Abstract
Objectives
Ureteral obstruction (UO) in cats causes acute kidney injury and typically requires surgical intervention. Information is required about potentially modifiable risk factors to inform prevention strategies.
Methods
A case-control study was performed to assess risk factors associated with feline UO. Cases were defined as cats with either of the following: (1) ureteral obstruction (ureteroliths: 13/18; unknown: 5/18) confirmed with pyelography; or (2) a creatinine concentration >140 µmol/l with both UO (ureteroliths: 6/10; blood clots: 3/10; pyonephrosis: 1/10) and pyelectasia ⩾5 mm on abdominal ultra sonography. Controls were defined as cats without evidence of UO on history, physical examination and abdominal ultrasound. Age, sex, breed (domestic shorthair/longhair), diet (predominantly dry, mixed or predominantly moist food), housing (indoors or mixed) and plasma total calcium were evaluated for their association with UO using multivariable logistic regression. A receiver operator characteristic (ROC) curve was created to evaluate the predictive ability of the final model.
Results
In total, 168 cats (28 cases, 140 controls) were included. Age, sex, breed, housing and total calcium were not significantly associated with UO; however, diet was. Compared with cats eating a predominantly moist food diet, cats fed a predominantly dry food diet were 15.9 times more likely to develop a UO (95% confidence interval 2.9–295; P = 0.009). There was no difference in the association between diet and UO in cats fed a mixed diet vs cats fed a predominantly moist food diet (P = 0.25). The area under the ROC curve was 72%.
Conclusions and relevance
Changes in diet formulation could provide a simple and economical method to reduce the risk of UO.
Keywords: Ureteral obstruction, risk factors, diet, acute kidney injury
Introduction
Ureteral obstruction (UO) is a serious and potentially life-threatening disease that is being recognised with increasing frequency in cats. 1 UO is most often due to ureterolithiasis, ureteral strictures, solidified blood calculi and infection.1–3 UO causes an obstructive nephropathy and acute kidney injury (AKI). 4 Without major surgical intervention, UO results in substantial kidney injury and, if bilateral or pre-existing kidney disease is present, can be fatal. Medical management of UO has a low (8–13%) success rate and recent guidelines advise against attempted medical management of feline ureteroliths owing to the risk of progressive kidney damage.1,5,6 The diagnosis, and medical and surgical management of UO has been reviewed, but there is little research into risk factors for UO.1,2,6–8
Surgical intervention for UO is associated with a substantial cost and risk of major complications in up to 46% of cats, and cats with AKI can be euthanased where the financial or logistical facilities are not available for surgical intervention.2,9 Complications reported with placement of a subcutaneous ureteral bypass (SUB) device include infection, minor and major technical difficulties at the time of surgery, deteriorating renal function, cardiopulmonary arrest and chronic kidney disease (CKD), with median survival times exceeding 2 years.2,9
Previous literature has identified correlations between diet, specifically urine-acidifying diets or feeding a single brand, breed, housing and hypercalcaemia with calcium oxalate urolith formation.10–14 For calcium oxalate uroliths in general, high-moisture diets are a key prevention strategy.6,14,15
A case-control study was designed with the aim of evaluating the potential association between husbandry factors, specifically diet, and UO in cats. Our hypothesis was that diets low in moisture (dry food) would be associated with an increased risk of UO than diets higher in moisture (typically, tinned foods).
Materials and methods
Medical records
Medical records from the Small Animal Specialist Hospital (SASH), a private referral and emergency veterinary hospital in Sydney (Australia) between August 2016 and October 2019 were reviewed. Permission to access patient records for this retrospective study was granted by the hospital, and client confidentiality was maintained at all times. All ultrasonographic reports completed for feline patients in this period were reviewed. Based on the history, clinical records and ultrasonographic reports, cats were categorised as cases or controls, or excluded from the study if records were incomplete or did not permit accurate categorisation.
