Abstract
Objectives
The aim of this study was to characterize gastrointestinal (GI) transit times and pH in healthy cats.
Methods
GI transit times and pH were measured in six healthy, colony-housed, purpose-bred spayed female cats using a continuous, non-invasive pH monitoring system in a sequential order design. For the first period (‘pre-feeding’), food was withheld for 20 h, followed by oral administration of a pH capsule. Five hours post-capsule administration, cats were meal-fed by offering them their daily allowance of food for 1 h. For the second period (‘post-feeding’), food was withheld for 24 h and cats were fed for 1 h, after which a pH capsule was orally administered. Studies in both periods were repeated three times. GI transit times and pH were compared between the two periods.
Results
The median transit times for the pre- and post-feeding periods, respectively, were: gastric – 94 mins (range 1–4101) and 1068 mins (range 484–5521); intestinal – 1350 mins (range 929–2961) and 1534 mins (range 442–2538); and GI – 1732 mins (range 1105–5451) and 2795 mins (range 926–6563). The median GI pH values for the first and second periods, respectively, were: esophageal – 7.0 (range 3.5–7.8) and 4.5 (range 2.9–6.4); gastric – 2.7 (range 1.7–6.2) and 2.0 (range 1.1–3.3); intestinal – 8.2 (range 7.6–8.7) and 7.8 (range 6.7–8.5); first-hour small intestinal – 8.2 (range 7.4–8.7) and 8.3 (range 7.9–8.6); and last-hour large intestinal – 8.5 (range 7.0–8.9) and 7.8 (range 6.3–8.7). Gastric (P <0.0020) and intestinal pH (P <0.0059) were significantly increased in the pre-feeding period compared with the post-feeding period.
Conclusions and relevance
Gastric and intestinal pH differed significantly when the capsule was administered 5 h prior to feeding compared with 1 h after feeding. Transit times for both periods showed high degrees of intra- and inter-individual variability.
Keywords: Motility, residence time, gastric transit, intestinal transit
Introduction
There are large knowledge gaps regarding gastrointestinal (GI) physiology in cats that need to be addressed in order to design and select drug regimens for cats. Indeed, there have been very few studies dedicated to characterizing GI transit times and pH, which has hampered our ability to apply the principles of pharmacokinetics to drug development and selection for cats. This has been particularly important for medications intended for oral administration. As a result of this deficiency, veterinarians occasionally treat cats as they would dogs, without consideration for important potential differences that could impact oral drug absorption. Drugs developed for dogs, or humans, cannot be assumed to be orally absorbed in cats with equal extent and rate. For example, some orally administered gastric acid suppressants designed for human use, which are also effective in dogs, fail to increase gastric pH in cats to the same degree, even when dosed identically.1–4 There are many other examples of drugs for which the oral absorption is lower in cats than in dogs.5,6 When these differences are known, dose adjustments can be made. But, for the vast majority of drugs, the differences are unknown and veterinarians are faced with prescribing these drugs at dosages with only extrapolations to guide them, risking adverse events and lack of efficacy. In order to remedy this knowledge gap, determination of basic physiologic parameters is needed. Better understanding of the GI transit times and pH in cats would help predict absorption of drugs with poor permeability characteristics.
Accordingly, the primary objective of this study was to characterize GI transit times and pH in healthy cats 5 h prior to feeding and 1 h post-feeding. Based on review of the limited literature in cats, 7 we hypothesized that GI transit times and pH would have a high degree of intra- and inter-individual variability.
