Abstract
Objectives
The aim of this study was to investigate the impact of tissue plasminogen activator (TPA) on the treatment of feline aortic thromboembolism (FATE).
Methods
Cats diagnosed with FATE involving ⩾2 limbs were enrolled in a prospective, multicenter, double-blinded, randomized, placebo-controlled study within 6 h of an event. Diagnosis was made by clinical findings and one confirmatory criterion. Cats received placebo or TPA (1 mg/kg/h with the first 10% by bolus). All cats received pain control and thromboprophylaxis. The primary outcome was a change from baseline in a published limb score at 48 h. Secondary outcomes included 48 h survival, survival to discharge and complication proportions. Statistical analyses included pattern-mixture models, logistic regression and Fisher’s exact, Student’s t- and Mann–Whitney–Wilcoxon tests.
Results
Based on a power analysis, 40 cats were enrolled; however, only 20 survived to 48 h (TPA, n = 12; placebo, n = 8 [P = 0.34]). There was a statistically significant improvement in limb scores compared with baseline for both groups (P <0.001). Limb score at 48 h was 1 point lower (better) in the TPA group (P = 0.19). Thrombolysis had no statistically significant effect on 48 h survival (P = 0.22). Lower affected limb lactate was associated with better 48 h survival (odds ratio 1.53, 95% confidence interval 1.08–2.17; P = 0.02). The survival to discharge rates were 45% (TPA) and 30% (placebo; P = 0.51). Complications in the TPA and placebo groups included acute kidney injury (22% and 19%, respectively; P = 1.00) and/or reperfusion injuries (33% and 19%, respectively; P = 0.45).
Conclusions and relevance
Survival and complication rates of acute FATE were not different with or without thrombolysis. High in-hospital mortality decreased the statistical power to detect a statistically significant difference between treatments with regard to our primary outcome.
Keywords: Thrombolysis, thrombosis, alteplase, rTPA, embolism, ischemic neuropathy
Introduction
The clinical syndrome of feline aortic thromboembolism (FATE) is initiated by the sudden migration of a left atrial thrombus into the systemic arteries.1,2 This leads to an acute disorder characterized by signs of ischemia, severe pain, paralysis and eventual rhabdomyolysis in the affected limb(s). The prognosis of FATE is considered poor, with the rate of euthanasia as high as 90% in some reports, often without any attempted treatment.3,4 With treatment, the published survival to discharge rates from retrospective studies are between 27% and 45%.3–7
Thrombolysis using tissue plasminogen activator (TPA) or other thrombolytic agents has been described since as early as the 1960s in FATE, and early treatment is the standard of care in many thromboembolic diseases in people, although rarely attempted in FATE.8–12 Combining 377 cases from the two largest retrospective studies in FATE, only 1% of cases received a thrombolytic (streptokinase in all four cases).3,6 Lack of efficacy and risks of reperfusion injury (RI) are common concerns surrounding thrombolysis in this syndrome.7,13,14 However, a recent multicenter retrospective study that investigated analgesia and thromboprophylaxis without thrombolysis revealed that 50% and 27% of cats treated for FATE will develop RI or acute kidney injury (AKI), respectively. No evidence of a difference in the proportion of cats developing RI or AKI was seen compared with the group that was treated with systemic intravenous (IV) TPA within 6 h of a FATE event. 1 Rapid and non-invasive FATE detection using thermal imaging and the clinical effectiveness of some thromboprophylactic agent(s) have recently been published, making early detection and long-term treatment options more available.15–17
A randomized, international, placebo-controlled prospective study was designed to assess the impact of early (within 6 h of a known event) thrombolysis with TPA on cats suffering from bilateral FATE. We hypothesized that early thrombolysis would be associated with a short-term improvement of motor function vs no thrombolysis. A secondary outcome was to prospectively describe the natural progression of FATE cats hospitalized for treatment of their disease, including 48 h survival and survival to discharge, as well as the identification of prognostic factors for survival.
Materials and methods
The study was conducted at four veterinary practices in two countries, including (in alphabetical order) Angell Animal Medical Center, Boston, MA, USA; North Carolina State University, Raleigh, NC, USA; The Ohio State University, Columbus, OH, USA; and VetAgroSup-Lyon Campus, Lyon, France, between 1 January 2015 and 1 August 2019.
The work described herein involved the use of owned animals and procedures that differed from established internationally recognized high standards of veterinary clinical care for the individual cat due to the randomized nature of the study. The study therefore had prior ethical approval from an established committee at each of the participating locations. Informed written consent was obtained from the owner or legal custodian of all cats described in this study for all procedure(s) undertaken.
