Abstract
Objectives
The duodenal papilla (DP) is an anatomical structure located in the duodenal wall, a few centimetres from the pylorus. In cats, the pancreatic and bile ducts merge as they enter the DP, and this explains why cats are more likely than dogs to have concomitant digestive, pancreatic and hepatic infections. Ultrasonography of the DP has been previously established in dogs but not in cats. The purpose of our prospective study was to describe the ultrasound features of the DP in 30 adult clinically healthy cats.
Methods
A full abdominal ultrasound was performed. Five measurements were recorded: the width and the height in a transverse section; the length and the height in a longitudinal section; and the thickness of the duodenal wall adoral to the DP in a longitudinal section. The subjective appearance (echogenicity and shape) of the DP was described.
Results
The dimensions of the DP were a mean ± SD width of 3.13 ± 0.68 mm and height of 2.47 ± 0.63 mm in the transverse section, and length of 3.98 ± 1.27 mm and height of 2.44 ± 0.57 mm in the longitudinal section. The DP was homogeneous, subjectively isoechoic to fat and had a round and oval shape in the transverse and longitudinal sections, respectively. There was no correlation between the DP measurements and the weight, age or sex of the cats. The animals that were fed a mixed diet had a longer DP than those fed dry food.
Conclusions and relevance
This study provides reference values for the dimensions of the DP, as well as information on its ultrasonographic appearance in clinically healthy adult cats. We did not find any correlation between the age of the cats and the size of the papilla, but the age range was small and another study in older cats should be undertaken to address this more thoroughly.
Keywords: Duodenal papilla, ultrasound, anatomy, duodenum
Introduction
The duodenal papilla (DP) is an anatomical structure of the proximal portion of the descending duodenal wall that lies on its internal dorsal side 1 approximately 2–3 cm distal to the pylorus. 2 Although the DP refers to the major papilla in 75% of cats, 1 an accessory pancreatic duct that reaches the duodenum at the minor DP, a few centimetres after the major DP, has been described in 25% of cats. 3 In cats, the pancreatic duct and the bile duct have a very short common portion and they merge as they enter the DP. This explains why cats are more likely than dogs to have concomitant digestive, pancreatic and hepatic infections. These diseases may affect the DP, changing its shape, size and echoic appearance, thus making it necessary to compare with a description of the normal DP.
Abdominal ultrasound is an inexpensive and non-invasive imaging method. The DP can be routinely assessed during an abdominal ultrasound examination. The descending duodenum can be imaged using a ventral approach, although a leftward tilt from dorsal recumbency is also possible and it offers better access to the DP. An intercostal approach can also be used with the help of the curvilinear probe providing a very small footprint. A fast of 12 h is recommended to avoid interference from the gastric contents, although these precautions do not ensure the absence of gas in the duodenum, which is the most common cause of artefacts. 2 The recumbency and the pressure on the probe can be modified to overcome this problem. A high-frequency linear transducer (7.5 MHz and higher) is recommended for evaluation of the DP, although a curvilinear probe (3–9 MHz) can also be used because it has a very small footprint when placed below the ribs. 2 Two methods can be used to assess the DP. The first method consists of locating the stomach and then the pylorus, while the second method involves locating the descending duodenum based on the location of the right kidney. 2 In the transverse plane, the DP is shaped like a ring and is located on the mesenteric side of the gastrointestinal tract. In the longitudinal plane, it has a tubular shape inside the descending duodenal wall, mainly within the muscular layer. 2
The dimensions of the DP were reported in a single study performed 20 years ago, which involved only four adult healthy cats. 4 The width and height of the DP measured in the transverse plane were 2.9–5.5 mm and 4 mm, respectively. In this study, all ultrasound evaluations were performed with a curvilinear probe.
The purpose of our study was to describe the ultrasonographic anatomy of the major DP in a larger population of healthy adult cats using a linear transducer. Additionally, the influence of age, sex, weight, lifestyle, and diet on the size and appearance of the DP were studied. We included a study of lifestyle, as well as type of diet, owing to the potential influence of parasitism and the type of food residues on the state of the DP. 4
We hypothesised that sex, age and weight do not significantly influence the dimensions of the major DP; and that diet has an impact on the size of the DP.