Materials and methods
Animals
The subjects of this study were six healthy, female spayed, purpose-bred domestic shorthair cats from a closed research colony at Texas A&M University, all aged approximately 4.5 years with a median body condition score (BCS) of 4 (range 4–6) using a nine-point scale (WSAVA Feline BCS System), and a median body weight of 3.9 kg (range 3.4–5.4) at the start of the study and 4.0 kg (range 3.6–5.4) at the end of the study. Cats had no history of clinical signs of GI disease, including vomiting, decreased appetite or diarrhea. Cats also had no abnormalities on historical blood tests performed annually prior to enrollment. In addition, the cats had no physical examination findings suggestive of systemic or GI disease, including a poor haircoat, a BCS of ⩽3/9, or an abnormal muscle condition score, abdominal palpation or thoracic auscultation. Finally, the cats had no abnormalities present on baseline blood test results (ie, complete blood count serum biochemistry profile and urinalysis) performed within 2 months of study entry. To minimize the confounding effect of stress on GI transit times and pH, the cats were maintained in their original colony housing throughout the study either individually or grouped, as previously housed, in stainless steel housing (approximately 8 ft 4 in × 7 ft 9 in × 8 ft 8 in) in a controlled environment with a 12 h light/dark cycle and access to natural light.The cats were fed their usual allotment of 50–60 g of their standard commercial diet free-choice (Purina Pro Plan Focus Adult Hairball Management Chicken and Rice formula dry cat food [Nestlé Purina PetCare], 3.7 kcal/g as fed; 38.8% metabolizable energy [ME] and 112 g/1000 kcal protein, 39.8% ME and 47 g/1000 kcal fat, 21.4% ME and 62 g/1000 kcal carbohydrate, 11 g/1000 kcal crude fiber) once a day on non-study dates. During the study, cats were meal-fed 50–60 g of their standard diet for 1 h to meet their historical daily intake. Cats were weighed before each feeding time. The feed was also weighed before and after the 1 h feeding time to calculate energy intake. Cats were provided water ad libitum, including during fasting periods.Cats were monitored a minimum of twice daily for changes in attitude or fecal consistency using the Purina Fecal Scoring System (Nestlé Purina PetCare) in accordance with the Institutional Animal Care and Use Committee (IACUC) protocol. Cats were cared for according to the principles outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (IACUC 2019-0322).
Study protocol
The Bravo pH monitoring system (Bravo pH capsule; Medtronic) was used to characterize GI transit times and pH, as previously described for dogs. 8 The pH capsule was detached from its delivery device following calibration and orally administered to determine GI transit times and pH. The pH capsule was orally administered with a syringe-style pet piller (Butler Sales Bullseye Pillgun), as previously described, 9 and followed by syringe administration of up to 15 ml of tap water to facilitate swallowing of the capsule. The procedure was repeated three times within each pre-feeding period and post-feeding period, with at least 1 month separating each repeated evaluation. Each capsule administration was performed using a cat bag and/or fear-free-based (Fear Free) techniques for restraint to minimize stress associated with pilling. The cats used in this study had been previously acclimated to handling and minimal restraint for other studies. For this study, the individuals not previously involved in the care of the cats that were directly involved in pilling and feeding the cats spent 3 months prior to the study to help the cats acclimate to them.
For the period of the study in which the capsule was administered prior to feeding (pre-feeding), food was withheld from the cats for approximately 20 h before the pH capsule was administered orally. Five hours after capsule administration, the cats were meal-fed their historical daily allowance for 1 h. For the period of the study in which the capsule was administered after feeding (post-feeding), food was withheld from the cats for 24 h prior to being meal-fed their historical daily allowance for 1 h, after which point the food was removed and the capsule was administered orally. During both periods, the food was weighed before and after the 1 h feeding period to calculate total food intake. Cats were fed the same food allotment every 24 h on subsequent days.
pH telemetry system
All pH capsules and receivers were calibrated as previously described, 9 according to the manufacturer’s instructions. The Bravo pH capsule (6 mm × 5 mm × 25 mm) records GI pH readings from 0 to 9 every 6 s. GI pH readings were initiated immediately after oral administration and acquired continuously until the capsule was eliminated from the cat’s body via the feces. The corresponding data receivers were kept on the side of each cat’s cage, out of the cat’s reach, during the data acquisition phase. The pH data were uploaded to a computer using a software package provided by the manufacturer (Polygram Net Software; Medtronic) every 48 h for each monitoring period. The same receiver was used to obtain data from the same cat for the next monitoring period.
The pH was used to determine the location of the capsule within the GI tract (Figure 1), as previously described. 10 Entry into the stomach from the esophagus was defined by a sharp and persistent decrease in pH <4. Gastric transit time was defined as the time of entry into the stomach to the time of entry into the small intestine, denoted by a rapid and persistent increase in the pH >4 accompanied by an increase of ⩾3 pH units. Intestinal transit time was defined as the time of entry into the small intestine followed by exit from the body, detected as a sharp decrease in pH. Because the capsule does not measure pressure and the transition from small intestine to large intestine could not be determined due to a similar pH, small and large intestinal transit times and pH were described together as intestinal transit time and intestinal pH. As a representation of proximal (likely small intestine) intestinal pH, data for 1 h following gastric transit were recorded. As a representation of distal (likely large intestine) intestinal pH, data for 1 h prior to capsule elimination were recorded.