To be eligible for enrolment, a cat needed to be able to be treated within 6 h of having a witnessed or known episode of FATE, regardless of the underlying etiology. This was determined if the owner noticed the cat suddenly vocalizing, being acutely down in the hindlimbs or was seen walking normally prior to the aforementioned time frame. The putative time from the onset of FATE to admission was determined based on history. Diagnosis was suspected based on clinical observations of any limb affected by the following five criteria: pale, cold, pulseless, painful and lacking motor function. At least one of the following additional criteria was used to confirm the diagnosis: lack of Doppler flow in the affected limbs, low glucose or high lactate in the affected limb compared with the non-affected limb, or vascular ultrasound.
Exclusion criteria for study entry were an inability to reasonably identify the onset of the FATE event, the involvement of only one limb or the inability to start treatment with TPA or placebo within 6 h of the FATE event. Additionally, cats in moribund condition or not expected to survive for the duration of the 48 h study (eg, due to shock) were not entered into the study. Cats could not be enrolled in the study more than once.
After determination that the cat could be enrolled in the study, the cat was randomized to receive either TPA or saline placebo IV. A dose of 1 mg/kg of TPA (maximum 6 mg) or an equivalent volume of 0.9% NaCl (1 ml/kg; maximum 6 ml) was administered (Alteplase [Cathflo activase]; Genentech). A web-based, centralized electronic randomization center was used (https://vet.osu.edu/tpa-registration). According to a previously published protocol, TPA or a corresponding volume of 0.9% NaCl would be given over 1 h, with 10% of the volume injected as an initial bolus.1,10 Repeated dosing was not allowed. Owners and all personnel (eg, emergency and critical care clinicians, cardiologists and veterinary technicians) involved in case management, including the person administrating the TPA/placebo and people involved in limb scoring, were blinded to group allocation.
Pain control and thromboprophylaxis were standardized across all institutions. Pain management included opiate analgesics such as fentanyl or buprenorphine. Thromboprophylaxis was started after the study drug was administered using a low molecular weight heparin (LMWH) such as enoxaparin at 1 mg/kg SC q8–12h, or an equivalent dosage of an alternative LMWH such as dalteparin. Clopidogrel was administered at 17.5 mg/cat PO q24h within 12 h of admission. Therapy for congestive heart failure (CHF) and other cardiac medications was permitted according to clinician’s preference. Cats alive at 12 h had a predetermined recheck of packed cell volume (PCV), total plasma protein (TP), serum electrolytes and renal function tests.
The primary outcome for this study was the change over time in a previously described limb score based on physical examination findings during a 48 h period (Table 1).1,7 Limb score was calculated immediately prior to TPA or placebo administration and 6, 12, 24 and 48 h thereafter. Improvement of limb function was defined as a decrease in limb scores ⩾3 between the initial and the last limb score performed. Clinical improvement was defined as an improvement of the limb score, or if motor function improved as recorded in the medical record. Secondary outcomes were 48 h survival and survival to discharge. Short-term outcomes were chosen because the short half-life of thrombolytics (ie, 5 mins) would theoretically only impact short-term outcome, and this approach reduced the risks of non-thrombolysis factors influencing outcome. 18 Demographic, physical examination and clinicopathologic variables, complications and other outcomes were recorded.
Table 1.
Limb score based on physical examination1,7
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Limb function score | Pulse present, strong motor activity | Pulse present, weak motor activity | Pulse present, no motor activity |
No pulse, no motor activity |
Score is determined for each limb and added. Minimum would be 4 if all limbs are normal; maximum would be 16 if all four limbs have no motor activity and no pulse
As cats in CHF receiving diuretics would likely see increases in serum creatinine, AKI was defined as a 100% increase of serum creatinine concentration between two time points, as previously described.1,19,20 RI was defined as an increase in serum potassium above the institutional laboratory reference interval without a concurrent rise in blood urea nitrogen and creatinine, as previously described. 1 When a rise in creatinine preceded a rise in potassium, it was classified as AKI. When a rise in potassium preceded the rise in creatinine, it was classified as RI. If both rises were concurrent or too challenging to appropriately reconcile, it was classified as both AKI and RI but analyzed with the AKI group. 1
Sample size calculations were performed for the primary outcome measured (ie, change of limb score) with the assumption that a linear mixed model procedure would be used for data analysis. For a balanced design (1:1 allocation) of 40 total cats, the statistical power to detect a change in limb score of ⩾3 was estimated to be >80%. For the survival to 48 h outcome, 20 cats per treatment group were projected to detect a doubling of survival proportions (80% vs 40%; with a two-tailed statistical power of 76%; and a difference of 80% vs 35% with a two-tailed statistical power of 84.5%). These outcomes were considered clinically relevant by the investigators a priori.
Statistical analyses
The normality of continuous data was tested using the d’Agostino–Pearson test. Normally distributed data are presented with mean ± SD, and non-normally distributed data are presented as median (range). Data are presented as median (range) if one of the groups (ie, TPA or placebo) was non-normally distributed. All analyses were undertaken on an intention-to-treat basis. Differences in nominal data between TPA patients and the placebo group were tested using a χ2 or Fisher’s exact test (if a cell had a number <5) for categorical data. Continuous data were compared using a Student’s t-test or a Mann–Whitney–Wilcoxon test, as appropriate for distribution.
