, R. P. Goswami, P. N. Gupta, S. K. Saha, and N. Ali. 1999. Immunoglobulin subclass distribution and diagnostic value of Leishmania donovani antigen-specific immunoglobulin G3 in Indian kala-azar patients. Clin. Diagn. Lab. Immunol. 6:231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anam, K., F. Afrin, D. Banerjee, N. Pramanik, S. K. Guha, R. P. Goswami, S. K. Saha, and N. Ali. 1999. Differential decline in Leishmania membrane antigen-specific immunoglobulin G (IgG), IgM, IgE, and IgG subclass antibodies in Indian kala-azar patients after chemotherapy. Infect. Immun. 67:6663-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atta, A. M., A. D'Oliveira, Jr., J. Correa, M. L. Atta, R. P. Almeida, and E. M. Carvalho. 1998. Anti-leishmanial IgE antibodies: a marker of active disease in visceral leishmaniasis. Am. J. Trop. Med. Hyg. 59:426-430. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid, Y. 2003. The role of CD4+ CD25+ regulatory T cells in Leishmania infection. Expert. Opin. Biol. Ther. 3:875-885. [DOI] [PubMed] [Google Scholar]
- 6.da Matta, V. L., S. Hoshino-Shimizu, R. Dietze, and C. E. Corbett. 2000. Detection of specific antibody isotypes and subtypes before and after treatment of American visceral leishmaniasis. J. Clin. Lab. Anal. 14:5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Defrance, T., B. Vanbervliet, F. Briere, I. Durand, F. Rousset, and J. Banchereau. 1992. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J. Exp. Med. 175:671-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhar, S., I. Kaur, G. Dawn, S. Sehgal, and B. Kumar. 1995. Post-kala-azar dermal leishmaniasis mimicking leprosy: experience with 4 patients, with some unusual features in 1. Lepr. Rev. 66:250-256. [DOI] [PubMed] [Google Scholar]
- 9.el Hassan, A. M., H. W. Ghalib, E. E. Zijlstra, I. A. Eltoum, M. Satti, M. S. Ali, and H. M. Ali. 1992. Post-kala-azar dermal leishmaniasis in the Sudan: clinical features, pathology and treatment. Trans. R. Soc. Trop. Med. Hyg. 86:245-248. [DOI] [PubMed] [Google Scholar]
- 10.el Hassan, A. M., F. A. Hashim, M. Abdullah, E. E. Zijlstra, and H. W. Ghalib. 1993. Distinguishing post-kala-azar dermal leishmaniasis from leprosy: experience in the Sudan. Lepr. Rev. 64:53-59. [DOI] [PubMed] [Google Scholar]
- 11.el Hassan, A. M., and E. A. Khalil. 2001. Post-kala-azar dermal leishmaniasis: does it play a role in the transmission of Leishmania donovani in the Sudan? Trop. Med. Int. Health 6:743-744. [DOI] [PubMed] [Google Scholar]
- 12.el-Hassan, A. M., H. W. Ghalib, E. E. Zijlstra, I. A. El-toum, M. S. Ali, and H. M. Ahmed. 1990. Post-kala-azar dermal leishmaniasis in the absence of active visceral leishmaniasis. Lancet 336:750. [DOI] [PubMed] [Google Scholar]
- 13.Fargeas, C., M. Hommel, R. Maingon, C. Dourado M. Monsigny, and R. Mayer. 1996. Synthetic peptide-based enzyme-linked immunosorbent assay for serodiagnosis of visceral leishmaniasis. J. Clin. Microbiol. 34:241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg, V. K., S. Agrawal, S. Rani, A. Joshi, A. Agarwalla, M. L. Das, and S. Koirala. 2001. Post-kala-azar dermal leishmaniasis in Nepal. Int. J. Dermatol. 40:179-184. [DOI] [PubMed] [Google Scholar]