Figure 1.
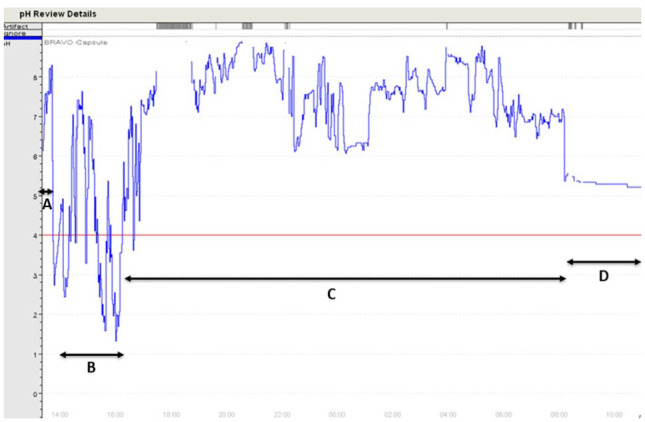
Gastrointestinal pH monitoring in a cat following oral administration of the pH capsule. pH and time are reported on the y- and x-axes, respectively. (A) Capsule in the esophagus. (B) Gastric transit time starts at a sharp and persistent decrease of pH <4. (C) Intestinal transit time starts at a sharp and persistent increase of pH >4 accompanied by an increase of ⩾3 pH units. (D) Capsule eliminated in the feces, shown by a second sharp decrease in pH that remains steady between a pH of 4 and 6
Statistical analysis
Normality was assessed using Shapiro–Wilk W tests. Non-parametric data are reported as median (range). Descriptive statistics of the GI transit time and pH values were calculated. Pairwise Spearman’s rank correlation coefficient was used to compare GI transit time and pH during the pre-feeding and post-feeding periods. Wilcoxon signed-rank tests were performed to compare pre-feeding and post-feeding cats’ GI transit times and pH since the data were not normally distributed. Coefficient of variation (CV) was used to assess intra-individual (within cats: reproducibility) and inter-individual (across all cats; consistency) variability. For intra-individual variability, a CV >0.10 was considered high, and for inter-individual variability, a CV >0.15 was considered high. If there was a missing value for either period, its paired value for the other period would also not be used, for they were considered an ineffective pair to use in the test. Statistical significance was defined as P <0.05.
Results
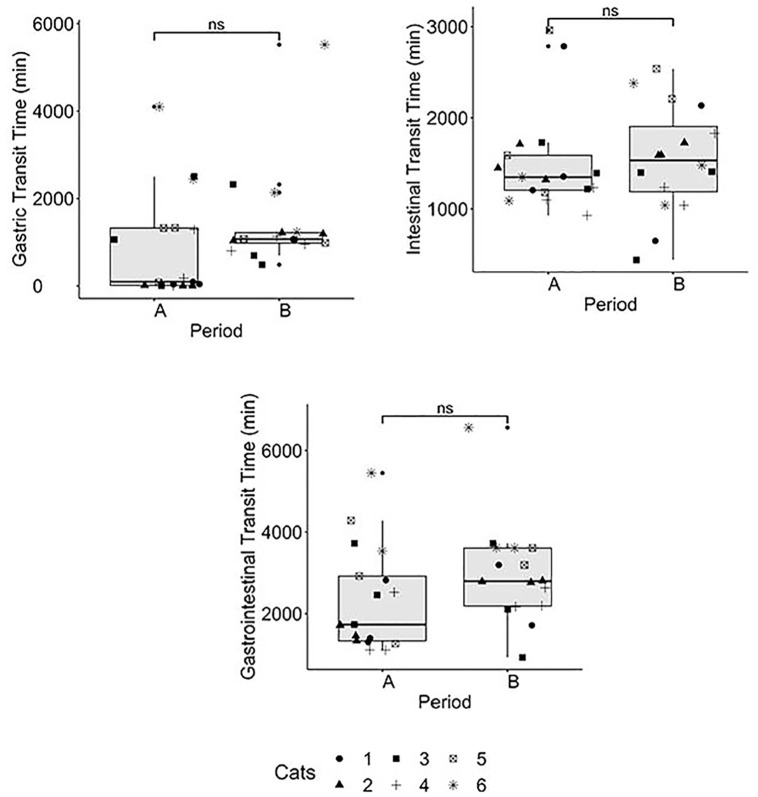
Two cats vomited the capsule during the post-feeding capsule administration period, and one cat was non-compliant to pilling, and so to avoid causing stress, the third capsule administration in the pre-feeding period was not completed. No cat developed diarrhea (Purina fecal score ⩾5) throughout the study. During the post-feeding period, all cats ate >67% of their maintenance energy requirement (MER) based on historical daily intake on all replicates. The majority of the cats met their MER with the exception of one cat that consistently ate 75% of its historical MER on all occasions during the post-feeding period. The median daily food intake was 96 kcal (range 87–222) per day. The GI transit times and pH in the pre-feeding and post-feeding periods are summarized in Figures 2 and 