Abstract
Objectives
The aim of this study was to evaluate the association between meningeal enhancement (MgE) and cerebrospinal fluid (CSF) analysis results, their individual association with bacteriology results from affected ear samples and whether these test results influenced clinicians’ therapeutic choice in cats with otitis media and interna (OMI).
Methods
This was a multicentre retrospective study carried out over an 8-year period. Cats diagnosed with OMI, with or without a nasopharyngeal polyp, leading to peripheral vestibular signs were included. Only cats for which MRI with postcontrast T1-weighted sequences and CSF analyses available were included. Cats with intra-axial MRI lesions or empyema were excluded.
Results
Fifty-eight cats met the inclusion criteria. MgE was reported in 26/58 cases, of which nine had an abnormal CSF result (increased total nucleated cell count [TNCC] or total protein); 32/58 cases had no MgE, of which 10 showed abnormal CSF results. There was no association between bacteriology results (external ear canal or bulla) and MgE or abnormal CSF results. CSF abnormalities were statistically significantly more common in acute cases (n = 16/37) than in chronic cases (n = 3/21; Fischer’s test P = 0.04). Prednisolone was prescribed in 10/16 cases with increased TNCC. Among the 42 cases with normal TNCC, 15 received prednisolone and 13 received non-steroidal anti-inflammatory drugs. Various antimicrobial drugs were prescribed in 53/58 cats. Duration of antimicrobial treatment was similar, regardless of positive bacterial culture (5.58 vs 4.22 weeks), abnormal CSF (5.83 vs 4.76 weeks) or MgE (5.33 vs 4.90 weeks).
Conclusions and relevance
No association was found between the CSF and MgE results. Furthermore, no association was found between MgE, CSF or bacteriology findings. In addition, abnormal CSF results might lead the clinician to treat with corticosteroids, but they did not have any impact on duration of antimicrobial treatment. CSF abnormalities were seen significantly less frequently in chronic cases. The outcome tended to be poorer when MgE was detected on MRI.
Keywords: Otitis media and interna, peripheral vestibular signs, MRI meningeal enhancement, cerebrospinal fluid
Introduction
Otitis media and interna (OMI) has been reported to be the cause of vestibular signs in 43–63% of cats with peripheral vestibular signs (PVS).1,2 OMI in cats is most commonly associated with inflammation caused by upper respiratory infection that has extended through the auditory tube or a nasopharyngeal polyp.1,3,4 It occurs less frequently as a consequence of an otitis externa or neoplasia. Bacterial isolates from OMI include Staphylococcus species, Streptococcus species, Pasteurella multocida, Escherichia coli, Enterococcus species and, less frequently, Mycoplasma species, Corynebacterium species and other bacterial species.1,4–8 Apart from bacteria, other infectious agents can be involved in cats, especially fungi (Malassezia species). 8 Occasionally, a nasopharyngeal polyp – a non-neoplastic, inflammatory growth that arises from the middle ear or auditory tube – can be responsible for OMI.1,2,9,10 Diagnosis of otitis media and bacterial infection in the bulla can be achieved via cytological and/or bacterial culture of material retrieved via myringotomy or a surgical procedure such as bulla osteotomy.1,8,9 Cats with PVS are clinically recognised with the presence of at least one of the following clinical signs: ipsilateral head tilt; jerk nystagmus; tight circling; positional strabismus and/or vestibular ataxia; as well as the absence of any neurological signs suggestive of intracranial disease.11–13 PVS reflect the involvement of the inner ear, while the presence of facial nerve deficit and/or Horner syndrome indicates involvement of the middle ear.11–13
MRI is a sensitive method with which to diagnose OMI, particularly for inner ear visualisation.1,7,14 The fluid composition of endo- and perilymph allows for good visualisation of this inner ear part in a fluid-sensitive sequence such as T2-weighted (T2W) images or in fluid-attenuated inversion recovery (FLAIR).14,15 A marked hyperintensity compared with adjacent structures is present on T2W images, while suppression is visible in FLAIR.14,15 Postcontrast T1-weighted (T1W) images may show an abnormality consistent with inflammatory changes in the inner ear. 1 Typical changes raising the suspicion of OMI include isointense material in the bulla on T1W images and hyperintense on T2W images. On postcontrast T1W images, a peripheral enhancement along the inner surface of the tympanic bulla can be observed.7,16 A laminated appearance of the mucosa of the tympanic bulla on T2W images has also been described. 17 A reduced signal intensity on T2W or an increased signal intensity on FLAIR images from the intralabyrinthine fluid is an MRI finding suggestive of otitis interna.14,16,18 Owing to the anatomical proximity, intracranial extension of OMI can lead to a meningeal enhancement (MgE) on MRI after intra-venous administration of paramagnetic contrast medium. 19 Anatomically, perilymph and cerebrospinal fluid (CSF) are connected via the cochlear aqueduct. 20 Therefore, CSF analysis is another important diagnostic tool for cats with OMI and is reported to be more sensitive than MRI in identifying intracranial inflammatory processes.7,15,21 Thus, an abnormal MRI and/or CSF analysis can provide useful clinical information on the presence of a concurrent meningitis and may influence treatment.