Cases
Cases were cats treated at SASH for complete or partial UO by all possible causes between August 2016 and October 2019. Cases had either the following: (1) UO confirmed with pyelography at the time of SUB placement (patients had UO due to ureterolithiasis or suspected solidified blood clots; there were no cases confirmed to be due to stricture, infection or neoplasia in this cohort); or (2) azotaemia, defined as creatinine >140 µmol/l on a benchtop analyser (IDEXX Catalyst), and (i) physical UO observed on ultrasonography and (ii) pyelectasia ⩾5 mm with concurrent hydronephrosis or hydroureter associated with the visualised ureterolith or soft tissue structure.6,8,16–18 The cut-off of 5 mm of pyelectasia was based on consensus recommendations for the diagnosis of UOs. 6 The width of the renal pelvis was measured on a transverse plane ultrasound image at the level of the renal hilus.
Controls
Controls were cats that had undergone abdominal ultrasonography that did not have ultrasonographic evidence of UO. Cats with ultrasonographic evidence of non-obstructive ureteroliths or nephroliths were excluded. Cats with ultrasonographic abnormalities of their upper urinary tract that were not clearly attributable to another disease process were excluded. Cats with ultrasonographic findings consistent with infiltrative renal neoplasia, polycystic kidney disease and CKD that also had a history compatible with chronic disease were included. Cats that had AKI where the underlying cause was not determined and therefore UO remained a possible diagnosis were excluded.
Data extracted from medical records included the following: signalment; dietary information; housing information; biochemistry results where available (creatinine, plasma total calcium and serum ionised calcium); ultrasound findings; results of pyelography (where available); and diagnosis. The specific confounding factors were chosen as they are either typical potential confounders in case-control studies (age, breed and sex) and based on a review of the literature relevant to uroliths in cats.10–12 Information regarding drinking behaviour and measured water intake was not available and could therefore not be assessed in relation to the prevalence of UO. Ultrasonographic findings were based on contemporaneous reports completed by a board-certified radiologist.
Based on a review of medical records, diet was classified as predominantly dry, mixed or predominantly moist food. The diet was considered predominantly dry or predominately moist if the diet was >75% of that food. Dietary information was gathered from historical records. If there was insufficient information to classify the diet type, the owner was contacted to gain further information and, if this was not possible, these cats were excluded. The exact diet (brand, variety) was recorded, where available, but in most instances only diet type (amount of wet/dry) had been recorded. Housing was categorised as indoor only or indoor and outdoor access.
Statistical analyses
Descriptive statistics were determined for age, sex, breed (pedigree and crosses, or domestic), diet, housing and total plasma calcium concentration, stratified by the presence of absence of a UO.
Logistic regression analyses were performed in R (https://www.R-project.org/) to evaluate the potential relationship between diet, housing and total calcium concentration and UO while accounting for the potential confounding factors of age, sex and breed. All variables were initially assessed in a univariable binary logistic regression model and potential explanatory variables with an initial P value <0.5 were entered into a multivariable logistic regression model. A stepwise backwards selection protocol was followed. The significance of each explanatory variable was assessed by the Wald test. Explanatory variables that were not statistically significant were removed from the model, one at a time, beginning with the least significant, until the estimated regression coefficients for the retained variables were significant (P ⩽0.05).
The significance of a group of coefficients (ie, diet) was assessed using a χ2 distributed likelihood ratio test comparing the model containing the grouped coefficients (ie, diet) with a null model (ie, containing no explanatory variables).
The results of the final model are reported in terms of adjusted odds ratios for each explanatory variable. The overall significance of the final model was assessed with the likelihood ratio test. A receiver operating characteristic (ROC) curve was constructed to describe the model’s predictive ability to discriminate between cats with and without a UO. The area under the curve (AUC) provides a measure of the overall fit of the model.
Results
In total, 168 (94 females, 74 males) cats were included in the study (Table 1). Only two cats had an outdoor-only lifestyle and both cats were reclassified as mixed housing for statistical analyses. Total calcium was within the reference interval for all cats except for a single cat in the control group, which had a plasma total calcium of 3.1 mmol/l and a serum ionised calcium of 1.56 mmol/l. Seventy-six cats were pedigree and 92 were domestic shorthair/longhair.
Table 1.
