Abstract
Several small clinical trials have indicated that antibiotic treatment of Chlamydia pneumoniae infection is associated with a better outcome in patients with coronary artery disease (CAD). It has not been demonstrated whether antibiotic treatment eradicates C. pneumoniae from vascular tissue. The aim of the present study was to assess the effect of clarithromycin on the presence of C. pneumoniae in the vascular tissue of patients with CAD. Patients who had CAD and who were waiting for coronary artery bypass graft surgery were enrolled in a randomized, double-blind, placebo-controlled trial. Patients were treated with clarithromycin at 500 mg or placebo once daily from the day of inclusion in the study until surgery. Several vascular tissue specimens were obtained during surgery. The presence of C. pneumoniae in vascular tissue specimens was examined by immunohistochemical staining (IHC) and two PCR assays. Chlamydia immunoglobulin G (IgG) titers were determined by an enzyme-linked immunosorbent assay at the time of inclusion in the study and 8 weeks after surgery. A total of 76 patients were included, and 180 vascular tissue specimens were obtained (80 specimens from the group treated with clarithromycin and 100 specimens from the group treated with placebo). Thirty-five patients received clarithromycin (mean duration, 27 days; standard deviation [SD], 12.2 days), and 41 patients received placebo (mean duration, 27 days; SD, 13.9 days). IHC detected the C. pneumoniae major outer membrane protein antigen in 73.8% of the specimens from the group treated with clarithromycin and 77.0% of the specimens from the group treated with placebo (P was not significant). Chlamydia lipopolysaccharide antigen was found in only one specimen from the group that received placebo. C. pneumoniae DNA was not detected in any specimen. Baseline Chlamydia-specific IgG titers were equally distributed in both groups and were not significantly different after treatment. There was no indication of an active C. pneumoniae infection in vascular tissue. Chlamydia-specific IgG titers remained unchanged throughout the study in both the antibiotic- and the placebo-treated patients.
Many risk factors for atherosclerosis have been identified. However, atherogenesis is not fully understood, and recently, infectious pathogens, particularly Chlamydia pneumoniae, have been considered potential risk factors for atherosclerosis (6). It has been proposed that during respiratory tract infection C. pneumoniae reaches vascular tissue via infected leukocytes. In vascular tissue, C. pneumoniae can infect atheroma-associated cells and can induce inflammatory cytokine production and smooth muscle cell proliferation (16). C. pneumoniae may also cause endothelial cell dysfunction and promote the secretion of matrix-degrading metalloproteinases that destabilize the atherosclerotic plaque (16, 18). Chlamydial lipopolysaccharide (LPS) and the 60-kDa chlamydial heat shock protein may contribute to atherogenesis by promoting foam cell formation, lipoprotein oxidation, and proinflammatory cytokine activation (16).
Some seroepidemiologic studies have found an association between C. pneumoniae and coronary artery disease (CAD). Prospective serologic studies, however, failed to demonstrate any association (5). Further indications that C. pneumoniae might play a role in atherogenesis came from studies that identified C. pneumoniae in vascular tissue by PCR, immunohistochemical staining (IHC), electron microscopy, and culture (6). However, the results of those studies are inconsistent, and huge variations in detection rates have been reported (6, 8).
The results of some small clinical trials that showed beneficial effects from antibiotic treatment encouraged many groups to further investigate the effects of antibiotic treatment on the secondary prevention of cardiovascular events (9, 10, 13). Those studies were based on the hypothesis that antibiotic treatment for C. pneumoniae infection eradicates the organism from the vascular wall in patients with CAD. This would end the infectious process, which would stabilize atheromas and decrease cardiovascular events. However, it has not been studied whether antibiotic treatment eradicates C. pneumoniae from the vascular tissue of patients with CAD. We initiated a placebo-controlled, double-blind, randomized clinical trial to investigate the effect of clarithromycin treatment on the presence of C. pneumoniae in vascular tissue and on circulating Chlamydia-specific immunoglobulin G (IgG) antibody levels in patients with CAD.
MATERIALS AND METHODS
Study population.