The relationship between MgE and abnormal CSF in cats with OMI has not yet been investigated; their association with bacteriology results and therapeutic management has also not been studied. The aims of this study were to describe the association between MgE and CSF analysis results, their individual association with bacteriology results from affected ear samples and the influence of the above with therapeutic choice in cats with OMI. We hypothesised that: (1) MgE is associated with CSF abnormalities; (2) positive bacteriology is more common if MgE and/or CSF abnormalities are present; and (3) positive bacteriology is associated with the choice and length of antimicrobial and/or anti-inflammatory treatment.
Materials and methods
Selection criteria
Data were retrospectively collected from six different referral centres across Europe (Vetsuisse-Faculty, University of Bern; University of Veterinary Medicine Hannover; Royal Veterinary College London [RVC]; School of Veterinary Medicine, University of Glasgow; Queen’s Veterinary School Hospital, University of Cambridge; and Royal [Dick] School of Veterinary Studies, University of Edinburgh) over an 8-year period (January 2012–December 2020). Only client-owned cats with PVS that underwent MRI (with postcontrast images) and CSF analysis as part of the diagnostic work-up were selected for this study. Inclusion criteria were: (1) clinical signs consistent with peripheral vestibular lesion localisation; (2) a diagnosis of OMI with or without the presence of a nasopharyngeal polyp based on MRI findings; and (3) the absence of an intra-axial abnormality or imaging findings consistent with empyema on MRI.
Retrospective information collected from the medical records included signalment, history, treatment prior to and after referral, right-, left-sided or bilateral PVS, MRI and CSF findings, bacteriology results from the affected ear and outcome.
MRI
MRI of the skull was obtained under general anaesthesia, using anaesthetic protocols at the discretion of the anaesthesiologist in charge. High-field MRI was used at all institutions (except for one cat) and varied between centres: a Philips Panorama HFO 1.0 T (Philips Medical Systems Nederland) for Bern; a Philips Achieva 3.0 T (Philips Medical Systems) for Hannover; 1.5T (Intera; Philips Medical Systems) for RVC; 1.5T (Magnetom Essenza; Siemens) for Glasgow, 0.27 T (Esaote VetMR Grande) or 1.5 T (Phillips Achieva; Phillips Healthcare) for Cambridge; and 1.5 T (Intera; Philips Medical Systems) for Edinburgh. Although MRI protocols varied between centres, at least T1W and T2W postcontrast images were available in all cats (gadoteric acid 0.2 mmol/kg IV [Dotarem; Guerbert Laboratories] for Bern and Hannover; gadoterate meglumine 0.1 mmol/kg IV [Dotarem; Guerbet] for RVC; gadobutrol 0.1 mmol/kg [Gadovist; Bayer] for Glasgow and Cambridge; and gadopentate dimeglumine 0.1 mmol/kg IV [Magnevist; Bayer] for Edinburgh). All MRIs were evaluated by a board-certified veterinary radiologist or a board-certified veterinary neurologist. Information about OMI with or without nasopharyngeal polyp and MgE were collected directly from MRI reports.
CSF analysis
Abnormal CSF was defined as a total nucleated cell count (TNCC) ⩾5 leukocytes/µl and/or increased total protein >0.3 g/l for cisterna magna samples and >0.45 g/l for lumbar samples. Albuminocytological dissociation was defined as an increase in total protein without an increased TNCC. Neutrophilic (respectively monocytic) pleocytosis was identified if neutrophils constituted >70% (respectively monocytes) in CSF with an abnormal TNCC. 22
Medical treatment
Antimicrobials were categorised as the first or second line of treatment. First-line treatment included amoxicillin–clavulanic acid, cephalosporin, metronidazole, clindamycin and doxycycline. Second-line treatment contained marbofloxacin, enrofloxacin, cefovecin, pradofloxacin and cefixime. 23
Anti-inflammatory drugs were categorised as steroidal (eg, prednisolone and dexamethasone) or non-steroidal (eg, meloxicam and robenacoxib).
Statistical analysis
The presence or absence of MgE on MRI, CSF results and bacteriology culture results were compared using a χ2 test. The choice of treatment based on MgE, CSF and bacteriology results were compared using a χ2 test or a Fisher’s exact test if a group contained fewer than five cats. Duration of treatment was compared using a Student’s t-test. Test values were performed in a two-sided manner and a P value ⩽0.05 was considered statistically significant. All analyses were performed using R version 3.6.3.
Results
A total of 58 cats met the inclusion criteria. Domestic shorthair was the most common breed (n = 32). Other breeds included Maine Coon (n = 9), Siamese (n = 4), British Shorthair (n = 2), Burmese (n = 2), Russian Blue (n = 2), Bengal (n = 1), Egyptian Mau (n = 1), Norwegian Forest Cat (n = 1), Ocicat (n = 1), Persian (n = 1), Ragdoll (n = 1) and Snowshoe (n = 1). There were 27 females (four intact, 23 spayed) and 31 males (four intact, 27 castrated; male: female sex ratio = 1.15). The mean age of the cats was 6.9 years (median 7.3, range 3.7 months to 14.7 years). Half of the cases had left vestibular signs at time of presentation. Clinical signs were acute (⩽14 days) in 37 cats and chronic (>14 days) in 21 cats. Before presentation, 32 cats were given medical treatment. Nine cases received antimicrobial treatment alone, eight had antimicrobial treatment with non-steroidal anti-inflammatory drugs (NSAIDs), nine had an antimicrobial treatment with corticosteroids, and two had an antimicrobial treatment, NSAIDs and corticosteroids. Five cats received only corticosteroids and one only an NSAID. All clinical information is available in the table in the supplementary material.