3, respectively. The median transit times for the pre-feeding and post-feeding periods, respectively were: gastric – 94 mins (range 1–4101) and 1068 mins (range 484–5521); intestinal – 1350 mins (range 929–2961) and 1534 mins (range 442–2538); and GI – 1732 mins (range 1105–5451) and 2795 mins (range 926–6563). Although no significant differences (Table 1) were observed, median transit times were increased from the pre-feeding to the post-feeding periods. Five of six cats had at least one replicate where the esophageal retention of the capsules was ⩾15 mins in the pre-feeding period (five cats; 8/17 times) or post-feeding (four cats; 6/18 times) period, respectively. After pilling, the capsules remained in the esophagus for 5 h and then passed into the stomach during feeding time 3/17 times in the pre-feeding period. When these replicates were removed from the analysis of the pre-feeding period, the median (and range) gastric transit time decreased from 94 mins (range 1–4104) to 60 mins (1–4104). The capsule passed immediately into the stomach after pilling 3/17 times in the pre-feeding period and 9/18 times in the post-feeding period. The capsule passed through the esophagus and stomach and almost immediately into the small intestine 3/17 times in the pre-feeding period and 0/17 times in the post-feeding period. There was a notable, but non-significant, difference (P = 0.0938) in gastric transit time between the pre-feeding and post-feeding periods (Table 1, Figure 2).
Figure 2.
Box plot of each gastrointestinal transit time physiological measurement when the capsule was administered pre- or post-feeding. Symbols represent individual cats. Experiments for the pre-feeding period were performed three times in five cats and twice in the remaining cat, and for the post-feeding period three times in all six cats. ns = non-significant; A = pre-feeding capsule period; B = post-feeding capsule period
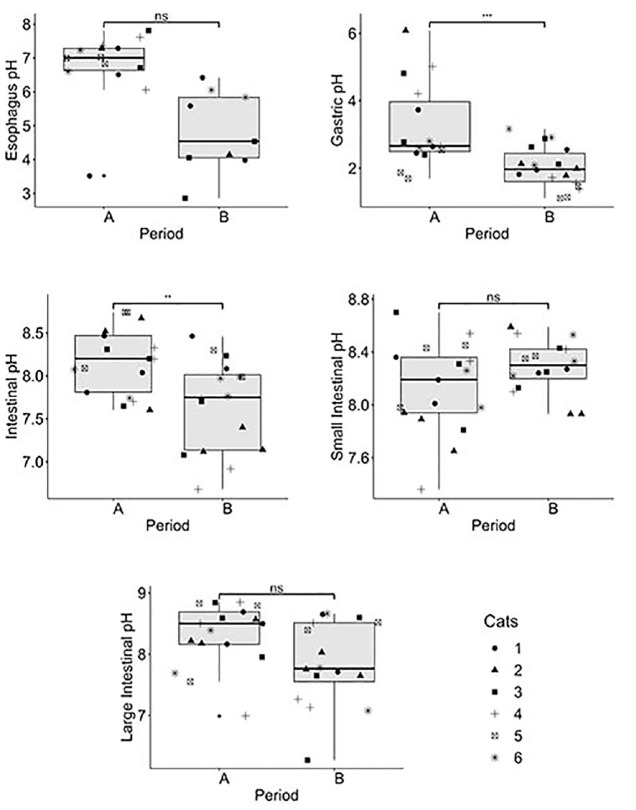
Figure 3.
Box plot of each gastrointestinal pH physiological measurement when the capsule was administered pre- or post-feeding. Symbols represent individual cats. Experiments for the pre-feeding period were performed three times in five cats and twice in the remaining cat, and for the post-feeding period three times in all six cats. *P <0.05; **P <0.01; ***P <0.001. ns = non-significant; A = pre-feeding capsule period; B = post-feeding capsule period
Table 1.