Between July 1999 and July 2001, patients who had documented CAD and who were scheduled for coronary artery bypass graft (CABG) surgery were invited to participate in the study. The invitation to be included in the study was made during attendance at the preoperative outpatient clinic at the Department of Thoracic Surgery of the Amphia Hospital, Breda, The Netherlands. Exclusion criteria included (i) concomitant administration of terfenadine, rifabutin, or cisapride; (ii) antibiotic therapy with a macrolide, tetracycline, or quinolone within the 3 months prior to inclusion in the study or during the study period; (iii) renal failure (serum creatinine levels, above 150 μmol/liter); (iv) elevated liver function test results (alanine aminotransferase level, >55 U/liter; aspartate aminotransferase level, >45 U/liter; total bilirubin level, >27 μmol/liter; or alkaline phosphatase level, >180 U/liter); and (v) not taking adequate birth control precautions for female patients capable of childbearing.
After the patients gave informed consent, they were randomized in a double-blind, placebo-controlled trial. Patients received, from the day of inclusion until the day of surgery, a daily dose of slow-release clarithromycin (500 mg) or a placebo tablet (slow-release clarithromycin tablets and matching placebo tablets were obtained from Abbott Laboratories Ltd., Queenborough, England).
An independent pharmacist dispensed either clarithromycin or placebo tablets according to a computer-generated randomization table, which stratified the patients in groups of 10. The researcher responsible for seeing the patients allocated the next available number to a patient on the patient's entry into the trial and provided the patient the corresponding tablets. The code was revealed to the researcher once recruitment, data collection, and laboratory analysis were complete. The local Medical Ethics Committee approved the study.
Clinical specimens.
During CABG surgery, specimens were obtained from coronary atheromas, obstructed old coronary grafts, the mammary artery, and then the saphenal vein, when possible. All vascular specimens were divided into two portions, one for IHC and one for PCR. Samples for IHC were routinely fixed in 10% buffered formalin until further research. Samples for PCR were transported at 4°C in 200 μl of lysis buffer (1 M Tris [pH 7.0], 0.5 mM EDTA, 5 M NaCl, 1% sodium dodecyl sulfate, 20 mg of proteinase K per ml) and were processed within 24 h.
Ten milliliters of blood was obtained from each patient on the day of inclusion and 8 weeks after surgery. The blood was stored at 4°C immediately after collection and was centrifuged within 2 h. The serum was then stored at −20°C pending further testing.
Laboratory methods. (i) Serology.
Chlamydia-specific IgG antibody titers were determined by a recombinant enzyme-linked immunosorbent assay (rELISA; Medac GmbH, Hamburg, Germany), according to the instructions of the manufacturer. This rELISA uses a recombinant Chlamydia-specific LPS fragment as the antigen. An IgG titer of ≥1:100 was considered a positive result.
(ii) IHC.
Cross-sections of each vascular tissue specimen embedded in paraffin wax were stained with hematoxylin-eosin. For each cross-section, the lumen area, the circumference of the internal elastic lamina, and the area encompassed by it were evaluated. Antigens were detected in 4-μm sections by IHC as described by Meijer et al. (19). Two monoclonal antibodies were used in the IHC: the species-specific monoclonal antibody RR-402 against the C. pneumoniae major outer membrane protein (MOMP; Washington Research Foundation, Seattle, Wash.) (25) and the Chlamydia genus-specific anti-LPS monoclonal antibody 16.3B6 (produced by the National Institute of Public Health and the Environment, Bilthoven, The Netherlands). HEp-2 cells (CCL23; American Type Culture Collection) infected with C. pneumoniae strain TW-183 were used as the positive control, and mock-infected HEp-2 cells were used as the negative control.
The specimens were evaluated microscopically by one experienced technician. Specimens were considered positive for C. pneumoniae antigen when a clear dot-like cell-associated staining was observed (8).
PCR. (i) Specimen processing.
Within 24 h after surgery, DNA was extracted from the clinical specimens by use of a QIAamp DNA mini kit (Qiagen Inc., Valencia, Calif.), according to the instructions of the manufacturer. A control was included with every four clinical specimens in the extraction procedure.
(ii) Real-time PCR.