Forty-five cats (78%) were diagnosed with OMI alone (without a nasopharyngeal polyp) (Figures 1 and 2): three based on histology of material obtained from bulla osteotomy, three based on findings during bulla osteotomy, 26 based on otoscopy and cytology results obtained through myringotomy, and 13 were suspected on MRI only. The remaining cats (n = 13/58 [22%]) were diagnosed with OMI secondary to a polyp (Figures 3 and 4): six based on histology of material obtained from bulla osteotomy, two based on findings during bulla osteotomy, two based on otoscopy and/or cytology results obtained through myringotomy, and two were suspected on MRI only.
Figure 1.
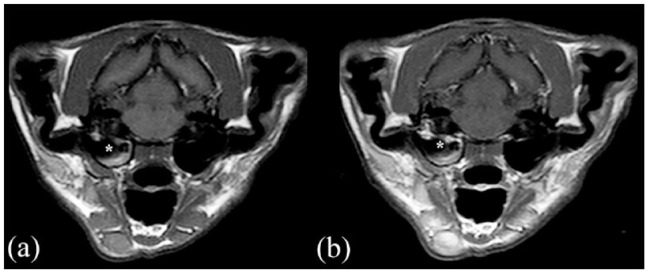
MRI (Philips Panorama HFO 1.0 T) transverse images in (a) T1-weighted (T1W) sequence and (b) T1W postcontrast sequence of a cat presented with otitis media interna without polyp (*) and without meningeal enhancement
Figure 2.
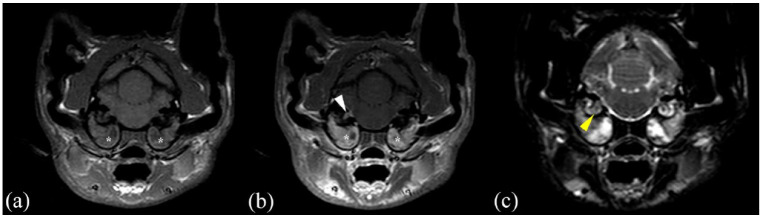
MRI (Philips Panorama HFO 1.0 T) transverse images in (a) T1-weighted (T1W) sequence, (b) T1W postcontrast sequence and (c) T2W sequence of a cat presented with bilateral otitis media interna without polyp (*), with meningeal and vestibulocochlear nerve enhancement (white arrowhead) and otitis interna (yellow arrowhead)
Figure 3.
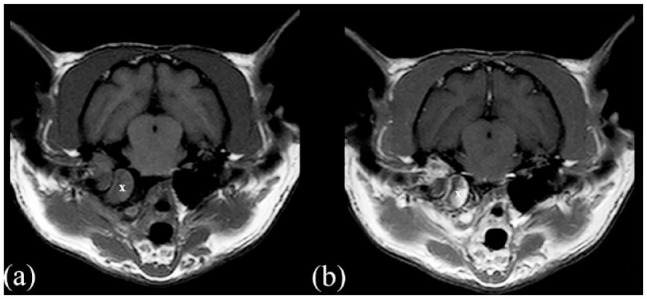
MRI (Philips Panorama HFO 1.0 T) transverse images in (a) T1-weighted (T1W) sequence and (b) T1W postcontrast sequence of a cat presented with otitis media interna associated with a polyp (X) and without meningeal enhancement
Figure 4.
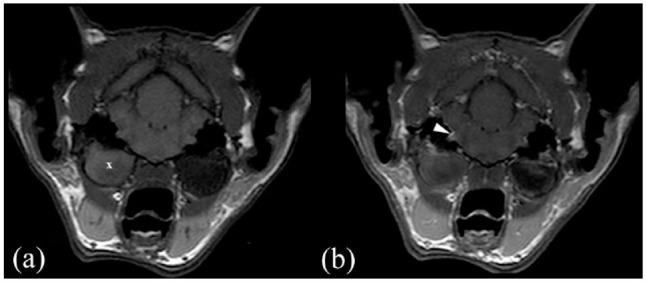
MRI (Philips Panorama HFO 1.0 T) transverse images in (a) T1-weighted (T1W) sequence and (b) T1W postcontrast sequence of a cat presented with otitis media interna associated with a polyp (X) and with meningeal and vestibulocochlear nerve enhancement (white arrowhead)
Meningeal contrast enhancement was present in 26 cases (45%; Figure 2b and Figure 4b), while 32 cases (55%) did not show MgE (Figure 1b and Figure 3b). CSF analysis was abnormal in 19 cats (33%): 11 with only increased TNCC, five with both increased TNCC and total protein, and three with albuminocytological dissociation. Increased TNCC ranged between five and 1205 leukocytes/µl (median 12); increased total protein in CSF ranged between 0.34 and 0.77 g/l (median 0.56). Neutrophilic pleocytosis was seen in nine cases, monocytic pleocytosis was seen in one case and mixed-cell pleocytosis in six cases.
Nine cats (16%) presented with both MgE and abnormal CSF (five with only increased TNCC, two with both increased TNCC and total protein and two with albuminocytological dissociation). MgE was detected in 17 cats (29%) with normal CSF. Abnormal CSF was seen in 10/32 cats (31%) without MgE: six with only increased TNCC, three with both increased TNCC and total protein, and one with albuminocytological dissociation. No significant association (χ2 test, P = 0.79) was found between the CSF results and MgE findings (Table 1).