- 15.Gascan, H., J. F. Gauchat, M. G. Roncarolo, H. Yssel, H. Spits, and J. E. de Vries. 1991. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by interleukin 4 and a signal provided by activated CD4+ T cell clones. J. Exp. Med. 173:747-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasim, S., A. M. Elhassan, E. A. Khalil, A. Ismail, A. M. Kadaru, A. Kharazmi, and T. G. Theander. 1998. High levels of plasma IL-10 and expression of IL-10 by keratinocytes during visceral leishmaniasis predict subsequent development of post-kala-azar dermal leishmaniasis. Clin. Exp. Immunol. 111:64-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghalib, H. W., M. R. Piuvezam, Y. A. Skeiky, M. Siddig, F. A. Hashim, A. M. el-Hassan, D. M. Russo, and S. G. Reed. 1993. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J. Clin. Investig. 92:324-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh, A. K., S. Dasgupta, and A. C. Ghose. 1995. Immunoglobulin G subclass-specific antileishmanial antibody responses in Indian kala-azar and post-kala-azar dermal leishmaniasis. Clin. Diagn. Lab. Immunol. 2:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girgla, H. S., R. A. Marsden, G. M. Singh, and T. J. Ryan. 1977. Post-kala-azar dermal leishmaniasis. Br. J. Dermatol. 97:307-311. [DOI] [PubMed] [Google Scholar]
- 20.Goswami, R. P., B. Bairagi, and P. K. Kundu. 2003. K39 strip test—easy, reliable and cost-effective field diagnosis for visceral leishmaniasis in India. J. Assoc. Physicians India 51:759-761. [PubMed] [Google Scholar]
- 21.Halder, J. P., K. C. Saha, and A. C. Ghose. 1981. Serological profiles in Indian post-kala-azar dermal leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 75:514-517. [DOI] [PubMed] [Google Scholar]
- 22.Ismail, A., A. M. El Hassan, K. Kemp, S. Gasim, A. E. Kadaru, T. Moller, A. Kharazmi, and T. G. Theander. 1999. Immunopathology of post kala-azar dermal leishmaniasis (PKDL): T-cell phenotypes and cytokine profile. J. Pathol. 189:615-622. [DOI] [PubMed] [Google Scholar]
- 23.Ismail, A., A. Kharazmi, H. Permin, and A. M. El-Hassan. 1997. Detection of Leishmania in tissues of patients with post-kala-azar dermal leishmaniasis using a specific monoclonal antibody. Trans. R. Soc. Trop. Med. Hyg. 91:283-285. [DOI] [PubMed] [Google Scholar]
- 24.Jeannin, P., S. Lecoanet, Y. Delneste, J. F. Gauchat, and J. Y. Bonnefoy. 1998. IgE versus IgG4 production can be differentially regulated by IL-10. J. Immunol. 160:3555-3561. [PubMed] [Google Scholar]
- 25.Jensen, A. T., S. Gasim, T. Moller, A. Ismail, A. Gaafar, M. Kemp, A. M. el Hassan, A. Kharazmi, T. M. Alce, D. F. Smith, and T. G. Theander. 1999. Serodiagnosis of Leishmania donovani infections: assessment of enzyme-linked immunosorbent assays using recombinant L. donovani gene B protein (GBP) and a peptide sequence of L. donovani GBP. Trans. R. Soc. Trop. Med. Hyg. 93:157-160. [DOI] [PubMed] [Google Scholar]
- 26.Kaul, P., N. Malla, S. Kaur, R. C. Mahajan, and N. K. Ganguly. 2000. Evaluation of a 200-kDa amastigote-specific antigen of L. donovani by enzyme-linked immunosorbent assay (ELISA) for the diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 94:173-175. [DOI] [PubMed] [Google Scholar]
- 27.Kumar, R., K. Pai, K. Pathak, and S. Sundar. 2001. Enzyme-linked immunosorbent assay for recombinant K39 antigen in diagnosis and prognosis of Indian visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 8:1220-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-276. [PubMed] [Google Scholar]
- 29.McKarns, S. C., J. J. Letterio, and N. E. Kaminski. 2003. Concentration-dependent bifunctional effect of TGF-beta 1 on immunoglobulin production: a role for Smad3 in IgA production in vitro. Int. Immunopharmacol. 3:1761-1774. [DOI] [PubMed] [Google Scholar]