Descriptive statistics of cats with and without ureteral obstruction (UO)
| UO (n = 28) | No UO (n = 140) | |
|---|---|---|
| Median (IQR) age (years) | 8.0 (5.7–10.9) | 10.0 (5.0–13.0) |
| Female/male (n) | 15/13 | 79/61 |
| Domestic/pedigree (n) | 15/13 | 77/63 |
| Indoor/outdoor (n) | 16/10 | 86/49 |
| Moist/mixed/dry diet (n) | 1/10/17 | 28/82/30 |
| Cat with plasma tCa levels available (n) | 22 | 88 |
| Median (IQR) tCa (mmol/l) | 2.3 (2.17–2.45) | 2.23 (2.09–2.40) |
| Cats with creatinine levels available (n) | 28 | 98 |
| Median (IQR) creatinine (mmol/l) | 751 (415–1240) | 140 |
IQR = interquartile range; tCa = total calcium
Cases
A total of forty-three cats were initially reviewed for inclusion, with 15 subsequently excluded. Two of these cats had a diagnosis of UO confirmed by pyelography but were excluded owing to insufficient historical information. The remaining 13 cats were suspected to have a UO but were excluded as they did not fulfil all three criteria and did not have confirmatory pyelography. Typically, these cats were severely azotaemic, with marked pyelectasia and hydronephrosis, but no physical obstruction, identified on imaging. Other excluded cats without confirmatory pyelography had evidence of a UO with pyelectasia <5 mm.
Twenty-eight cats were included in this study (median age 8 years; interquartile range [IQR] 5.7–10.9 [female:male ratio 15:13]). These cases had complete UO confirmed by pyelography (n = 18) or the combination of azotaemia, pelvic dilatation ⩾5 mm with concurrent hydronephrosis or hydroureter with a visualised physical obstruction (n = 10). For those cats that were managed medically and therefore did not have pyelography performed, differentiation between complete and partial obstruction was not possible. Obstructions were attributed to nephrolith or ureteroliths (n = 19), suspected blood clot (n = 4) or pyonephrosis (n = 1). The cats that had a suspected blood clot had a soft tissue structure at the site of obstruction, with the main differentials being a dried solidified blood calculi or unidentified ureterolith. These cats did not have radiographs performed. Five cats that had an obstruction confirmed with pyelography did not have the cause identified. There were no cases due to strictures or neoplasia. Creatinine concentration was determined in all cats (median 751 mmol/l; IQR 415–1240). Most (n = 22/28) cats had total plasma total calcium concentration determined (median 2.3 mmol/l; IQR 2.17–2.45).
Pedigree breeds/crosses included Devon Rex (n = 1), Ragdoll (n = 3), Ragdoll cross (n = 2), Burmese (n = 2), Chantilly (n = 1), British Shorthair (n = 1), Australian Mist (n = 1) and Norwegian Forest Cat (n = 1); the remaining 15 cats were domestic shorthair/longhair. All cats within the case group were neutered.
Seven cats had bilateral UO. The pelvic diameter associated with the UO ranged from 2 mm to 27 mm (median 6.25 mm; IQR 5–9.25) and was ⩾5 mm for 24 cats (the remaining four cats had an obstruction confirmed with pyelography).
Eighteen cats had placement of a SUB device after pyelography. The remaining 10 cats were medically managed for their UO owing to financial or other constraints. Of these, one was euthanased owing to a poor response to treatment, two were euthanased within 24 h of diagnosis, four were medically managed successfully and three were lost to follow-up after continued care at the referring veterinarian after diagnosis.
Twenty-seven of 28 cases had a urine culture performed. Three of these were positive and the other 24 were negative. Two cats had positive cultures of Escherichia coli. In one of these cats, pyelonephritis was considered the primary disease process and the azotaemia resolved with medical management. The other cat had SUB placement for persistent obstruction. In the third cat, a positive urine culture of Salmonella species was suspected to be a contaminant, and a SUB was placed surgically. Additional comorbidities of cats that had surgery included congestive heart failure (n = 1), triaditis (n = 1) and upper respiratory tract infection (n = 1). The comorbidities of cats that were medically managed included inflammatory bowel disease (n = 2) and diabetes mellitus (n = 1).