Table 1.
Association between cerebrospinal fluid (CSF) and meningeal enhancement (MgE) findings (χ2 test, P = 0.79)
| CSF+ | CSF− | |
|---|---|---|
| MgE+ | 9 | 17 |
| MgE− | 10 | 22 |
CSF+ = abnormal CSF analysis; CSF− = normal CSF analysis; MgE+ = presence of meningeal enhancement; MgE− = absence of meningeal enhancement
CSF abnormalities were more statistically significantly detected more often in acute cases (n = 16/37) compared with chronic cases (n = 3/21; Fisher’s test, P = 0.04), while MgE was similar in acute (n = 15/37) and chronic (n = 11/21) cases. Furthermore, none of the chronic cases presented with abnormal CSF without MgE (Table 2). No association was found between the use of anti-inflammatory drugs before MRI and CSF analysis (Table 3), or the presence or absence of a polyp (Table 4) and MgE or CSF abnormalities.
Table 2.
Association between cerebrospinal fluid (CSF) and meningeal enhancement (MgE) findings divided between acute and chronic cases
CSF+ = abnormal CSF analysis; CSF− = normal CSF analysis; MgE+ = presence of meningeal enhancement; MgE− = absence of meningeal enhancement
Fisher’s test
Table 3.
Association between cerebrospinal fluid (CSF) and meningeal enhancement (MgE) findings according to the presence or absence of anti-inflammatory treatment before presentation
| Anti-inflammatory | No anti-inflammatory | P value* | |||
|---|---|---|---|---|---|
| CSF+ | CSF− | CSF+ | CSF− | ||
| MgE+ | 3 | 8 | 6 | 9 | 0.91 |
| MgE− | 4 | 10 | 6 | 12 | |
| P value | 0.5 | ||||
CSF+ = abnormal CSF analysis; CSF− = normal CSF analysis; MgE+ = presence of meningeal enhancement; MgE− = absence of meningeal enhancement
χ2 test
Table 4.
Association between cerebrospinal fluid (CSF) and meningeal enhancement (MgE) findings according to the presence or absence of a polyp
CSF+ = abnormal CSF analysis; CSF− = normal CSF analysis; MgE+ = presence of meningeal enhancement; MgE− = absence of meningeal enhancement
χ2 test
Fisher’s test
Bacterial culture was performed in 45/58 cases. Samples were collected from bulla osteotomy in 13 cases, myringotomy in 28 and the external ear canal in four. Negative bacterial culture was observed in 33/45 cases, of which 13 (39%) received antimicrobial treatment before sampling. The percentages of negative bacterial culture was 70% in acute (21/30) and 73% in chronic (n = 11/15) cases.
Twelve cases showed a positive bacterial culture: six for Staphylococcus species, three for Pasteurella species, one for Streptococcus canis, one for Actinomyces pyogenes and one with both Streptococcus equi subspecies zooepidemicus and Staphylococcus felis. Of these, seven (58%) received antimicrobial treatment before sampling.
The results of the bacterial culture depending on presence/absence of MgE and normal/abnormal CSF findings are summarised in Table 5. No statistical association was found between a positive bacterial culture and MgE (χ2, P = 0.82) or CSF results (χ2 test, P = 0.15). One observation was that if no MgE was seen on MRI and no abnormality was detected on CSF analysis, the likelihood of getting a negative ear sample bacterial culture from myringotomy or bulla osteotomy was only about 12% (n = 2/17). One cat had a positive culture from CSF (Clostridium beijerinkii and Enterococcus faecalis), despite no positive bacterial culture ear sample from myringotomy and clindamycin treatment for 3 days.
Table 5.
Association between meningeal enhancement (MgE) and cerebrospinal fluid (CSF) results and bacterial culture results from ear canal, myringotomy or bulla osteotomy
| MgE+ | MgE− | CSF+ | CSF− | |
|---|---|---|---|---|
| Culture positive | 5 (1 EC + 2M + 2 BO) | 7 (2 EC + 4 M + 1 BO) | 6 (3 EC + 3 M) | 6 (3 M + 3 BO) |
| Culture negative | 15 (10 M + 5 BO) | 18 (1 EC + 12 M + 5 BO) | 9 (7 M + 2 BO) | 24 (1 EC + 15 M + 8 BO) |
| P value* | 0.82 | 0.15 | ||
MgE+ = presence of meningeal enhancement; MgE− = absence of meningeal enhancement; CSF+ = abnormal CSF analysis; CSF− = normal CSF analysis; EC = ear canal; M = myringotomy; BO = bulla osteotomy
χ2 test
Twenty-five cats received corticosteroids and 13 received NSAIDs after diagnosis. The choice of anti-inflammatory drugs according to MgE, CSF or bacteriology findings is summarised in Table 6. No statistically significant difference (MgE: χ2 test, P = 0.53; CSF: Fisher’s test, P = 0.08; bacteriology: Fisher’s test, P = 0.62) was identified, although corticosteroids seem to have been chosen more often in the case of abnormal CSF results (11 cases vs 1 case). Fifty-four cats (93%) received antimicrobial treatment after diagnosis. Twenty-six of 54 (48%) had started antimicrobial treatment prior to referral, while it was started by the referral centre after diagnosis in 28 cases (52%). Thirty-nine received first-line anti-microbials, seven received second-line and eight received both. The duration of antimicrobial treatment depending on MgE, CSF or bacteriology findings are summarised in Table 7. Duration of antimicrobial treatment tended to be longer in the case of positive cultures (5.58 vs 4.22 weeks) or when CSF findings were abnormal (5.83 vs 4.76 weeks), although this difference was not statistically significant (Student’s t-test).