Results of the Wilcoxon signed-rank test for when the capsule was administered in the pre-feeding and post-feeding periods
| GI measurement | Effective pairs | Test statistic | P value | Significantly different | |
|---|---|---|---|---|---|
| Transit time (TT) | Gastric TT (mins) | 15 | 30 | 0.0938 | † |
| Intestinal TT (mins) | 15 | 67 | 0.7120 | ||
| Gastrointestinal TT (mins) | 15 | 34 | 0.1475 | ||
| pH value | Esophageal pH | 8 | 32 | 0.0587 | † |
| Gastric pH | 15 | 115 | 0.0020 | * | |
| Intestinal pH | 15 | 109 | 0.0059 | * | |
| Small intestinal pH | 15 | 48 | 0.5133 | ||
| Large intestinal pH | 15 | 88 | 0.1183 | ||
0.001 < P <0.01
0.05 < P <0.1
Experiments for the pre-feeding period were performed three times in five cats and twice in the remaining cat, and for the post-feeding period three times in all six cats. An effective pair is defined by successful pairing of two treatments without any missing value in either period
The median GI pH values for the pre-feeding and post-feeding capsule administration periods, respectively, were: esophageal – 7.0 (range 3.5–7.8) and 4.5 (range 2.9–6.4); gastric – 2.7 (range 1.7–6.2) and 2.0 (range 1.1–3.3); intestinal – 8.2 (range 7.6–8.7) and 7.8 (range 6.7–8.5); first-hour small intestinal –8.2 (range 7.4–8.7) and 8.3 (range 7.9–8.6); and last-hour large intestinal – 8.5 (range 7.0–8.9) and 7.8 (range 6.3–8.7). The median pH values were increased in the pre-feeding vs the post-feeding period, except for in the proximal intestine. There was a significant difference between the pre-feeding and post-feeding period for gastric pH (P <0.002) and intestinal pH (P <0.0059), with both being increased in the pre-feeding period. No buffering effect of feeding was detected.
Intra- and inter-individual variation for GI transit times and pH in the both the pre-feeding and post-feeding periods are presented in Table 2. Intra-individual CVs for transit times ranged from 0.26 to 0.92, while inter-individual CVs ranged from 0.21 to 1.60. Gastric pH had higher degrees of intra- and inter-individual CVs vs intestinal, proximal 1 h and distal 1 h intestinal pH.
Table 2.
Coefficient of variation (CV) for intra- and inter-individual variability for each gastrointestinal (GI) physiologic measurement during pre- or post-feeding capsule administration
| GI measurement | Period | CV | ||
|---|---|---|---|---|
| Intra-individual | Inter-individual | |||
| Transit time (TT) | Gastric TT (mins) | A | 0.92* | 1.23* |
| B | 0.32* | 0.56* | ||
| Intestinal TT (mins) | A | 0.26* | 0.21* | |
| B | 0.35* | 0.28* | ||
| GI TT (mins) | A | 0.39* | 0.46* | |
| B | 0.27* | 0.30* | ||
| pH value | Esophageal pH | A | 0.13* | 0.08 |
| B | 0.16* | 0.21* | ||
| Gastric pH | A | 0.24* | 0.40* | |
| B | 0.15* | 0.28* | ||
| Intestinal pH | A | 0.05 | 0.03 | |
| B | 0.04 | 0.06 | ||
| Small intestinal pH | A | 0.04 | 0.02 | |
| B | 0.02 | 0.01 | ||
| Large intestinal pH | A | 0.06 | 0.02 | |
| B | 0.08 | 0.04 | ||
Experiments for the pre-feeding period were performed three times in five cats and twice in the remaining cat, and for the post-feeding period three times in all six cats
Intra-individual CV >0.10 or inter-individual CV >0.15 A = pre-feeding capsule period; B = post-feeding capsule period
Discussion
Fewer drugs are US Food and Drug Administration-approved for cats compared with dogs. 11 Drugs developed for dogs, or humans, cannot be assumed to be orally absorbed in cats with an equal extent or rate. In fact, there are examples of drugs in which the oral absorption is lower in cats than in dogs (eg, itraconazole 6 and robenacoxib 12 ), leading to a need for drug modification or alteration of dosage recommendations for cats. Determination of basic physiologic parameters, including GI transit times and pH, would improve prescribing recommendations and guide the development of oral drugs specifically intended for cats. Accordingly, we used a non-invasive, continuous pH monitoring system, previously used in dogs, to better characterize these basic physiologic parameters8,13 in cats.