- 30.Mittal, R., P. N. Behl, and G. Srivastava. 2002. Post-kala-azar dermal leishmanasis occurring after 10 years of treated kala azar. Int. J. Dermatol. 41:875-876. [DOI] [PubMed] [Google Scholar]
- 31.Muigai, R., G. S. Gachihi, C. N. Oster, J. B. Were, P. M. Nyakundi, C. N. Chunge, G. Kirigi, and J. R. Rashid. 1991. Post-kala-azar dermal leishmaniasis: the Kenyan experience. East Afr. Med. J. 68:801-806. [PubMed] [Google Scholar]
- 32.Napier, L. E., and C. R. Das Gupta. 1930. A clinical study of post-kala azar dermal leishmaniasis. Indian Med. Gaz. 65:249-257. [PMC free article] [PubMed] [Google Scholar]
- 33.Ogawa, J., A. Sasahara, T. Yoshida, M. M. Sira, T. Futatani, H. Kanegane, and T. Miyawaki. 2004. Role of transforming growth factor-beta in breast milk for initiation of IgA production in newborn infants. Early Hum. Dev. 77:67-75. [DOI] [PubMed] [Google Scholar]
- 34.Osman, O. F., L. Oskam, N. C. Kroon, G. J. Schoone, E. T. Khalil, A. M. El-Hassan, E. E. Zijlstra, and P. A. Kager. 1998. Use of PCR for diagnosis of post-kala-azar dermal leishmaniasis. J. Clin. Microbiol. 36:1621-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pal, A., K. Mukerji, D. Basu, K. Naskar, K. K. Mallick, and K. Ghosh,. 1991. Evaluation of direct agglutination test (DAT) and ELISA for serodiagnosis of visceral leishmaniasis in India. J. Clin. Lab. Anal. 5:303-306. [DOI] [PubMed] [Google Scholar]
- 36.Punnonen, J., G. Aversa, B. G. Cocks, and J. E. de Vries. 1994. Role of interleukin-4 and interleukin-13 in synthesis of IgE and expression of CD23 by human B cells. Allergy 49:576-586. [DOI] [PubMed] [Google Scholar]
- 37.Ramesh, V., and A. Mukherjee. 1995. Post-kala-azar dermal leishmaniasis. Int. J. Dermatol. 34:85-91. [DOI] [PubMed] [Google Scholar]
- 38.Ramesh, V., and N. A. Singh. 1999. Clinical and histopathological study of macular type of post-kala-azar dermal leishmaniasis. Trop. Doct. 29:205-207. [DOI] [PubMed] [Google Scholar]
- 39.Ramesh, V., R. S. Misra, U. Saxena, and A. Mukherjee. 1993. Post-kala-azar dermal leishmaniasis: a clinical and therapeutic study. Int. J. Dermatol. 32:272-275. [DOI] [PubMed] [Google Scholar]
- 40.Ramesh, V., U. Saxena, R. S. Misra, and A. Mukherjee. 1991. Post-kala-azar dermal leishmaniasis: a case report strikingly resembling lepromatous leprosy. Lepr. Rev. 62:217-221. [DOI] [PubMed] [Google Scholar]
- 41.Ravindran, R., K. Anam, B. C. Bairagi, B. Saha, N. Pramanik, S. K. Guha, R. P. Goswami, D. Banerjee, and N. Ali. 2004. Characterization of immunoglobulin G and its subclass response to Indian kala-azar infection before and after chemotherapy. Infect. Immun. 72:863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolland-Burger, L., X. Rolland, C. W. Grieve, and L. Monjour. 1991. Immunoblot analysis of the humoral immune response to Leishmania donovani infantum polypeptides in human visceral leishmaniasis. J. Clin. Microbiol. 29:1429-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan, J. R., A. M. Smithyman, G. H. Rajasekariah, L. Hochberg, J. M. Stiteler, and S. K. Martin. 2002. Enzyme-linked immunosorbent assay based on soluble promastigote antigen detects immunoglobulin M (IgM) and IgG antibodies in sera from cases of visceral and cutaneous leishmaniasis. J. Clin. Microbiol. 40:1037-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salotra, P., A. Raina, and V. Ramesh. 1999. Western blot analysis of humoral immune response to Leishmania donovani antigens in patients with post-kala-azar dermal leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 93:98-101. [DOI] [PubMed] [Google Scholar]