Table 6.
Choice of anti-inflammatory drug depending on the meningeal enhancement (MgE), cerebrospinal fluid (CSF) and bacterial culture (right) results
| MgE+ | MgE− | CSF+ | CSF− | Culture positive | Culture negative | |
|---|---|---|---|---|---|---|
| Corticosteroids | 10 | 15 | 11 | 14 | 7 | 13 |
| NSAIDs | 5 | 8 | 1 | 12 | 2 | 8 |
| None | 11 | 9 | 7 | 13 | 3 | 12 |
| P value | 0.53* | 0.08 † | 0.62 † | |||
MgE+ = presence of meningeal enhancement; MgE− = absence of meningeal enhancement; CSF+ = abnormal CSF analysis; CSF− = normal CSF analysis; NSAIDs = non-steroidal anti-inflammatory drugs
χ2 test
Fisher’s test
Table 7.
Comparison of antibiotic treatment duration (in weeks) based on meningeal enhancement (MgE), cerebrospinal fluid (CSF) or bacterial culture results
| Positive | Negative | P value* | |
|---|---|---|---|
| Culture | 5.58 | 4.22 | 0.27 |
| CSF | 5.83 | 4.76 | 0.17 |
| MgE | 5.33 | 4.90 | 0.80 |
Student’s t-test
The association between the CSF results, MgE and outcome are provided in Table 8. A good outcome was defined as an improvement of clinical signs (and euthanasia unrelated to the disease after several months). A poor outcome was defined by a lack of improvement or euthanasia. Although cases with MgE tended to have a poorer outcome (n = 5/18) than those without MgE (n = 3/26), the difference was not statistically significant (Fisher’s test, P = 0.24). No statistically significant association was found between outcome and CSF abnormalities (Fisher’s test, P = 1). Bulla osteotomy was performed in 14 cases (eight with polyps and six with OMI), between 1 and 78 days after diagnosis (median 5). Delayed bulla osteotomies were due to the absence of improvement or relapse of clinical signs after initial medical management. Improvement of neurological signs after surgical management was seen in 11 cases (length of follow-up varied between 1 and 104 weeks, including five cases with >8 weeks of follow-up), relapse in one (3 months postoperatively) and three cases were lost to follow-up. None of the surgical cases was euthanased for reasons related to OMI. Medical management resulted in improvement in 23/44 cases (duration of follow-up varied between 1 and 78 months, including 12 cases with >8 weeks of follow-up). Two cases improved and were euthanased for unrelated reasons 4 months (carcinoma) and 17 months (polyarthritis) after the diagnosis of OMI, respectively. Three cases did not improve after 1 month, but their owners declined surgery. Four cases were euthanased following diagnosis or several weeks after, with no improvement on medical treatment. One case showed intermittent vestibular signs and was euthanased 20 months after diagnosis owing to seizure-like episodes. Eleven cases were lost to follow-up.
Table 8.
Association between cerebrospinal fluid (CSF) results, meningeal enhancement (MgE) and outcome in 44 cats*
CSF+ = abnormal CSF analysis; CSF− = normal CSF analysis MgE+ = presence of meningeal enhancement; MgE− = absence of meningeal enhancement
Eleven cats were lost to follow-up
Improvement or euthanasia unrelated to the disease
No improvement or euthanasia
Fisher’s test
Discussion
The findings of this study show that in a cohort of 58 cats with PVS diagnosed with OMI, MgE is seen in approximately 50% of cases; however, only 27% of these cases had an abnormal CSF result. There was no association between MgE and abnormal CSF results. Chronic cases had significantly fewer abnormal CSF findings. When a treatment was given, its duration was similar regardless of a positive bacterial culture, abnormal CSF analysis or MgE. Cases with MgE tend to have a poorer outcome.