Materials and methods
Study design
In our prospective study, cats were recruited from the pets of the students at the Oniris Veterinary School. The cats’ owners were contacted by email. Each owner who wished to involve their animal in the study was provided with an information sheet on the study’s objectives and methods. In addition, an informed consent form, setting out the terms of the study (in particular, the need to clip the animal’s abdomen), was provided, which the owners then signed. Abdominal ultrasound examinations took place at the University Veterinary Hospital Center of Nantes between January and June 2018.
Case selection
To be selected for the study, cats had to meet the inclusion criteria. They had to be between 1 and 10 years old; neutered and unneutered male and female cats were accepted; cats with a history of digestive illness (gastrointestinal, hepatobiliary or pancreatic diseases) were excluded from the study; and cats were not to have undergone any medical treatment or have shown any clinical signs of any kind for 6 months to be included in the study.
Cats were excluded from this study if they had a significant abnormality at the clinical examination or at the abdominal ultrasound examination (lymphadenopathy, bile duct dilatation or biliary sludge).
Each cat was fasted for 12 h before the ultrasound examination.
Image acquisition
The same Esaote MyLab70 XVG ultrasound system was used throughout the study. Each cat underwent a full abdominal ultrasound examination. A linear transducer (6–18 MHz) was used to evaluate the DP and the descending duodenum. The frequency was manually adjusted for each cat, in order to optimise the image quality.
Three staff members of the Diagnostic Imaging Unit of the University Veterinary Hospital Center of Nantes participated in the study. For each cat, only one staff member carried out the full-body ultrasound examination.
Records
The medical records for each included cat were collected by a single observer. The recorded data were as follows: breed, sex, neuter status, age, body weight, history and clinical signs. A clinical examination was performed for each cat 1 h before or after the abdominal ultrasound. The recorded clinical data comprised rectal temperature, heart rate, mucous membrane colour, respiratory rate and delay of capillary refill. Pulmonary and heart auscultation, abdominal palpation and lymph node examination were carried out.
For the ultrasound examination, each cat was clipped between the xiphoid process and the pubis, and coupling gel was applied to the skin. The ultrasound examinations were performed with the animals in dorsal recumbency. At the discretion of the imaging staff, the cat could be gently shifted onto its left side in order to obtain the best images. No sedation was used for the full abdominal ultrasonographic examination.
The wall thickness of the duodenum was evaluated and measured by placing callipers on the outer side of the serosa and on the mucosal inner border adoral to the DP in a longitudinal section (Figure 1). The size of the DP was evaluated by measurement of the width and the height in a single transverse plane and the length and the height in a single longitudinal plane (Figure 2). For each slide, the echogenicity of the DP was subjectively compared to that of the surrounding fat, which means that no scale was used in order to grade the echogenicity. The description of echogenicity therefore corresponds only to a subjective comparison with that of fat.
Figure 1.
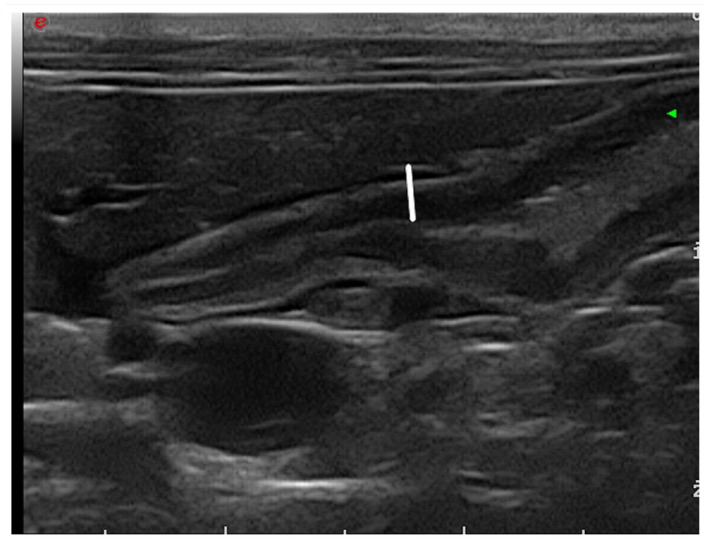
Measurements of the thickness of the duodenal wall adoral to the duodenal papilla in the longitudinal plane
Figure 2.