A real-time PCR assay specific for C. pneumoniae and designed to detect the VD4 variable domain of the ompA gene was performed. Oligonucleotide primers included the VD4 forward primer (5′-TCC GCA TTG CTC AGC C-3′), the VD4 reverse primer (5′-AAA CAA TTT GCA TGA AGT CTG AGA A-3′), and a VD4-specific probe (5′-FAM-TAA ACT TAA CTG CAT GGA ACC CTT CTT TAC TAG G-TAMRA, where FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine) (30).
A universal internal control was used to monitor the clinical specimens for the possible presence of PCR inhibitors. This internal control sample consisted of a whole-virus preparation of phocid herpesvirus type 1 (PhHV-1) (12), which was added to the original clinical sample at a final concentration of approximately 5,000 to 10,000 DNA copies per ml. Primers PhHV-F1 (5′-GGG CGA ATC ACA GAT TGA ATC-3′) and PhHV-R1 (5′-GCG GTT CCA AAC GTA CCA A-3′) and a probe (5′-VIC-TTT TTT ATG TGT CCG CCA CCA TCT GGA TC-TAMRA-3′, where VIC is 6-carboxy-fluorescein) were used to amplify PhHV-1, which for uninhibited samples had cycle threshold value of approximately 30. Amplification was carried out with both C. pneumoniae- and PhHV-1-specific primers and probes in a multiplex PCR.
The reaction mixtures were prepared with a 96-well MicroAmp optical plate (Applied Biosystems) by addition of 5 μl of extracted DNA to 45 μl of a PCR mixture containing 1× TaqMan universal PCR master mixture (Applied Biosystems), 600 nM VD4 forward primer, 300 nM VD4 reverse primer, 150 nM FAM-labeled fluorescent C. pneumoniae-specific probe, 5 μl of PhHV-1 (whole virus), 400 nM forward primer PhHV-F1, 400 nM reverse primer PhHV-R1, and 150 nM VIC-labeled PhHV-1-specific probe. The 96-well plate was centrifuged at 1,000 × g for 1 min at room temperature in a swing-out rotor to remove the small air bubbles in the reaction vessels. Amplification and detection were performed with an ABI Prism 7900HT sequence detection system (Applied Biosystems) by using the standard protocols of the manufacturer. The PCR cycling program consisted of 2 min at 50°C, 10 min at 95°C, and 50 cycles of 15 s at 95°C and 1 min at 60°C. Each run contained (i) negative controls (one for every four extracted DNA samples), (ii) a positive control series containing known amounts of C. pneumoniae (5, 2, and 1 inclusion-forming units), and (iii) a negative mixture control. A specimen was considered positive for C. pneumoniae if the fluorescence was above the cycle threshold limit. Specimens were considered negative for C. pneumoniae if the internal control was positive with a cycle threshold value of ≤35.
Industry-developed RUO-PCR.
The presence of C. pneumoniae DNA in specimens was also examined by an industry-developed research-use-only LCx C. pneumoniae PCR (RUO-PCR; Diagnostics Division, Abbott Laboratories, Abbott Park, Ill.). Abbott Laboratories personnel performed the RUO-PCR assay at Abbott Laboratories as described earlier (7). Briefly, an activation mixture was prepared by mixing equal volumes of LCx Activation Reagent II and LCx C. pneumoniae oligonucleotide mixture. Forty microliters of the freshly prepared activation mixture and 30 μl of the purified DNA samples were subsequently added to the appropriate LCx amplification vial. The total reaction volume was 200 μl. Amplification was carried out with a 480 thermocycler (Perkin-Elmer, Norwalk, Conn.) under the following conditions: 97°C for 2 min; 40 cycles of 97°C for 30 s, 59°C for 30 s, and 72°C for 30 s; and finally, 1 cycle of 97°C for 5 min and 12°C for 5 min. PCR products were detected with an LCx analyzer. Samples yielding a rate over 100 cps were considered C. pneumoniae positive. This cutoff was determined by testing titrated C. pneumoniae isolates and uninfected HEp-2 cell DNA multiple times (7).
Specimens were coded, and all detection experiments were performed blind. The code was revealed when the study was completed.
Statistical analysis.
All baseline characteristics were analyzed by a χ2 test or a Student's t test, when appropriate. A P value of <0.05 was considered statistically significant. The SPSS (version 11.0) statistical software package was used for all calculations.