The use of MRI to investigate the cause of PVS is common practice in clinical neurology, particularly if concurrent meningitis is suspected. Administration of a gadolinium-based contrast medium in MRI has been demonstrated as a more sensitive method to diagnose experimental bacterial meningitis in dogs vs MRI without contrast or postcontrast CT. 23 However, only severe meningeal inflammation at necropsy was correlated with MRI findings, while mild inflammation was not detected in MRI, leading to the conclusion that the absence of meningeal enhancement does not rule out bacterial meningitis. 24 False-positive results of diffuse MgE on MRI without CSF abnormalities have also been described previously. 25 MRI also allows for a better evaluation of fluid-containing inner ear structures than CT, and otitis interna can be assessed on T2W images.16,26 More recently, Keenihan et al and d’Anjou et al compared MR sequences for detecting MgE in dogs.27,28 Postcontrast T1W and T1W fat suppression were found to be the sequences of choice to detect meningeal inflammation. In previous studies in cats with OMI, only one case with focal meningitis resembled our population of cats with MgE, without intra-axial lesions or empyema. This case had an abnormal CSF analysis. 1 In dogs, few studies describe naturally occurring OMI leading to meningeal enhancement in T1W images after gadolinium administration, but no large study focusing on this aspect has been carried out; therefore, its clinical consideration remains unknown.16,29,30
Despite the first hypothesis that MgE is associated with abnormal CSF findings, a discrepancy between MgE and CSF results was found in 47% of cases. Indeed, MgE was seen more frequently in cases with unremarkable CSF analysis (65%), and abnormal CSF can be found without MgE in up to 31% of cases. To the best of our knowledge, no previous report of cats with OMI has evaluated the association between MRI findings and CSF analysis. In previous reports of cats diagnosed with OMI and intra-axial lesions or empyema on MRI, abnormal CSF was detected in 22/25 cases in which CSF analysis was performed.1,7,31 MgE was specifically described in one of these studies, where none of the five cats with chronic vestibular clinical signs had MgE, while all of the six acute or subacute cases did. 7 The prevalence of MgE is in contrast with our results, in which 11/21 chronic cases and 15/35 acute cases showed MgE. When available, abnormal CSF analysis was detected in all acute or subacute cases (5/5) and only in 1/4 chronic cases. 7 These results reflect our findings with significantly fewer CSF abnormalities in chronic cases. Previous treatment with anti-inflammatory drugs or the presence of a polyp did not affect the presence/absence of MgE or CSF results.
The second hypothesis was that there would be an association between MgE and/or CSF abnormalities and a positive bacteriology culture. In the case of absent MgE and normal CSF, the likelihood of a positive bacterial culture from myringotomy or bulla osteotomy was low (~12%). Even if this result is not statistically significant, it raises awareness in the clinician of the possible need to alter antimicrobial treatment after receiving culture results. Bacteriology results between acute or chronic cases did not differ significantly.
Finally, we hypothesised that a positive bacterial culture would influence the choice and duration of antimicrobial and/or anti-inflammatory treatment. Owing to the retrospective and multicentric aspect of our study, medical management was variable, making investigation of the final hypothesis difficult. Generally, long-term (4–8 weeks), broad-spectrum antimicrobial treatment or, ideally, a treatment based on an in vitro antimicrobial sensitivity profile, is recommended to treat OMI in cats and dogs. 8 In this study, the duration of treatment was slightly longer in cases of positive bacterial culture than in cases of negative bacterial growth (5.58 weeks vs 4.22 weeks), although the difference was not statistically significant. The similar duration of treatment may be due to the initial prescription, with no improvement in clinical signs or a lack of short-term follow-up by the referring centre. Also, if anti-inflammatory drugs were to be implemented after abnormal CSF results, clinicians tended to use corticosteroids more frequently. These results could reflect a clinician’s preference for corticosteroids in the case of central nervous system inflammation. However, clinicians need to remember the lack of CSF abnormalities in chronic cases, despite meningeal inflammation.
Culture results from samples taken from the external ear canal have to be interpreted with caution. Common microorganisms can be detected in the tympanic bulla of healthy cats in up to 25% of cases. 32 Bacteria have been previously cultured from 48% of healthy canine external ears. 33 Moreover, up to 67% of myringotomies performed via video-otoscopy might be contaminated, even if microorganisms were detected in only 15.4% of samples. 34 The presence or absence of bacteria on culture should not be considered as critical in formulating a treatment plan as the type of bacteria that are cultured (ie, whether they are likely of external ear canal origin and/or possible iatrogenic contaminants vs a likely cause of middle-ear infection). The lack of a cultured infectious agent in our case series with the presumed presence of OMI raises the question of a purely inflammatory mechanism leading to otitis interna.
The age of the cats, uni- or bilateral vestibular signs of distribution, proportion of OMI with or without polyps, and type of bacteria cultured were similar to previously published literature.1,2,13,35 In this cohort, cases with identified MgE tended to have a poorer prognosis than those without MgE; however, this difference was not statistically significant, and an abnormal CSF result was not associated with any difference in outcome. This finding might help clinicians to anticipate and adapt the treatment for such cases. Surgical treatment with bulla osteotomy was performed in 14 cats (24%), including four in which it was performed several weeks after diagnosis. This is different to other published studies in which none of the cases with vestibular signs underwent surgery, and up to 30% of otitis media cases without neurological signs received bulla osteotomy.1,4 In another study focusing on OMI in cats with intracranial complications, ventral bulla osteotomy was performed more often (12/18 cases [67%]). 31 Surgical management led to an improvement in neurological status in all cases (n = 13/13), while medical management showed an improvement in 23/31 cases (74%). These results are slightly better than those for cats with OMI and intracranial complications. 31
There are several limitations to this study. Firstly, its retrospective nature is associated with incomplete data and did not allow for the long-term follow-up of cases. A multicentre study with different MRI equipment, clinicians and protocols will result in differences in the evaluation of MRI and the clinical management of cases. Concerning recruitment, cases without MRI and/or CSF analysis were excluded, which may have biased the study population towards potentially more severely affected cases. Clinicians may decide against CSF analyses in those cases where clear MgE is seen on MRI, biasing the population towards a higher number of cases without MgE. A similar bias could also have affected the choice of treatment in the cases included in the present study. Moreover, the use of medication prior to presentation might have affected the results. We decided to exclude all cases that presented with intra-axial lesions or empyema on MRI, despite presumed peripheral vestibular lesion localisation as brainstem signs may not be clinically obvious in the neurological examination and might have affected the CSF results.36,37 In dogs, it has been shown that bacterial culture obtained via myringotomy can be contaminated by bacteria from the external ear canal, which could be one limitation to our bacteriology results. 34 The time of acquisition in dogs and higher dose of gadolinium in humans may induce false-negative MgE.28,38 False-positive MgE may also occur. 25
Conclusions
In this study, no association was found between MgE and CSF results. Nearly half of cases (47%) showed a discrepancy between MRI and CSF findings. Additionally, the lack of MgE in MRI does not rule out the presence of a meningitis pathologically. Hence, CSF analysis may be useful to detect the presence of possible concurrent meningitis in cats with OMI. CSF findings and MgE results were not associated with the likelihood of a positive or negative middle-ear bacterial culture. Abnormal CSF results seemed to influence the clinicians’ choice of anti-inflammatory drugs with a preference for glucocorticoids over NSAIDs. Abnormal CSF results were seen less frequently in chronic cases than in acute cases. Additionally, the identification of an abnormal CSF analysis did not seem to influence the duration of antimicrobial treatment, which remains the mainstay for this presumed infectious disease. Outcome tended to be poorer when MgE was detected on MRI.