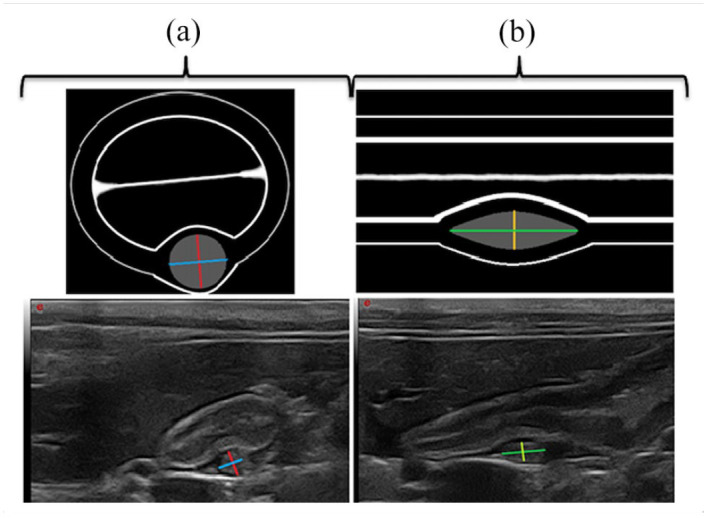
Measurements of the duodenal papilla dimension on illustration (top half) and on ultrasonographic images (bottom half). The measurements recorded were (a) the width (blue) and the height (red) in the transverse plane and (b) the length (green) and the height (yellow) in the longitudinal plane
We studied the relationship between the type of food and the dimensions of the DP. The cats were divided into two groups: group 1 was fed only kibble (dry food; n = 21/30); group 2 was fed mixed food (dry + wet food; n = 9/30).
Finally, we studied the influence of lifestyle on the size and the ultrasound appearance of the DP. The study population was classified into two groups: cats living strictly indoors in the year before the ultrasound examination and cats with access to the outdoors, even if only occasionally, over the same period.
Statistical analysis
Microsoft Office Excel 2007 was used to calculate the mean ± SD of the various data. The means were compared by Student’s t-tests. Before the Student’s t-tests, a Fisher’s test was performed to compare the variances of the two samples studied.
The following statistical tests were performed with R software: Shapiro–Wilk tests, to determine the normality of a variable; Wilcoxon tests, to compare two means when the variables did not follow normal laws; and ANOVA and linear regressions, to study the quantitative variables. Finally, a Fisher’s exact test was used to compare the distribution of the proportions. The error threshold used was 5% (P = 0.05). The 95% confidence intervals are reported in the histograms. 5
Results
Study population
Thirty-six cats were recruited into the study. Two were excluded as they had a gastrointestinal disease, and one because it was undergoing treatment at the time of the abdominal ultrasound. None of the animals was excluded owing to clinical abnormalities. Three cats were excluded owing to abnormalities discovered during the abdominal ultrasound (lymphadenopathy, bile duct dilatation and biliary sludge).
Ultimately, 30 cats were included in the study: 25 domestic shorthair (DSH), two Siamese, one Ragdoll, one Scottish Fold and one crossed Norwegian Forest Cat. All cats were neutered; 18 were male and 12 were female. Mean age was 3.6 years ± 2.4 years. The age of the youngest cat was 1 year and 14 days, and the oldest was 9 years and 10 months.
None of the animals lived exclusively outdoors: nine were strictly indoor cats and 21 had outdoor access.
Mean weight was 4.2 ± 0.73 kg (range 2.8–5.8). Mean weight was 4.52 kg for the male cats and 3.63 kg for the female cats.
The DP was localised in all of the cats. Five measurements were taken for all of the cats: four dimensions of the DP (the length and the height in a longitudinal section, the width and the height in a transverse section) (Figure 2); and the thickness of the duodenal wall adoral to the DP in a longitudinal section (Figure 1). The mean data are summarised in Table 1.