- 45.Salotra, P., G. Sreenivas, A. A. Nasim, B. V. Subba Raju, and V. Ramesh. 2002. Evaluation of enzyme-linked immunosorbent assay for diagnosis of post-kala-azar dermal leishmaniasis with crude or recombinant k39 antigen. Clin. Diagn. Lab. Immunol. 9:370-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salotra, P., G. Sreenivas, G. P. Pogue, N. Lee, H. L. Nakhasi, V. Ramesh, and N. S. Negi. 2001. Development of a species-specific PCR assay for detection of Leishmania donovani in clinical samples from patients with kala-azar and post-kala-azar dermal leishmaniasis. J. Clin. Microbiol. 39:849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salotra, P., G. Sreenivas, V. Ramesh, and S. Sundar. 2001. A simple and sensitive test for field diagnosis of post kala-azar dermal leishmaniasis. Br. J. Dermatol. 145:630-632. [DOI] [PubMed] [Google Scholar]
- 48.Shiddo, S. A., G. Huldt, L. A. Nilsson, O. Ouchterlony, and R. Thorstensson. 1996. Visceral leishmaniasis in Somalia. Significance of IgG subclasses and of IgE response. Immunol. Lett. 50:87-93. [DOI] [PubMed] [Google Scholar]
- 49.Sundar, S., and M. Rai. 2002. Laboratory diagnosis of visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 9:951-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sundar, S., D. K. More, M. K. Singh, V. P. Singh, S. Sharma, A. Makharia, P. C. Kumar, and H. W. Murray. 2000. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 31:1104-1107. [DOI] [PubMed] [Google Scholar]
- 51.Sundar, S., S. G. Reed, S. Sharma, A. Mehrotra, and H. W. Murray. 1997. Circulating T helper 1 (Th1) cell- and Th2 cell-associated cytokines in Indian patients with visceral leishmaniasis. Am. J. Trop. Med. Hyg. 56:522-525. [DOI] [PubMed] [Google Scholar]
- 52.Thakur, C. P., and K. Kumar. 1992. Post-kala-azar dermal leishmaniasis: a neglected aspect of kala-azar control programmes. Ann. Trop. Med. Parasitol. 86:355-359. [DOI] [PubMed] [Google Scholar]
- 53.Veeken, H., K. Ritmeijer, J. Seaman, and R. Davidson. 2003. Comparison of an rK39 dipstick rapid test with direct agglutination test and splenic aspiration for the diagnosis of kala-azar in Sudan. Trop. Med. Int. Health 8:164-167. [DOI] [PubMed] [Google Scholar]
- 54.Zijlstra, E. E., N. S. Daifalla, P. A. Kager, E. A. Khalil, A. M. el-Hassan, S. G. Reed, and H. W. Ghalib. 1998. rK39 enzyme-linked immunosorbent assay for diagnosis of Leishmania donovani infection. Clin. Diagn. Lab. Immunol. 5:717-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zijlstra, E. E., A. M. el-Hassan, and A. Ismael. 1995. Endemic kala-azar in eastern Sudan: post-kala-azar dermal leishmaniasis. Am. J. Trop. Med. Hyg. 52:299-305. [DOI] [PubMed] [Google Scholar]
- 56.Zijlstra, E. E., A. M. Musa, E. A. Khalil, I. M. el-Hassan, and A. M. el-Hassan. 2003. Post-kala-azar dermal leishmaniasis. Lancet Infect. Dis. 3:87-98. [DOI] [PubMed] [Google Scholar]
- 57.Zijlstra, E. E., E. A. Khalil, P. A. Kager, and A. M. el-Hassan. 2000. Post-kala-azar dermal leishmaniasis in the Sudan: clinical presentation and differential diagnosis. Br. J. Dermatol. 143:136-143. [DOI] [PubMed] [Google Scholar]
- 58.Zwingenberger, K., G. Harms, C. Pedrosa, S. Omena, B. Sandkamp, and S. Neifer. 1990. Determinants of the immune response in visceral leishmaniasis: evidence for predominance of endogenous interleukin 4 over interferon-gamma production. Clin. Immunol. Immunopathol. 57:242-249. [DOI] [PubMed] [Google Scholar]