Supplemental Material
Table of cases.
Footnotes
Accepted: 25 August 2022
Supplementary material: The following file is available online:
Table of cases.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognized high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Guillaume F Dutil  https://orcid.org/0000-0001-6295-5601
https://orcid.org/0000-0001-6295-5601
Nick J Grapes  https://orcid.org/0000-0002-8849-5508
https://orcid.org/0000-0002-8849-5508
Steven De Decker  https://orcid.org/0000-0002-2505-2152
https://orcid.org/0000-0002-2505-2152
Rodrigo Gutierrez-Quintana  https://orcid.org/0000-0002-3570-2542
https://orcid.org/0000-0002-3570-2542
Kiterie Faller  https://orcid.org/0000-0002-4525-7059
https://orcid.org/0000-0002-4525-7059
References
- 1. Negrin A, Cherubini GB, Lamb C, et al. Clinical signs, magnetic resonance imaging findings and outcome in 77 cats with vestibular disease: a retrospective study. J Feline Med Surg 2010; 12: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grapes NJ, Taylor-Brown FE, Volk H, et al. Clinical reasoning in feline vestibular syndrome: which presenting features are the most important? J Feline Med Surg 2021; 23: 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harvey RG, ter Haar G. Ear, nose and throat diseases of the dog and cat. Boca Raton, FL: CRC Press, 2016. [Google Scholar]
- 4. Swales N, Foster A, Barnard N. Retrospective study of the presentation, diagnosis and management of 16 cats with otitis media not due to nasopharyngeal polyp. J Feline Med Surg 2018; 20: 1082–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ackermann AL, Lenz JA, May ER, et al. Mycoplasma infection of the middle ear in three cats. Vet Dermatol 2017; 28: 417–e102. DOI: 10.1111/vde.12437. [DOI] [PubMed] [Google Scholar]
- 6. Henneveld K, Rosychuk RAW, Olea-Popelka FJ, et al. Corynebacterium spp. in dogs and cats with otitis externa and/or media: a retrospective study. J Am Anim Hosp Assoc 2012; 48: 320–326. [DOI] [PubMed] [Google Scholar]
- 7. Sturges BK, Dickinson PJ, Kortz GD, et al. Clinical signs, magnetic resonance imaging features, and outcome after surgical and medical treatment of otogenic intracranial infection in 11 cats and 4 dogs. J Vet Intern Med 2006; 20: 648–656. [DOI] [PubMed] [Google Scholar]
- 8. Gotthelf LN. Diagnosis and treatment of otitis media in dogs and cats. Vet Clin North Am Small Anim Pract 2004; 34: 469–487. [DOI] [PubMed] [Google Scholar]
- 9. Vernau KM, LeCouteur RA. Feline vestibular disorders. Part II: diagnostic approach and differential diagnosis. J Feline Med Surg 1999; 1: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kudnig ST. Nasopharyngeal polyps in cats. Clin Tech Small Anim Pract 2002; 17: 174–177. [DOI] [PubMed] [Google Scholar]
- 11. Cook LB. Neurologic evaluation of the ear. Vet Clin North Am Small Anim Pract 2004; 34: 425–435. [DOI] [PubMed] [Google Scholar]
- 12. Garosi LS, Lowrie ML, Swinbourne NF. Neurological manifestations of ear disease in dogs and cats. Vet Clin North Am Small Anim Pract 2012; 42: 1143–1160. [DOI] [PubMed] [Google Scholar]
- 13. Rossmeisl JH. Vestibular disease in dogs and cats. Vet Clin North Am Small Anim Pract 2010; 40: 81–100. [DOI] [PubMed] [Google Scholar]
- 14. Castillo G, Parmentier T, Monteith G, et al. Inner ear fluid-attenuated inversion recovery MRI signal intensity in dogs with vestibular disease. Vet Radiol Ultrasound 2020; 61: 531–539. [DOI] [PubMed] [Google Scholar]
- 15. Foth S, Meller S, De Decker S, et al. Unilateral decrease in inner ear signal in fluid-attenuated inversion recovery sequences in previously suspected canine idiopathic vestibular syndrome. Vet J 2021; 277: 105748. DOI: 10.1016/j.tvjl.2021.105748. [DOI] [PubMed] [Google Scholar]
- 16. Garosi LS, Dennis R, Penderis J, et al. Results of magnetic resonance imaging in dogs with vestibular disorders: 85 cases (1996–1999). J Am Vet Med Assoc 2001; 218: 385–391. [DOI] [PubMed] [Google Scholar]
- 17. Dvir E, Kirberger RM, Terblanche AG. Magnetic resonance imaging of otitis media in a dog. Vet Radiol Ultrasound 2000; 41: 46–49. [DOI] [PubMed] [Google Scholar]
- 18. Bischoff MG, Kneller SK. Diagnostic imaging of the canine and feline ear. Vet Clin North Am Small Anim Pract 2004; 34: 437–458. [DOI] [PubMed] [Google Scholar]