Table 1.
Mean ± SD and median and range for each measurement of the duodenal papilla (DP) and the thickness of the descending duodenal wall in 30 healthy clinically adult cats
| Dimensions (mm) | Mean ± SD | Median (range) |
|---|---|---|
| Width in the transverse plane of the DP | 3.13 ± 0.68 | 3.15 (1.7–4.6) |
| Height in the transverse plane of the DP | 2.47 ± 0.63 | 2.55 (0.8–3.6) |
| Length in the longitudinal plane of the DP | 3.98 ± 1.27 | 3.75 (2.4–7.7) |
| Height in the longitudinal plane of the DP | 2.44 ± 0.57 | 2.4 (1–4.1) |
| Thickness of the descending duodenal wall | 2.95 ± 0.51 | 3.0 (1.8–3.7) |
The Shapiro–Wilk normality test indicated a normal distribution for width in a transverse section (P = 0.8684), the height in a transverse and a longitudinal section (P = 0.5016 and P = 0.1975, respectively), and the thickness of the duodenal wall (P = 0.7677). The length was the only variable not to follow a normal distribution.
Measurements were performed by the three observers on still images. No significant difference was observed for the four measurements.
In the transverse plane, the shape of the DP by ultrasound was mostly round (18/30) or oval (11/30) and rarely almond-shaped (1/30). It was homogeneous (23/30) and isoechoic to the fat (12/30) or hypoechoic (12/30), and less frequently heterogeneous (7/30) and hyperechoic (6/30) to fat. In the longitudinal plane, the shape was mostly oval (19/30), but also round or almond-shaped (4/30) and tapered (7/30). It was homogeneous (17/30) or heterogeneous (13/30) and isoechoic to the fat (19/30) and less frequently hyperechoic (7/30) or hypoechoic (4/30) to the fat.
Variation
Evaluation of the distribution between the dimensions of the DP and the cats’ weights indicated that there was no correlation between these variables, irrespective of sex (P >0.05 for all dimensions). Similarly, no correlation was found between the shape or the echogenicity of the DP and body weight.
No correlation was found between the size of the DP and age or between the shape/echogenicity of the DP and age. Lastly, a Student’s t-test showed that there was no significant difference between the papilla size in females and the DP size in males. In the same way, a Wilcoxon test showed that there was no a correlation between sex and DP dimensions.
A Student’s t-test was performed to evaluate the correlation between diet and DP size. For the width and the height in the transverse plane, this showed that there was no correlation with the type of food. A Wilcoxon test showed that there was no correlation between the height in the longitudinal plane and the type of food. However, a significant difference was found between the cats that only ate dry food and the cats that ate mixed food in terms of the length in the longitudinal plane vs the cats that ate dry and wet food, with the length of the DP being greater than that of cats that only ate dry food. No correlation was found between the shape and echogenicity of the DP and the type of food consumed.
Finally, we studied the influence of lifestyle on the size and ultrasound appearance of the DP. According to the Shapiro–Wilk tests, all subcategories had a normal distribution (P >0.05). Student’s t-tests were performed to determine whether lifestyle was associated with significant differences for each dimension of the DP. We uncovered two correlations: the width in the transverse section and the length in the longitudinal section were significantly greater in indoor cats. However, no significant difference was noted between the two heights. Regarding the shape and appearance of the DP, there did not appear to be a significant difference between indoor cats compared with those with a mixed lifestyle, whether in the transverse or in the longitudinal section.
Discussion
The purpose of our study was to describe the ultrasonographic features of the DP in 30 adult clinically healthy cats and to determine whether there was a correlation between the size, shape and echogenicity of the DP and the weight, age, sex or type of food consumed.
The cats’ owners targeted during our recruitment were all veterinary students, which allowed us to have complete records and a detailed history for each animal. In addition, they were already familiar with certain methods of our study (clipping, positioning and restraining of the animals). A total of 30 cats were included in our study. In 2001, Etue et al 4 carried out an ultrasound study aimed at describing the appearance of the pancreas and associated anatomical structures, including the DP, in 20 healthy cats. The DP was observed and thus measured in only four animals. In 2016, Mortier et al 6 published a study describing the ultrasound appearance of the major DP in 40 dogs free of digestive disorders.