RESULTS
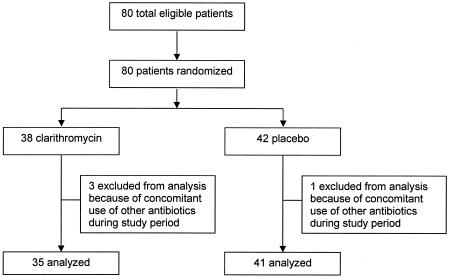
Figure 1 shows that a total of 80 patients who had CAD and who were waiting for CABG surgery were enrolled in the study. Four patients who concomitantly used other antibiotics during the study period were excluded from this treatment analysis due to possible additive effects. Thirty-five patients were randomly assigned to receive clarithromycin, and 41 were randomly assigned to receive placebo. Table 1 shows that the baseline patient characteristics were well balanced between the two treatment groups. The mean number of treatment days for both groups was 27, as indicated by the number of tablets used.
FIG. 1.
Trial profile.
TABLE 1.
Baseline patient characteristics
| Characteristica | Clarithromycin group (n = 35) | Placebo group (n = 41) |
|---|---|---|
| Male patients (no. [%]) | 32 (91.4) | 35 (85.3) |
| Mean (SD) age (yr) | 65 (8.6) | 65 (9.3) |
| Mean (SD) wt (kg) | 84 (19.4) | 81 (12.1) |
| Mean (SD) ht (cm) | 170 (20.2) | 174 (9.9) |
| Mean (SD) NYHA score | 3 (0.9) | 3 (0.7) |
| Mean (SD) no. of treatment days | 27 (12.2) | 27 (13.9) |
| Current smoker (no. [%] of patients) | 5 (14.3) | 4 (9.8) |
| Smoker in past (no. [%] of patients) | 27 (77.1) | 31 (75.6) |
| Medical history (no. [%] of patients) | ||
| COPD | 6 (17.1) | 3 (7.3) |
| Diabetes mellitus | ||
| Type I | 2 (5.7) | 1 (2.4) |
| Type II | 4 (11.4) | 8 (19.5) |
| Hypercholesterolaemia | 20 (57.1) | 27 (65.9) |
| Malignancy | 3 (8.6) | 1 (2.4) |
| CVA or TIA | 2 (5.7) | 6 (14.6) |
| Pectoral angina | 33 (94.3) | 40 (97.6) |
| Myocardal infarction | 20 (57.1) | 23 (56.1) |
| Valvular insufficience | 3 (8.6) | 1 (2.4) |
| Hypertension | 18 (51.4) | 25 (61.0) |
| Earlier vascular surgery | 10 (28.6) | 15 (36.6) |
| Family medical history (no. [%] of patients) | ||
| Cardiovascular disease | 29 (82.9) | 31 (75.6) |
| Diabetes mellitus | 7 (20.0) | 6 (14.6) |
| Medication (no. [%] of patients) | ||
| Statins | 21 (60.0) | 27 (65.9) |
| Antihypertensive drugs | 35 (100) | 41 (100) |
Baseline patient characteristics were not significantly different between the treatment groups. Abbreviations: NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; CVA, classification for angina pectoris; CVA, cerebrovascular accident; TIA, transient ischemic attack.
During CABG surgery, 180 vascular tissue specimens were obtained, including coronary atheromas (n = 31), obstructed old CABG specimens (n = 12), mammary artery specimens (n = 66), and saphenal vein specimens (n = 71). All atheromas and obstructed old CABG specimens showed histological signs of inflammation and advanced atherosclerosis (thickened intima, plaques with thrombus, lymphocytes, foam cells, and surface defects). The other specimens showed normal to slightly thickened vascular walls.
The results of IHC are presented in Table 2. The C. pneumoniae MOMP antigen was found in the majority of specimens of both study groups. Chlamydia LPS antigen was found only once, in an atheroma from a patient in the placebo group. There was no significant difference in the presence of antigens between the two groups. The real-time PCR and the RUO-PCR failed to detect C. pneumoniae DNA in the 180 vascular tissue specimens.
TABLE 2.