Controls
In total, 140 cats were categorised as controls (median age 10 years; IQR 5.0–13.0 [female:male ratio 79:61]). These cats typically underwent ultrasonography for evaluation of vomiting, chronic enteropathy, weight loss and CKD. Most (n = 98/140) cats had creatinine concentration determined (median 140 mmol/l; IQR 100–187). Most (n = 88/140) cats had plasma total calcium levels available (median 2.23 mmol/l; IQR 2.09–2.40).
Breeds included British Blue (n = 1), Chinchilla (n = 1), Ragdoll (n = 8), Ragdoll cross (n = 2), British Shorthair (n = 2), Burmese (n = 14), Siamese (n = 4), Devon Rex (n = 5), Himalayan (n = 3), Russian Blue (n = 6), American Shorthair (n = 1), Bengal (n = 2), Tonkinese (n = 1), Maine Coon (n = 1), Exotic Shorthair (n = 2), Birman (n = 1), Ocicat (n = 1), Siberian (n = 1), Chantilly (n = 1) and Cornish Rex (n = 1), with the remaining 77 cats being domestic shorthair/longhair. Two males in the control group were intact; the remaining 138 cats were neutered.
Statistical analyses
Age (P = 0.46), sex (P = 0.78), breed (P = 0.89), calcium (P = 0.42) and housing (P=0.83) were not significantly associated with the risk of UO when assessed independently in bivariate logistic regression analysis (Table 2).
Table 2.
Risk factors for ureteral obstruction in 168 cats
| Variable (reference category) | Coefficient (SE) | P value | OR (95% CI) |
|---|---|---|---|
| Age | –0.0317 (0.0433) | 0.46 | 0.97 (0.89–1.05) |
| Sex (female) Male |
–0.116 (0.46) | 0.78 | 0.89 (0.39–2.03) |
| Breed (domestic shorthair/longhair) Pedigree |
0.0576 (0.415) | 0.89 | 1.06 (0.46–2.39) |
| Housing (indoor only) Indoor/outdoor housing |
0.0925 (0.441) | 0.83 | 1.09 (0.45–2.57) |
| Diet (predominantly moist) Mixed Predominantly dry |
1.23 (1.07) 2.76 (1.06) |
0.25 0.0093 |
3.41 (0.61–64) 15.87 (2.95–295) |
| Total calcium | 0.715 (0.883) | 0.42 | 2.05 (0.37–12.3) |
Regression coefficients and standard errors from bivariate logistic regression
SE = standard error; OR = odds ratio; CI = confidence interval
Based on the bivariate regression results, diet, age and total calcium were entered into a multivariable model. In the final model, only diet was significantly associated with UO (Figure 1). Diet overall was significantly associated with UO (χ2 (2) = 17.9, P <0.001). Compared with cats eating a predominantly moist food diet, cats fed a predominantly dry food diet were 15.9 times more likely to develop a UO (95% confidence interval [CI] 2.9–295; P = 0.009). There was no difference in the association between diet and UO in cats fed a mixed or predominantly moist food diet (P = 0.25) (Table 2). The AUC of the ROC curve was 72% (95% CI 63–81.5) (Figure 1).
Figure 1.
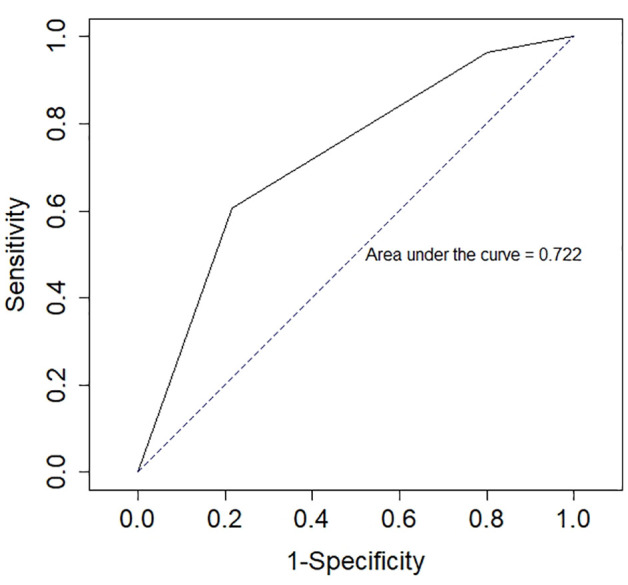
Receiver operator characteristics curve (ROC) for a logistic model evaluating the relationship between diet and ureteral obstruction in 168 cats
Discussion
Cats fed diets with a high moisture content, such as tinned food and mixtures of tinned and dry food, were significantly less likely to have a UO than cats fed an exclusively dry food diet.