Feeding is a reported cause of a delayed gastric transit time (GTT) and, as a result, total transit time in humans and dogs.8,10,13,14 Although we were unable to detect a significant difference due to the wide variability among replicates and cats, we also observed a marked, albeit non-significant, difference in GTT and GI transit time when the capsule was administered 5 h pre-feeding vs 1 h post-feeding. In a study conducted in healthy Beagles using the Heidelberg capsule, large non-digestible particles (tablets) emptied from the stomach during the inter-digestive migrating motility complex (MMC) after liquid and digestible solids were emptied during the digestive phase. 15 In a study conducted in humans, a non-digestible wireless telemetric motility capsule (SmartPill Motility Testing; Medtronic) also predominantly emptied in phase III MMC. 16 This could be the reason for the similarly prolonged GTT in cats in which the non-digestible Bravo pH capsule exits the stomach during the giant migrating complexes 17 of the inter-digestive phase.
With the exception of the proximal intestine immediately following gastric exit of the capsule, feeding was observed to cause a decrease in pH along the GI tract and there was no apparent buffering effect of food in the stomach. Gastric and total intestinal pH were significantly increased in the pre-feeding capsule administration period (2.7 and 8.2, respectively) vs the post-feeding (2.0 and 7.8, respectively) period. The lack of a gastric buffering effect of feeding in our study, which presumably is more influenced by luminal contents with the oral administration of the capsule, agrees with a previous study in which the pH capsule was adhered to the cats’ gastric mucosa. 2 Our findings are also similar to those reported in dogs, where a buffering effect of food is not routinely observed.8,13 This is in contrast to the buffering effect often observed in non-human primates 18 and humans.19,20 The decrease in gastric pH with feeding cats in this study may have been related to the relatively high protein content of the cats’ diet or inter-species differences in meal-stimulated gastric acid output.The oral bioavailability of a drug is dependent on the extent of its absorption, which can be affected by both the solubility of the drug and the intestinal permeability. Food can have a profound effect on oral bioavailability owing to the solubility of the drug. For example, drugs that are lipid-soluble have enhanced solubility when coadministered with a fatty meal. The gastric contents in a fasted state would not be enough to promote the absorption of such a drug. Therefore, in the majority of cases, the composition of the meal and the gastric emptying time contribute more to drug absorption than the gastric pH. However, there are several instances where a lack of a buffering or neutralizing effect of food could play a role on the rate of drug absorption. A lack of a buffering effect with food could lead to increased residual gastric acid and more efficient dissolution and absorption of basic drugs. In contrast, animals with a buffering/neutralizing effect of food would experience an increase in gastric pH resulting in a decreased absorption rate of a poorly soluble weak base. Alternatively, prolonged gastric retention and increased gastric acidity could lead to degradation of an unstable drug. Thus, the impact of the buffering effect on drug absorption is largely dependent on the drug’s characteristics. 21
The median total intestinal pH was relatively consistent throughout the study, with a median of 8.2 (range 7.6–8.7) in the pre-feeding period and 7.8 (range 6.7–8.5) in the post-feeding period. Our data are in contrast to those of the study by Brosey et al, 22 which reported a much lower intestinal pH in the duodenum (range 4.9–6.7), jejunum (range 5.9–7.6), ileum (range 5.1–7.6), proximal colon (range 5.0–6.3) and distal colon (range 4.4–5.7). In the referenced study, a single pH measurement was recorded from luminal contents obtained from each section of the intestinal tract with a micro-pH probe. 22 Although we could not precisely identify the intestinal location of the capsule, our study is a more complete assessment of the cats’ intestinal pH as the methodology used in our study recorded the pH every 6 s, allowing for the analysis of thousands of data points and was performed in three replicates in each cat. Alternatively, these differences could be related to differences in microbial environment of the cats or dietary composition.