Results of IHC of the vascular tissue specimens obtained from the study patients
| Specimen and antigen | No. of specimens positive/no. of specimens tested (%)a
|
P valueb | |
|---|---|---|---|
| Clarithromycin group | Placebo group | ||
| Coronary atheroma | |||
| MOMP | 10/14 (71.4) | 10/17 (58.8) | 0.47 |
| LPS | 0/14 (0) | 1/17 (5.9) | 0.36 |
| Old coronary graftc | |||
| MOMP | 6/6 (100) | 5/6 (83.3) | 0.30 |
| LPS | 0/6 (0) | 0/6 (0) | 1.0 |
| Mammary artery | |||
| MOMP | 22/28 (78.6) | 30/38 (78.9) | 0.97 |
| LPS | 0/28 (0) | 0/38 (0) | 1.0 |
| Saphenal vein | |||
| MOMP | 21/32 (65.6) | 32/39 (82.1) | 0.11 |
| LPS | 0/32 (0) | 0/39 (0) | 1.0 |
| Total | |||
| MOMP | 59/80 (73.7) | 77/100 (77.0) | 0.69 |
| LPS | 0/80 (0) | 1/100 (1.0) | 1.0 |
More than one specimen was obtained from some patients.
A P value <0.05 is considered statistically significant.
Obstructed old bypass graft from earlier CABG procedure.
Chlamydia-specific IgG antibody titers were measured upon inclusion in the study (baseline serology) and 8 weeks after the completion of treatment. Baseline serology was positive for 81.6 and 73.8% of the patients in the clarithromycin group and the placebo group, respectively. The corresponding rates 8 weeks after treatment were 78.9 and 66.7%, respectively. Clarithromycin had no significant effect on Chlamydia-specific IgG antibody titers. In both treatment groups, the Chlamydia-specific IgG antibody titers after treatment were not significantly different from the baseline titers.
DISCUSSION
Macrolide antibiotics, including clarithromycin, are active against C. pneumoniae. The hypothesis that C. pneumoniae infection is a risk factor for atherosclerosis has led to clinical trials of macrolide treatment in patients with CAD (13). Human placebo-controlled trials have been performed to investigate the clinical effects of antibiotics in patients with vascular disease. These studies follow the hypothesis that macrolides will kill C. pneumoniae in the vascular wall, which will subsequently end the infectious process. Thereby, the plaque will be stabilized, which will result in fewer complications of atherosclerosis. However, whether antibiotic treatment has an effect on the presence of the microorganism in vascular tissue has not been studied well.
In the present study, vascular tissue specimens were tested by an automated real-time PCR which combines amplification, hybridization, and quantitative product detection. The specimens were also tested by an industry-developed RUO-PCR. Both methods failed to detect C. pneumoniae DNA in any specimen. This indicates that there is no evidence of active C. pneumoniae infection in the vascular tissue of CAD patients. This questions the use of antibiotics for this indication.
Melissano et al. (21) evaluated the effect of roxithromycin on C. pneumoniae in carotid atheromas. Those investigators concluded that roxithromycin treatment was effective in eradicating C. pneumoniae from carotid atheromas, since C. pneumoniae DNA was detected in 31% (5 of 16) of the atheromas in the roxithromycin group and in 75% (12 of 16) of the atheromas in the control group. However, they used a conventional seminested PCR assay to detect C. pneumoniae DNA. Conventional PCR assays are unstandardized and are known to produce conflicting results, including false-positive results (2, 3). Also, that small trial was unblinded, which limits the accuracy of the results. Efforts have recently been focused on the establishment of quantitative real-time PCR and RUO-PCR technologies. The first reports on evaluations of these tests suggest that they are sensitive, specific, and reproducible (4, 7). The conclusion from our DNA detection experiments must be that there was no active C. pneumoniae infection in the vascular tissues of patients with CAD.
The results of IHC were different. A high prevalence of C. pneumoniae MOMP antigen was found in both groups. Chlamydia LPS antigen was detected by IHC in only one specimen from the placebo group. These results are consistent with the findings of other investigators. The abundance of MOMP antigen and the low rate of detection of LPS antigen in vascular tissue specimens have been reported before (6, 19, 20). IHC is a valuable technique but is limited by the need for the subjective reading and interpretation of the results. These remain difficult because of background staining and nonspecific staining with antigenic components in vascular tissue specimens, such as inflammatory cells and tissue components (24, 27, 29). It is also possible that components of Chlamydia-like microorganisms described recently can cause cross-reactivity to the monoclonal antibodies used in IHC (19). Recently, the results of a study by Hoymans et al. (14) suggested that a positive IHC result was obtained because of nonspecific cross-reactivity between MOMP antibodies with plaque constituents, such as ceroid deposits.