UOs are typically considered as being due to ureteroliths, although dried solidified blood clots, ureteral strictures and infections are all potential causes of benign UO. Uroliths from the upper urinary tract are less often available for analysis than lower urinary tract uroliths, but the existing evidence indicates that the vast majority of stones from the upper urinary tract to be calcium oxalate, with up to 98% reported to contain calcium oxalate. 5 This contrasts with cystoliths where struvite and calcium oxalate cystoliths are reported to occur in similar frequency (44% and 40%, respectively). 19 The incidence of calcium oxalate ureteroliths has been increasing over recent decades, with up to a 50-fold increase in incidence reported over the late 20th century.5,19–21 There have not been any recent studies evaluating ureterolith composition, but it is likely that most ureteroliths are calcium oxalate and identifying risk factors for calcium oxalate urolithiasis will reduce the risk of UO.
Previous studies have identified an association between housing, breed, sex and calcium and calcium oxalate uroliths. Male cats, indoor cats and Persian and Himalayan cats are more likely to have calcium oxalate uroliths.10–14 There was no association with sex or breed and UO in this study. Multiple studies have evaluated dietary factors, specifically protein and fat content, on the influence of calcium oxalate stone formations in cats.22,23 Despite this, the underlying mechanism for calcium oxalate urolith formation is not completely understood. 8 Feeding a diet with a minimum moisture content of 73% resulted in reduced calcium oxalate relative supersaturation in urine, which supports this study’s findings of an increased likelihood of UO in cats fed predominantly dry food.14,15
Given the technical difficulty in retrieving upper urinary uroliths, previous studies of risk factors for uroliths have generally evaluated lower urinary tract uroliths.10–12 It is possible that there are differences between the risk factors for upper and lower urinary tract uroliths; however, it is reasonable to suspect that the risk factors for upper and lower urinary tract calcium oxalate urolithiasis are similar. Indoor housing may have a greater influence on cystolith than ureterolith formation if housing is associated with reduced voiding and increased urine stasis. There was no association between housing and UO in our population. Genetic differences may vary between geographical locations.
Total plasma calcium was not associated with UO in this study, unlike previous studies, which have shown an association between hypercalcaemia and calcium oxalate stone formation.11–14 This could be a genuine difference due to geographical or husbandry factors. Alternatively, the potential significance of calcium could have been under-recognised because ionised calcium concentrations were not routinely determined. The ability of total calcium to accurately predict ionised hypercalcaemia is poor and hypercalcaemia may only become evident post-treatment for obstruction; therefore, preoperative total calcium measurements may have underestimated the prevalence of hypercalcaemia in this study.24–26
Dietary changes to cause urine acidification has been the primary method to prevent and manage struvite urolithiasis in cats. 6 However, it has been shown that diets which promote the formation of acidic urine in cats also promote the formation of calcium oxalate uroliths.11,12 Unfortunately, we were not able to reliably assess the proportions of cats in either group on urine-acidifying diets in our population.
It would have been interesting to assess a potential relationship between CKD and UO as CKD has been associated with nephroliths in cats. 27 Unfortunately, the presence of pre-existing CKD, although often suspected, was not easy to determine in cats presenting with AKI due to UO.