We decided not to anaesthetise our cats to mitigate any increased risks associated with sedation, as well as to increase the willingness of the students to volunteer their cats for the study. The major disadvantage was the increase in ultrasound time depending on the animal’s patience. In addition, we were able to visualise the DP in all cats. Therefore, this choice appeared to be entirely suitable.
Regarding the inclusion criteria, we chose cats aged between 1 and 10 years. This age group corresponds to adult cats that have not yet aged significantly. This age group should also be compared with those preferentially affected by pathologies of the DP. In terms of cholangitis, pancreatitis, inflammatory bowel disease (IBD) and hepatobiliary diseases, very young adults (<2 years of age) are at relatively low risk, while cats >2 years of age and <10 years of age are most often affected by these pathologies.7–17
The majority of the cats in our study were DSHs; only 16.7% were of other breeds or mixed breeds. We compared these proportions with those of all of the cats received at the veterinary hospital between 2017 and 2018. DSH cats accounted for 84.7% of the cats; the remainder were purebred cats or crossbreeds. These proportions are therefore comparable to those of our study.
All cats included in the study were neutered, probably because the included cats belonged to veterinary students. There was an equivalent proportion of males and females. For neutrophilic cholangitis, there does not appear to be a predisposition based on sex, while males are more affected by the lymphocytic form. 8 Other studies did not find a sex predisposition, irrespective of the form of cholangitis,10,18 pancreatitis 12 and IBD. 15 With regard to hepatobiliary neoplasms, males appear to be twice as affected as females, according to Patnaik et al. 19
A study involving a larger number of males and females, with a group of asymptomatic animals and a group with pancreatic, hepatobiliary or intestinal disorders, might provide a clearer picture regarding a possible predisposition of males.
We did not perform any blood tests, although several specific tests would have allowed for the identification of any bio-clinical signs of digestive disease (feline pancreatic lipase, alkaline phosphatase, alanine aminotransferase, bilirubin, etc). The absence of a blood test therefore represents a limitation of our study, but performing a blood test was not possible owing to its invasive nature and budget limitations. However, the cats did not exhibit any clinical signs at the time of the examination and during the 3 months of follow-up.
Ultrasound examination of the abdomen ruled out some of the animals in our study. Three of the cats were excluded owing to abnormalities detected by the abdominal ultrasound (lymphadenopathy, bile duct dilatation and biliary sludge). Unlike the study by Mortier et al, 6 we chose to examine abdominal organs other than those that are part of the digestive system and its appendages in order to select animals unrestricted by the maximum number of conditions detectable by ultrasound examination.
During the study, only a single ultrasound machine was used. To visualise the DP, only a linear probe was used, while the rest of the abdomen could be imaged either with a linear probe or with a convex probe. The use of a high-frequency linear probe (up to 18 MHz) allowed higher-quality DP images to be obtained. To reduce the duration of the procedure and to avoid sedation, we only used a linear probe instead of performing both measurements with the convex and linear probes.
The DP could be located in all of the scanned cats. Fasting of all of the animals made examination of the DP easier, as did the small size of the cats. In other descriptive ultrasound studies of an organ or structure, several measurements are taken of the same dimension, of the same animal and by the same operator, to ensure repeatability. This was the case for the study by Mortier et al, 6 in which all length, width and height measurements of the major DP were taken three times for each dog, with the ultrasound probe being repositioned between each measurement. This was not undertaken in our study so as to limit the examination time. We did not test the reproducibility of the measurements in our study owing to the time constraints on the examination and because the three manipulators were not available simultaneously.
Measuring the thickness of the intestinal wall needs to be carried out carefully in order to avoid a tangential cut, as this would distort the measurement. The duodenum wall thickness was evaluated and measured as described by Nyland and Mattoon. 20 However, the tubular shape of the intestine is simpler than that of the DP. For the majority of cats, the DP measurements were performed on only one transverse section and a single longitudinal section. Given its ovoid geometry (Figures 3 and 4), there can be a risk of underestimating the length and the height of the DP in a longitudinal section. Similarly, in a transverse section, the width and the height of the DP may be underestimated.