In the present study, we could not demonstrate any effect of clarithromycin on the presence of C. pneumoniae MOMP antigen in vascular tissue. A similar finding has been demonstrated in studies with animal models. Azithromycin treatment was not associated with the elimination of chlamydial antigen from vascular tissue specimens of rabbits infected with C. pneumoniae (22). Also, in a mouse model, azithromycin treatment did not affect the presence of C. pneumoniae in the aorta, lung, or spleen (26).
Clarithromycin treatment had no effect on Chlamydia-specific IgG antibody titers, and we found no significant difference between the baseline titers and the titers measured 8 weeks after treatment. Circulating Chlamydia-specific antibody titers have been used as a marker of the response to antibiotic treatment. Gupta et al. (9) reported that azithromycin had a significant effect on Chlamydia-specific IgG antibody titers. However, our study supports the results of other trials (1, 15, 17, 23, 28) that have found that antibiotic therapy does not influence Chlamydia-specific antibody titers in patients with vascular disease.
This is the first placebo-controlled, double-blind, randomized clinical trial to assess the effect of antibiotic treatment on the presence of C. pneumoniae in vascular tissue. The major finding in this study is that C. pneumoniae was not present in vascular tissue, including atherosclerotic plaques of patients with advanced CAD. Only the MOMP antigen was found. Since C. pneumoniae is no longer present in vascular tissue, it is unlikely that antibiotic treatment will have any effect in patients with advanced atherosclerosis. This explains the results of many clinical trials (1, 11, 15, 17, 23, 28, 31) that have failed to demonstrate any beneficial effect of antibiotic treatment in patients with vascular disease.
REFERENCES
- 1.Anderson, J. L., J. B. Muhlestein, J. Carlquist, A. Allen, S. Trehan, C. Nielson, S. Hall, J. Brady, M. Egger, B. Horne, and T. Lim. 1999. Randomized secondary prevention trial of azithromycin in patients with coronary artery disease and serological evidence for Chlamydia pneumoniae infection: The Azithromycin in Coronary Artery Disease: Elimination of Myocardial Infection with Chlamydia (ACADEMIC) study. Circulation 99:1540-1547. [DOI] [PubMed] [Google Scholar]
- 2.Apfalter, P., F. Blasi, J. Boman, C. A. Gaydos, M. Kundi, M. Maass, A. Makristathis, A. Meijer, R. Nadrchal, K. Persson, M. L. Rotter, C. Y. Tong, G. Stanek, and A. M. Hirschl. 2001. Multicenter comparison trial of DNA extraction methods and PCR assays for detection of Chlamydia pneumoniae in endarterectomy specimens. J. Clin. Microbiol. 39:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apfalter, P., O. Assadian, F. Blasi, J. Boman, C. A. Gaydos, M. Kundi, A. Makristathis, M. Nehr, M. L. Rotter, and A. M. Hirschl. 2002. Reliability of nested PCR for detection of Chlamydia pneumoniae DNA in atheromas: results from a multicenter study applying standardized protocols. J. Clin. Microbiol. 40:4428-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apfalter, P., W. Barousch, M. Nehr, A. Makristathis, B. Willinger, M. Rotter, and A. M. Hirschl. 2003. Comparison of a new quantitative ompA-based real-time PCR TaqMan assay for detection of Chlamydia pneumoniae DNA in respiratory specimens with four conventional PCR assays. J. Clin. Microbiol. 41:592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloemenkamp, D. G., W. P. Mali, F. L. Visseren, and Y. van der Graaf. 2003. Meta-analysis of sero-epidemiologic studies of the relation between Chlamydia pneumoniae and atherosclerosis: does study design influence results? Am. Heart J. 145:409-417. [DOI] [PubMed] [Google Scholar]