One of the limitations of this study, given its retrospective nature, was the accuracy of owner recollection and description of diet type. However, this limitation would apply to both cases and controls. Ideally, randomised trials would be performed to assess if dietary water content or specific dietary components contribute to upper urinary tract calculi formation. Given the significant morbidity and mortality associated with UO, observational studies are more feasible in small animal medicine.
Data collection was performed manually by a single person who was not blinded to the hypothesis, which was a limitation of this study. Case-control studies are a valuable study methodology for uncommon outcomes such as UO and have substantial cost and time advantages over prospective or cohort studies. However, case-control studies are susceptible to bias. 28 Information regarding diet was already present in the medical record, so recall bias was not a concern. Information was deliberately collected from case records at a separate time to classification of cats to minimise misclassification bias. However, the authors were not blinded to the hypothesis prior to data collection and may have inadvertently introduced misclassification bias.
Conclusions
There was a significant association of UO with diet formulation. Cats fed a predominantly dry food diet had an increased likelihood of UO than cats fed a moist or mixed diet. The model had an acceptable ability to discriminate between cats with and without a UO based on diet alone, but obviously there are risk factors not identified in this study. Future studies could focus on identifying these novel risk factors. In the meantime, increased use of diets with a high moisture content could reduce the incidence of UO.
Footnotes
Accepted: 21 April 2021
Author note: This paper was presented, in part, at the 2020 ACVIM Forum and 2020 ANZCVS Science Week.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or nonexperimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Alexandra J Kennedy  https://orcid.org/0000-0002-4596-1812
https://orcid.org/0000-0002-4596-1812
References
- 1. Clarke D. Feline ureteral obstructions. Part 1: medical management. J Small Anim Pract 2018; 59: 324–333. [DOI] [PubMed] [Google Scholar]
- 2. Berent AC, Weisse CW, Bagley DH, et al. Use of a subcutaneous bypass device for treatment of benign ureteral obstruction in cats: 174 ureters in 134 cats (2009–2015). J Am Vet Med Assoc 2018; 253: 1309–1327. [DOI] [PubMed] [Google Scholar]
- 3. Zaid MS, Berent AC, Weisse CW, et al. Feline ureteral strictures: 10 cases (2007–2009). J Vet Intern Med 2011; 24: 660–795. [DOI] [PubMed] [Google Scholar]
- 4. Meldrum K. Pathophysiology of urinary tract obstruction. In: Wein A, Kavoussi L, Partin A. (eds). Campbell-Walsh urology. Philadelphia: PA: Elsevier, 2016, pp 1089–1103. [Google Scholar]
- 5. Kyles A, Hardie E, Wooden E, et al. Management and outcome of cats with ureteral calculi: 153 cases 1984–2002. J Am Vet Med Assoc 2005; 226: 937–944. [DOI] [PubMed] [Google Scholar]
- 6. Lulich JP, Berent AC, Adams LG, et al. ACVIM Small Animal Consensus Recommendations on the treatment and prevention of uroliths in dogs and cats. J Vet Intern Med 2016; 30: 1564–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clarke D. Feline ureteral obstructions. Part 2: surgical management. J Small Anim Pract 2018; 59: 385–397. [DOI] [PubMed] [Google Scholar]
- 8. Lamb CR, Cortellini S, Halfacree Z. Ultrasonography in the diagnosis and management of cats with ureteral obstruction. J Feline Med Surg 2018; 20: 15–-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kulendra NJ, Borgeat K, Syme H, et al. Survival and complications in cats treated with subcutaneous ureteral bypass. J Small Anim Pract 2021; 62: 4–11. [DOI] [PubMed] [Google Scholar]
- 10. Kirk CA, Ling GV, Franit CE, et al. Evaluation of factors associated with development of calcium oxalate urolithiasis in cats. J Am Vet Med Assoc 1995; 207: 1429–1434. [PubMed] [Google Scholar]