Figure 3.
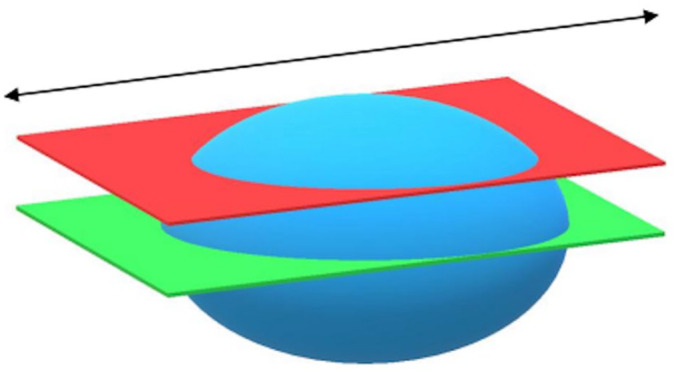
Illustration of duodenal papilla (blue) in a longitudinal plane: measurement of length. Green plane = correct cutting plane; red plane = incorrect cutting plane: length underestimate; black arrow = longitudinal axis of duodenum
Figure 4.
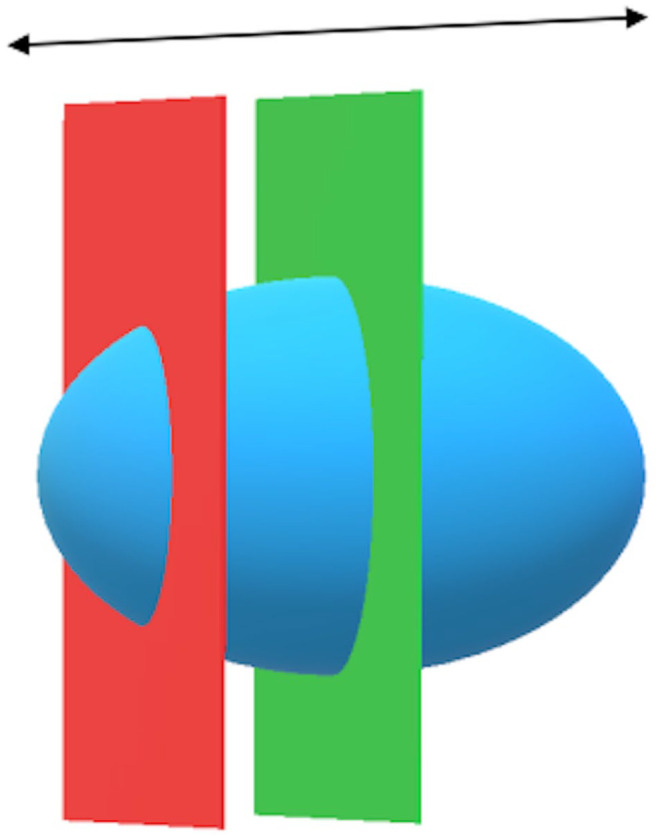
Illustration of duodenal papilla (blue) in a longitudinal plane: measurement of height. Green plane = correct cutting plane; red plane = incorrect cutting plane: length underestimate; black arrow = longitudinal axis of duodenum
With a mean of 2.95 ± 0.51 mm (range 1.8–3.7), our measurements of the thickness of the duodenal wall are comparable to those reported in the literature (2.2 mm in cats). 2
No correlation was found between the weight and the dimensions of the DP, contrary to the situation in dogs, for which weight correlates well with the dimensions of the major DP. 6 This could be explained by the small weight interval in the feline species. Indeed, in our study, the average weight was 4.2 ± 0.73 kg (range 2.8–5.8).