Intra- and inter-individual variation in GI transit times and pH has previously been described in the dog. For example, Mahar et al 8 observed high variability in gastric residence time in the fasted and fed state in dogs. Sagawa et al 13 observed higher inter-individual variability in fasted dogs. However, gastric pH was less variable in the fed state. Warrit et al 10 observed large variability between dogs and inconsistent gastric emptying following a meal. Dressman 19 discussed that gastric pH was more variable in the fed state in dogs. Despite our relatively homogeneous study group, we also observed a high degree of variability between the pre-feeding and post-feeding capsule administration periods for both transit times and pH, with an intra- and inter-individual CV range of 0.26–0.92 and 0.21–1.60, respectively, for transit time, and 0.02–0.24 and 0.01–0.40, respectively, for pH. This marked variability was observed even when controlling for diet, environment and genetic background. Factors such as range in body size and caloric and volume intake could explain the large variability in inter-individual variation, but the reasons for the large intra-individual variation are undetermined. Intra-individual variation in gastric transit time in the current study was larger than other studies evaluating gastric emptying in cats using methodology not based on the administration of indigestible, radiotelemetric materials such as ultrasound or scintigraphy.23,24 Thus, the larger intra-individual variation in our study may be related to a degree of randomness for when the capsule is propelled through the stomach.
Our study was limited to a small sample size of cats that were from the same research colony and of the same age. Cats were also fed the same dry diet once-daily and therefore we could not assess the effect of varying moisture or macronutrient composition on GI transit times or pH, nor could we comment on the effect of free-choice feeding as all cats were meal-fed. Restricted housing has previously been demonstrated8,10 to have an effect on gastric transit time and thus our results using cats from a research colony may not replicate that found in client-owned or free-roaming cats. Finally, meals were always given at a consistent time and were not timed with the presence of the capsule entering the stomach. In feeding cats at a consistent time of day throughout the study, we hoped to avoid the Pavlovian effect on GI pH and motility that has been previously described during the fasted state when the animal is anticipating the meal. 8 However, cats with prolonged esophageal retention time of the capsule, especially those in the ‘pre-feeding’ capsule administration period, could have had a dramatically different gastric retention time than that described if the meal had been withheld until the capsule had entered the stomach as food stimulates the post-prandial motor pattern. Finally, abdominal ultrasonography and evaluation of serum cobalamin and folate were not performed; therefore, we cannot discount the possibility of the presence of subclinical GI disease in the study cats. Additional studies, including those in client-owned cats of varying ages being meal and free-choice fed varying diets, with and without disease, and studies in healthy cats where the meal is timed with entry of capsule into the stomach, are warranted to evaluate the effect of these variables on GI transit times and pH. Additional studies are also needed in dogs using the same methodology and diet to allow for a more comprehensive comparison of results between these two species.
Conclusions
In this study, we report the GI transit times and pH in fully conscious healthy colony-housed cats using a non-invasive continuous pH monitoring tool. We hope that these data will better guide the development of oral drugs, specifically and solely for the cat and that it will serve as a platform for future studies evaluating the effect of different diet composition, meal size and frequency, and disease states on GI transit times and pH in cats.
Acknowledgments
The authors would like to thank Mary Lopez, Caleb Coursey and Victoria Mas for their assistance in this project.
Footnotes
Accepted: 15 September 2021
Author note: The contents of this publication are solely the responsibility of the authors and do not necessarily represent the views of the EveryCat Health Foundation, which supported the study. This study was presented at the 2020 ACVIM Forum On Demand. This research is included in Ms Naila Telles’ Masters thesis.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This project was supported by a Miller Trust Grant from the EveryCat Health Foundation (MT19-006).