- 6.Boman, J., and M. R. Hammerschlag. 2002. Chlamydia pneumoniae and atherosclerosis: critical assessment of diagnostic methods and relevance to treatment studies. Clin. Microbiol. Rev. 15:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernesky, M., M. Smieja, J. Schachter, J. Summersgill, L. Schindler, N. Solomon, K. Campbell, L. Campbell, A. Cappuccio, C. Gaydos, S. Chong, J. Moncada, J. Phillips, D. Jang, B. J. Wood, A. Petrich, M. Hammerschlag, M. Cerney, and J. Mahony. 2002. Comparison of an industry-derived LCx Chlamydia pneumoniae PCR research kit to in-house assays performed in five laboratories. J. Clin. Microbiol. 40:2357-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowell, S. F., R. W. Peeling, J. Boman, G. M. Carlone, B. S. Fields, J. Guarner, M. R. Hammerschlag, L. A. Jackson, C. C. Kuo, M. Maass, T. O. Messmer, D. F. Talkington, M. L. Tondella, S. R. Zaki, and C. pneumoniae Workshop Participants. 2001. Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada). Clin. Infect. Dis. 33:492-503. [DOI] [PubMed] [Google Scholar]
- 9.Gupta, S., E. W. Leatham, D. Carrington, M. A. Mendall, J. C. Kaski, and A. J. Camm. 1997. Elevated Chlamydia pneumoniae antibodies, cardiovascular events, and azithromycin in male survivors of myocardial infarction. Circulation 96:404-407. [DOI] [PubMed] [Google Scholar]
- 10.Gurfinkel, E., G. Bozovich, A. Daroca, E. Beck, B. Mautner, et al. 1997. Randomised trial of roxithromycin in non-Q-wave coronary syndromes: ROXIS pilot study. Lancet 350:404-407. [DOI] [PubMed] [Google Scholar]
- 11.Gurfinkel, E., G. Bozovich, E. Beck, E. Testa, B. Livellara, and B. Mautner. 1999. Treatment with the antibiotic roxithromycin in patients with acute non-Q-wave coronary syndromes. The final report of the ROXIS study. Eur. Heart J. 20:121-127. [DOI] [PubMed] [Google Scholar]
- 12.Harder, T. C., M. Harder, H. Vos, K. Kulonen, S. Kennedy-Stoskopf, B. Liess, M. J. Appel, and A. D. Osterhaus. 1996. Characterization of phocid herpesvirus-1 and -2 as putative alpha- and gammaherpesviruses of North American and European pinnipeds. J. Gen. Virol. 77:27-35. [DOI] [PubMed] [Google Scholar]
- 13.Higgins, J. P. 2003. Chlamydia pneumoniae and coronary artery disease: the antibiotic trials. Mayo Clin. Proc. 78:321-332. [DOI] [PubMed] [Google Scholar]
- 14.Hoymans, V. Y., J. M. Bosmans, D. Ursi, W. Martinet, F. L. Wuyts, E. Van Marck, M. Altwegg, C. J. Vrints, and M. M. Ieven. 2004. Immunohistostaining assays for detection of Chlamydia pneumoniae in atherosclerotic arteries indicate cross-reactions with nonchlamydial plaque constituents. J. Clin. Microbiol. 42:3219-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson, L. A., D. K. Stewart, S. P. Wang, D. B. Cooke, T. Cantrell, and J. T. Grayston. 1999. Safety and effect on anti-Chlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J. Antimicrob. Chemother. 44:411-414. [DOI] [PubMed] [Google Scholar]
- 16.Kalayoglu, M. V., P. Libby, and G. I. Byrne. 2002. Chlamydia pneumoniae as an emerging risk factor in cardiovascular disease. JAMA 288:2724-2731. [DOI] [PubMed] [Google Scholar]
- 17.Leowattana, W., K. Bhuripanyo, L. Singhaviranon, S. Akaniroj, N. Mahanonda, M. Samranthin, and S. Pokum. 2001. Roxithromycin in prevention of acute coronary syndrome associated with Chlamydia pneumoniae infection: a randomized placebo controlled trial. J. Med. Assoc. Thai. 84:S669-S675. [PubMed] [Google Scholar]
- 18.Liuba, P., P. Karnani, E. Pesonen, I. Paakkari, A. Forslid, L. Johansson, K. Persson, T. Wadstrom, and R. Laurini. 2000. Endothelial dysfunction after repeated Chlamydia pneumoniae infection in apolipoprotein E-knockout mice. Circulation 102:1039-1044. [DOI] [PubMed] [Google Scholar]