No significant correlation between the size of the DP and age was noted in our study. In dogs, this factor is also not significantly correlated with the dimensions of the major DP. 6 Thus, in cats, there is a weak but significant proportionality relationship between the diameter of the main pancreatic duct measured by ultrasound and the age: the diameter is greater in older cats. 21 This result was found in 73 cats, approximately 80% of which were under 10 years of age (ie, comparable to our population) but only when the duct was measured at the level of the body of the pancreas. It would be interesting to assess the same proportionality relationship by measuring the pancreatic duct as aboral as possible, before it enters the papilla. With regard to the duodenal wall, there is no correlation between the age of the animal and the thickness of the wall. 22
According to Mortier et al, 6 sex is not a factor that significantly influences the dimensions of the major DP. We obtained the same result in cats.
We also hypothesised that the type of food could have an impact on the size of the DP. Only the length in the longitudinal plane was found to be significantly correlated with the type of food consumed: the animals fed mixed foods had longer DPs than those fed only dry food. As the measurement of the length of the DP in a sagittal plane was the one with the greatest variability, we must be cautious about the conclusions. This result should be compared with the results of the study by Winter et al. 22 They used ultrasound to evaluate the thickness of each layer of the intestinal wall, and there was a significant correlation between the type of food and the thickness of the intestinal wall: cats consuming a mixture of dry and wet foods had a thicker wall than those that consumed only dry food. The difference in thickness may reflect more lymphoid tissue due to exposure to different food residues. The authors offer the following explanation: the difference in thickness may be due to larger lymphoid tissue as a result of exposure to different food residues, or the mucous membrane is thicker because more cells are needed to absorb the extra liquid from the food’s water content. Another hypothesis concerns the difference in carbohydrate content. Indeed, dry foods generally contain more carbohydrates than wet foods. Thus, the difference in thickness could be explained by a different response of intestinal villi depending on the carbohydrate content. 23 As the DP is composed of lymphoid tissue, it would be interesting to compare the ultrasound dimensions of the DP between two groups of cats fed with two different types of wet food, so as to assess whether there is a correlation with other dimensions.
We found two significant correlations with lifestyle: the width in the transverse plane and the length in the longitudinal plane were significantly greater in the indoor cats. The causal link between these results is unknown.
Conclusions
This study provides reference values for the dimensions of the DP, as well as its ultrasonographic appearance in clinically healthy adult cats. The DP dimensions included a width of 3.13 ± 0.68 mm and a height of 2.47 ± 0.63 mm in a transverse section, and a length of 3.98 ± 1.27 mm and a height of 2.44 ± 0.57 mm in a longitudinal section. There was no correlation between the DP measurements and the weight, age or sex. Most of the time the DP was isoechoic to fat and had a round shape and oval shape in transverse/longitudinal sections, respectively, and was homogeneous. Cats fed dry and wet food had a longer papilla than cats that ate only dry food. The width and the length of the DP were significantly greater in indoor cats. We did not find any correlation between the age of the cats and the size of the papilla, but our age interval was small and another study in elderly cats should be undertaken to address this more thoroughly.
Footnotes
Accepted: 19 January 2022
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognised high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Charlotte Coeuriot  https://orcid.org/0000-0002-7400-3007
https://orcid.org/0000-0002-7400-3007
References
- 1. Barone R. Anatomie comparée des Mammifères domestiques, Splanchnologie. Paris: Vigot, 1987, p 853. [Google Scholar]