Ethical approval: The work described in this manuscript involved the use of experimental animals and the study therefore had prior ethical approval from an established (or ad hoc) committee as stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: M Katherine Tolbert  https://orcid.org/0000-0001-8725-9530
https://orcid.org/0000-0001-8725-9530
References
- 1. Golly E, Odunayo A, Daves M, et al. The frequency of oral famotidine administration influences its effect on gastric pH in cats over time. J Vet Intern Med 2019; 33: 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parkinson S, Tolbert K, Messenger K, et al. Evaluation of the effect of orally administered acid suppressants on intragastric pH in cats. J Vet Intern Med 2015; 29: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sutalo S, Ruetten M, Hartnack S, et al. The effect of orally administered ranitidine and once-daily or twice-daily orally administered omeprazole on intragastric pH in cats. J Vet Intern Med 2015; 29: 840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ryan P, Odunayo A, Price J, et al. Comparative analysis of the effect of PO administered acid suppressants on gastric pH in healthy cats. J Vet Intern Med 2020; 34: 1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martinez MN, Court MH, Fink-Gremmels J, et al. Population variability in animal health: influence on dose-exposure-response relationships: Part I: drug metabolism and transporter systems. J Vet Pharmacol Ther 2018; 41: E57–E67. [DOI] [PubMed] [Google Scholar]
- 6. Mawby DI, Whittemore JC, Fowler LE, et al. Comparison of absorption characteristics of oral reference and compounded itraconazole formulations in healthy cats. J Am Vet Med Assoc 2018; 252: 195–200. [DOI] [PubMed] [Google Scholar]
- 7. Sparkes AH, Papasouliotis K, Barr FJ, et al. Reference ranges for gastrointestinal transit of barium-impregnated polyethylene spheres in healthy cats. J Small Anim Pract 1997; 38: 340–343. [DOI] [PubMed] [Google Scholar]
- 8. Mahar KM, Portelli S, Coatney R, et al. Gastric pH and gastric residence time in fasted and fed conscious Beagle dogs using the Bravo pH system. J Pharm Sci 2012; 101: 2439–2448. [DOI] [PubMed] [Google Scholar]
- 9. Tolbert MK, Olin S, MacLane S, et al. Evaluation of gastric pH and serum gastrin concentrations in cats with chronic kidney disease. J Vet Intern Med 2017; 31: 1414–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warrit K, Boscan P, Ferguson LE, et al. Minimally invasive wireless motility capsule to study canine gastrointestinal motility and pH. Vet J 2017; 227: 36–41. [DOI] [PubMed] [Google Scholar]
- 11. US Food and Drug Administration. Animal drugs. https://animaldrugsatfda.fda.gov/adafda/views/#/search (accessed April 12, 2021).
- 12. King JN, Jung M, Maurer MP, et al. Effects of route of administration and feeding schedule on pharmacokinetics of robenacoxib in cats. Am J Vet Res 2013; 74: 465–472. [DOI] [PubMed] [Google Scholar]
- 13. Sagawa K, Li F, Liese R, et al. Fed and fasted gastric pH and gastric residence time in conscious Beagle dogs. J Pharm Sci 2009; 98: 2494–2500. [DOI] [PubMed] [Google Scholar]
- 14. Koziolek M, Grimm M, Bollmann T, et al. Characterization of the GI transit conditions in Beagle dogs with a telemetric motility capsule. Eur J Pharm Biopharm 2019; 136: 221–230. [DOI] [PubMed] [Google Scholar]
- 15. Itoh T, Higuchi T, Gardner CR, et al. Effect of particle size and food on gastric residence time of non-disintegrating solids in Beagle dogs. J Pharm Pharmacol 1986; 38: 801–806. [DOI] [PubMed] [Google Scholar]
- 16. Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous smartpill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil 2008; 20: 311–319. [DOI] [PubMed] [Google Scholar]
- 17. Husnik R, Gaschen F. Gastric motility disorders in dogs and cats. Vet Clin North Am Small Anim Pract 2021; 51: 43–59. [DOI] [PubMed] [Google Scholar]
- 18. Chen EP, Mahar Doan KM, et al. Gastric pH and gastric residence time in fasted and fed conscious cynomolgus monkeys using the Bravo pH system. Pharm Res 2008; 25: 123–134. [DOI] [PubMed] [Google Scholar]
- 19. Dressman JB. Comparison of canine and human gastrointestinal physiology. Pharm Res 1986; 3: 123–131. [DOI] [PubMed] [Google Scholar]
- 20. Dressman JB, Berardi RR, Dermentzoglou LC, et al. Upper gastric (GI) pH in young, healthy men and women. Pharm Res 1990; 7: 756–761. [DOI] [PubMed] [Google Scholar]
- 21. Custodio JM, Wu C-Y, Benet LZ. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Drug Deliv Rev 2008; 60: 717–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brosey BP, Hill RC, Scott KC. Gastrointestinal volatile fatty acid concentrations and pH in cats. Am J Vet Res 2000; 61: 359–361. [DOI] [PubMed] [Google Scholar]
- 23. Husnik R, Fletcher JM, Gaschen L, et al. Validation of ultrasonography for assessment of gastric emptying time in healthy cats by radionuclide scintigraphy. J Vet Intern Med 2017; 3: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmitz S, Gotte B, Borsch C, et al. Direct comparison of solid-phase gastric emptying times assessed by means of a carbon isotope-labeled sodium acetate breath test and technetium 99mTc albumin colloid radioscintigraphy in healthy cats. Am J Vet Res 2014; 75: 648–652. [DOI] [PubMed] [Google Scholar]