- 19. Mellema LM, Samii VF, Vernau KM, et al. Meningeal enhancement on magnetic resonance imaging in 15 dogs and 3 cats. Vet Radiol Ultrasound 2002; 43: 10–15. DOI: 10.1111/j.1740-8261.2002.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 20. Salt AN, Hirose K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear Res 2018; 362: 25–37. DOI: 10.1016/j.heares.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bohn AA, Wills TB, West CL, et al. Cerebrospinal fluid analysis and magnetic resonance imaging in the diagnosis of neurologic disease in dogs: a retrospective study. Vet Clin Pathol 2006; 35: 315–320. [DOI] [PubMed] [Google Scholar]
- 22. Cook J, Levine G. Cerebrospinal fluid and central nervous system cytology. In: Diagnostic cytology and hematology of the dog and cat. 4th ed. St Louis, MO: Elsevier, 2014, pp 222–243. [Google Scholar]
- 23. Beco L, Guaguère E, Méndez CL, et al. Suggested guidelines for using systemic antimicrobials in bacterial skin infections: part 2 – antimicrobial choice, treatment regimens and compliance. Vet Rec 2013; 172: 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathews V, Kuharik M, Edwards M, et al. Dyke award. Gd-DTPA-enhanced MR imaging of experimental bacterial meningitis: evaluation and comparison with CT. Am J Roentgenol 1989; 152: 131–136. [DOI] [PubMed] [Google Scholar]
- 25. Ives EJ, Rousset N, Heliczer N, et al. Exclusion of a brain lesion: is intravenous contrast administration required after normal precontrast magnetic resonance imaging? J Vet Intern Med 2014; 28: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benigni L, Lamb C. Diagnostic imaging of ear disease in the dog and cat. In Pract 2006; 28: 122–130. [Google Scholar]
- 27. Keenihan EK, Summers BA, David FH, et al. Canine meningeal disease: associations between magnetic resonance imaging signs and histologic findings. Vet Radiol Ultrasound 2013; 54: 504–515. [DOI] [PubMed] [Google Scholar]
- 28. d’Anjou MA, Carmel ÉN, Blond L, et al. Effect of acquisition time and chemical fat suppression on meningeal enhancement on MR imaging in dogs. Vet Radiol Ultrasound 2012; 53: 11–20. [DOI] [PubMed] [Google Scholar]
- 29. Garosi LS, Dennis R, Schwarz T. Review of diagnostic imaging of ear diseases in the dog and cat. Vet Radiol Ultrasound 2003; 44: 137–146. [DOI] [PubMed] [Google Scholar]
- 30. Orlandi R, Gutierrez-Quintana R, Carletti B, et al. Clinical signs, MRI findings and outcome in dogs with peripheral vestibular disease: a retrospective study. BMC Vet Res 2020; 16: 159. DOI: 10.1186/s12917-020-02366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moore SA, Bentley RT, Carrera-Justiz S, et al. Clinical features and short-term outcome of presumptive intracranial complications associated with otitis media/interna: a multi-centre retrospective study of 19 cats (2009–2017). J Feline Med Surg 2019; 21: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klose TC, MacPhail CM, Schultheiss PC, et al. Prevalence of select infectious agents in inflammatory aural and nasopharyngeal polyps from client-owned cats. J Feline Med Surg 2010; 12: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuda H, Tojo M, Fukui K, et al. The aerobic bacterial flora of the middle and external ears in normal dogs. J Small Anim Pract 1984; 25: 269–274. [Google Scholar]
- 34. Reinbacher E, Kneissl S, Hirt R, et al. Myringotomy in dogs: contamination rate from the external ear canal – a pilot study. Vet Anim Sci 2020; 10: 100125. DOI: 10.1016/j.vas.2020.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosser EJ. Causes of otitis externa. Vet Clin Small Anim Pract 2004; 34: 459–468. [DOI] [PubMed] [Google Scholar]
- 36. Bongartz U, Nessler J, Maiolini A, et al. Vestibular disease in dogs: association between neurological examination, MRI lesion localisation and outcome. J Small Anim Pract 2020; 61: 57–63. [DOI] [PubMed] [Google Scholar]
- 37. Boudreau CE, Dominguez CE, Levine JM, et al. Reliability of interpretation of neurologic examination findings for the localization of vestibular dysfunction in dogs. J Am Vet Med Assoc 2018; 252: 830–838. [DOI] [PubMed] [Google Scholar]
- 38. Runge VM, Wells JW, Williams NM, et al. Detectability of early brain meningitis with magnetic resonance imaging. Invest Radiol 1995; 30: 484–495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table of cases.