- 19.Meijer, A., J. A. van der Vliet, P. J. M. Roholl, S. K. Gielis-Proper, A. de Vries, and J. M. Ossewaarde. 1999. Chlamydia pneumoniae in abdominal aortic aneurysms. Abundance of membrane components in the absence of heat shock protein 60 and DNA. Arterioscler. Thromb. Vasc. Biol. 19:2680-2686. [DOI] [PubMed] [Google Scholar]
- 20.Meijer, A., P. J. M. Roholl, S. K. Gielis-Proper, and J. M. Ossewaarde. 2000. Chlamydia pneumoniae antigens, rather than viable bacteria, persist in atherosclerotic lesions. J. Clin. Pathol. 53:911-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melissano, G., F. Blasi, G. Esposito, P. Tarsia, L. Dordoni, C. Arosio, Y. Tshomba, L. Fagetti, L. Allegra, and R. Chiesa. 1999. Chlamydia pneumoniae eradication from carotid plaques. Results of an open, randomised treatment study. Eur. J. Vasc. Endovasc. Surg. 18:355-359. [DOI] [PubMed] [Google Scholar]
- 22.Muhlestein, J. B., J. L. Anderson, E. H. Hammond, L. Zhao, S. Trehan, E. P. Schwobe, and J. F. Carlquist. 1998. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation 97:633-636. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor, C. M., M. W. Dunne, M. A. Pfeffer, J. B. Muhlestein, L. Yao, S. Gupta, R. J. Benner, M. R. Fisher, T. D. Cook, and Investigators in the WIZARD Study. 2003. Azithromycin for the secondary prevention of coronary heart disease events. The WIZARD study: a randomized controlled trial. JAMA 290:1459-1466. [DOI] [PubMed] [Google Scholar]
- 24.Ong, G., B. J. Thomas, A. O. Mansfield, B. R. Davidson, and D. Taylor-Robinson. 1996. Detection and widespread distribution of Chlamydia pneumoniae in the vascular system and its possible implications. J. Clin. Pathol. 49:102-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puolakkainen, M., J. Parker, C. C. Kuo, J. T. Grayston, and L. A. Campbell. 1995. Further characterization of Chlamydia pneumoniae specific monoclonal antibodies. Microbiol. Immunol. 39:551-554. [DOI] [PubMed] [Google Scholar]
- 26.Rothstein, N. M., T. C. Quinn, G. Madico, C. A. Gaydos, and C. J. Lowenstein. 2001. Effect of azithromycin on murine arteriosclerosis exacerbated by Chlamydia pneumoniae. J. Infect. Dis. 183:232-238. [DOI] [PubMed] [Google Scholar]
- 27.Shor, A., J. I. Philips, G. Ong, B. J. Thomas, and D. Taylor-Robinson. 1998. Chlamydia pneumoniae in atheroma: consideration of criteria for causality. J. Clin. Pathol. 51:812-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinisalo, J., K. Mattila, M. S. Nieminen, V. Valtonen, M. Syrjala, S. Sundberg, and P. Saikku. 1998. The effect of prolonged doxycycline therapy on Chlamydia pneumoniae serological markers, coronary heart disease risk factors and forearm basal nitric oxide production. J. Antimicrob. Chemother. 41:85-92. [DOI] [PubMed] [Google Scholar]
- 29.Taylor-Robinson, D., and B. J. Thomas. 1998. Chlamydia pneumoniae in arteries: the facts, their interpretation, and future studies. J. Clin. Pathol. 51:793-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tondella, M. L., D. F. Talkington, B. P. Holloway, S. F. Dowell, K. Cowley, M. Soriano-Gabarro, M. S. Elkind, and B. S. Fields. 2002. Development and evaluation of real-time PCR-based fluorescence assays for detection of Chlamydia pneumoniae. J. Clin. Microbiol. 40:575-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams, E. S., and J. M. Miller. 2002. Results from late-breaking clinical trial sessions at the American College of Cardiology 51st Annual Scientific Session. J. Am. Coll. Cardiol. 40:1-18. [DOI] [PubMed] [Google Scholar]