- 11. McClain HM, Barsanti JA, Bartges JW. Hypercalcemia and calcium oxalate urolithiasis in cats: a report of five cases. J Am Anim Hosp Assoc 1999; 35: 297–301. [DOI] [PubMed] [Google Scholar]
- 12. Thumchai R, Lulich JP, Osborne CA, et al. Epizootiologic evaluation of urolithiasis in cats: 3,498 cases (1982–1992). J Am Vet Med Assoc 1996; 208: 547–551. [PubMed] [Google Scholar]
- 13. De Brito Galvao JP, Parker V, Schenck PA, et al. Update on feline ionised hypercalcemia. Vet Clin North Am Small Anim Pract 2017; 47: 273–292. [DOI] [PubMed] [Google Scholar]
- 14. Smith BHE, Stenson AE, Markwell PJ. Urinary relative supersturations of calcium oxalate and struvite in cats are influenced by diet. J Nutrition 1998; 129: 2763S–2764S. [DOI] [PubMed] [Google Scholar]
- 15. Buckley CMF, Hawthorne A, Colyer A, et al. Effect of dietary water intake on urinary output, specific gravity and relative supersaturation for calcium oxalate and struvite in the cat. J Nutrition 2011; 106: S128–S130. [DOI] [PubMed] [Google Scholar]
- 16. Quimby JM, Dowers K, Herndon AK, et al. Renal pelvic and ureteral ultrasonographic characteristics of cats with chronic kidney disease in comparison with normal cats, and cats with pyelonephritis or ureteral obstruction. J Feline Med Surg 2017; 19: 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dirrig H, Lamb CR, Kulendra NJ, et al. Diagnostic imaging observations in cats treated with the subcutaneous ureteral bypass system. J Small Anim Pract 2020; 61: 24–31. [DOI] [PubMed] [Google Scholar]
- 18. The International Renal Interest Society. IRIS grading of AKI. http://www.iris-kidney.com/guidelines/grading.html (2019, accessed September 5, 2019).
- 19. Cannon AB, Westropp JL, Ruby AL, et al. Evaluation of trends in urolith composition in cats: 5,230 cases (1985–2004). J Am Vet Med Assoc 2007; 231: 570–576. [DOI] [PubMed] [Google Scholar]
- 20. Ettinger S, et al. Textbook of veterinary internal medicine. 8th ed. St Louis, MO: Elsevier, 2017. [Google Scholar]
- 21. Lekcharoensuk C, Osborne CA, Lulich JP, et al. Trends in the frequency of calcium oxalate uroliths in the upper urinary tract of cats. J Am Anim Hosp Assoc 2005; 41: 39–46. [DOI] [PubMed] [Google Scholar]
- 22. Lekcharoensuk C, Osborne CA, Lulich JP, et al. Association between dietary factors and calcium oxalate and magnesium ammonium phosphate urolithiasis in cats. J Am Vet Med Assoc 2001; 219: 1228–1237. [DOI] [PubMed] [Google Scholar]
- 23. Passlack N, Burmeier H, Brenten T, et al. Relevance of dietary protein concentration and quality as risk factors for the formation of calcium oxalate stones in cats. J Nutri Sci 2014; 3. DOI: 10.1017/jns.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schenck P, Chew P. Prediction of serum ionized calcium concentration by serum total calcium measurement in cats. Can J Vet Res 2010; 74: 209–213. [PMC free article] [PubMed] [Google Scholar]
- 25. Hodgson N, McMichael MA, Jepson RE, et al. Development and validation of a multivariable predictive model to estimate serum ionized calcium concentration from serum biochemical profile results in cats. J Vet Intern Med 2019; 33: 1943–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garcia M, Manassero M, Cannone-Guibert M, et al. Evolution of ionized calcium concentration over time in cats with ureteral obstruction: 39 cases – Research Communications of the 27th ECVIM-CA Congress. Intercontinental, Saint Julian’s, Malta, 14th to 16th September 2017. J Vet Intern Med 2018; 32: 525–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cleroux A, Alexander K, Beuchamp G, et al. Evaluation for association between urolithiasis and chronic kidney disease in cats. J Am Vet Med Assoc 2017; 250: 770–774. [DOI] [PubMed] [Google Scholar]
- 28. Shulz KF, Grimes DA. Case-control studies: research in reverse. Lancet 2002; 359: 431–434. [DOI] [PubMed] [Google Scholar]