- 2. Penninck D, D’Anjou M-A. Gastrointestinal tract. In: Penninck D, D’Anjou M-A. Atlas of small animal ultrasonography. 2nd ed. Oxford: John Wiley & Sons, 2015, pp 259–308. [Google Scholar]
- 3. Penninck D, D’Anjou M-A. Pancreas. In: Penninck D, D’Anjou M-A. (eds). Atlas of small animal ultrasonography, 2nd ed, Oxford: John Wiley & Sons, 2015, pp 309–330. [Google Scholar]
- 4. Etue S, Penninck D, Labato MA, et al. Ultrasonography of the normal feline pancreas and associated anatomic landmarks: a prospective study of 20 cats. Vet Radiol Ultrasound 2001; 42: 330–336. [DOI] [PubMed] [Google Scholar]
- 5. Thrusfield M. Veterinary epidemiology. 3rd ed. Oxford: Blackwell Science, 2007, p 610. [Google Scholar]
- 6. Mortier JR, Maddox TW, White GM, et al. Ultrasonographic appearance of the major duodenal papilla in dogs without evidence of hepatobiliary, pancreatic, or gastrointestinal tract disease. Am J Vet Res 2016; 77: 597–603. [DOI] [PubMed] [Google Scholar]
- 7. Marolf AJ, Leach L, Gibbons DS, et al. Ultrasonographic findings of feline cholangitis. J Am Anim Hosp Assoc 2012; 48: 36–42. [DOI] [PubMed] [Google Scholar]
- 8. Boland L, Beatty J. Feline cholangitis. Vet Clin North Am Small Anim Pract 2017; 47: 703–724. [DOI] [PubMed] [Google Scholar]
- 9. Center SA. Diseases of the gallbladder and the biliary tree. Vet Clin North Am Small Anim Pract 2009; 39: 543–598. [DOI] [PubMed] [Google Scholar]
- 10. Callahan Clark JE, Haddad JL, Brown DC, et al. Feline cholangitis: a necropsy study of 44 cats (1986–2008). J Feline Med Surg 2011; 13: 570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fragkou FC, Adamama-Moraitou KK, Poutahidis T, et al. Prevalence and clinicopathological features of triaditis in a prospective case series of symptomatic and asymptomatic cats. J Vet Intern Med 2016; 30: 1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armstrong PJ, Williams DA. Pancreatitis in cats. Top Comp Anim Med 2012; 27: 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jergens AE. Pancreatitis. In: Tilley LP, Smith FWK., Jr (eds). Blackwell’s five minute veterinary consult: canine and feline. 5th ed. Oxford: Wiley-Blackwell, 2011, pp 938–939. [Google Scholar]
- 14. Jergens AE. Feline idiopathic inflammatory bowel disease, what we know and what remains to be unravelled. J Feline Med Surg 2012; 14: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall EJ, German AJ. Diseases of the small intestine. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. Vol 2, 6th ed. Philadelphia: Elsevier Saunders, 2010, pp 1332–1378. [Google Scholar]
- 16. Willard MD, Fossum TW. Diseases of the extrahepatic biliary system. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. Vol 2, 6th ed. Philadelphia: Elsevier Saunders, 2010, pp 1478–1481. [Google Scholar]
- 17. Balkman C. Hepatobiliary neoplasia in dogs and cats. Vet Clin North Am Small Anim Pract 2009; 39: 617–625. [DOI] [PubMed] [Google Scholar]
- 18. Caney SMA, Gruffydd-Jones TJ. Feline inflammatory liver diseases. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. Vol 2, 6th ed. Philadelphia: Elsevier Saunders, 2010, pp 1448–1452. [Google Scholar]
- 19. Patnaik AK, Lieberrman PH, Erlandson RA, et al. Hepatobiliary neuroendocrine carcinoma in cats: a clinicopathologic, immunohistochemical, and ultrastructural study of 17 cases. Vet Pathol 2005; 42: 331–337. [DOI] [PubMed] [Google Scholar]
- 20. Nyland TG, Mattoon JS. Gastrointestinal tract. In: Nyland TG, Mattoon JS. (eds). Small animal diagnostic ultrasound. 3rd ed. London: Elsevier, 2002, pp 468–500. [Google Scholar]
- 21. Larson MM, Panciera DL, Ward DL, et al. Age-related changes in the ultrasound appearance of the normal feline pancreas. Vet Radiol Ultrasound 2005; 46: 238–242. [DOI] [PubMed] [Google Scholar]
- 22. Winter MD, Londono L, Berry CR, et al. Ultrasonographic evaluation of relative gastrointestinal layer thickness in cats without clinical evidence of gastrointestinal tract disease. J Feline Med Surg 2014; 16: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schreeg M. Histologic characterization of the major duodenal papilla of cats and correlation to the incidence of concurrent hepatic, pancreatic and intestinal inflammation and neoplasia. ACVP Annual Meeting; 2019. Nov 12; San Antonio, TX, USA. [Google Scholar]