The change in limb score was examined using ANOVA for repeated measures, with Bonferroni correction for cats that completed the 48 h study. To evaluate differences between groups for cats that did not complete the 48 h study, a pattern mixture model was used. This model is an extension of the linear mixed model and better handles missing data where the missingness is related to the value (eg, how worsening limb scores will mean a patient is more likely to die/be euthanized and so not have later observations). 21 The full model consisted of time from onset to administration, presence/absence of CHF, rectal temperature at admission, affected limb lactate value and geographic site of enrollment, along with the interactions between onset to administration time and TPA treatment as the main effect and outcome. Limb score was the dependent variable, treated as numeric. Model selection was performed via Satterthwaite’s method to approximate degrees of freedom.
Factors influencing 24 h survival, 48 h survival and survival to discharge were examined via multiple logistic regression. Rectal temperature, CHF, treatment group (TPA or placebo), geographic site of enrollment, 12 h venous lactate and affected limb lactate were included as independent variables with patient status (alive or dead/euthanized) as the dependent variable. Forward selection was carried out via the Akaike information criteria, with the minimal model being one with only treatment as an independent variable and the number of permitted variables being decided by the number of patients in the smaller of the outcome groups to allow 5–9 patients per variable. Patients with missing values in considered variables were not included in the forward selection procedure due to requirements of constant sample size for model comparisons. On selection of a model, previously excluded patients were reintroduced. Receiver operating characteristic (ROC) curves were calculated for factors shown to statistically influence survival outcomes. Statistical significance was set at P <0.05.
After enrolling the target number of 40 cats, the funding agency requested data analysis in a blinded fashion. The primary author (JG) was provided each patient’s treatment group as A or B, without the knowledge of treatment allocation. Statistical analysis was performed, the recruitment was stopped and the authors were then unblinded to treatment allocation.
Results
Demographics and patient characteristics
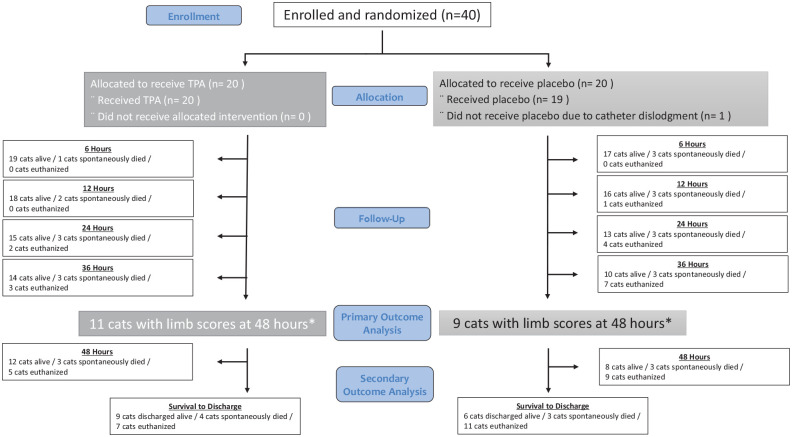
Forty cats were enrolled between January 2015 and July 2019, with 17 enrolled at North Carolina State University, nine at Lyon VetAgroSup, eight at The Ohio State University and six at Angell Animal Medical Center. All analyses were performed on 40 cats as the intention-to-treat group (Figure 1). Thirty-seven cats were in the per-protocol group: one cat in the TPA group had one limb affected and one received TPA with the FATE event happening 6.25 h prior to treatment. One cat in the placebo group never received the drug as the IV catheter became dislodged and the cat died shortly after admission.
Figure 1.
Patient flow diagram for 40 cats diagnosed with feline aortic thromboembolism (FATE) enrolled in a prospective study in a tissue plasminogen activator (TPA) group (20 cats) and saline placebo group (20 cats). *One cat in the TPA group was alive at 48 h but did not get a limb score; one cat in the placebo group had a limb score performed immediately before euthanasia at the 48 h mark
Affected breeds included 29 domestic shorthairs, four domestic mediumhairs, three domestic longhairs and one each of the following breeds: Sphynx, Bengal, Scottish Fold and Maine Coon. Thirty cats were castrated males and 10 cats were female, nine of which were spayed.
There were no significant differences in admission characteristics between TPA and placebo groups (Table 2). Three cats in the TPA group and one cat in the placebo group weighed >6 kg (P = 0.61).
Table 2.
Admission data and feline aortic thromboembolism (FATE) characteristics in 40 cats enrolled in a prospective, multicenter, blinded, randomized, placebo-controlled study within 6 h of an event
| Variable | Tissue plasminogen activator group (n = 20) | Placebo group (n = 20) | P value |
|---|---|---|---|
| Age (years) | 8.5 ± 3.9 | 10.5 ± 3.4 | 0.10 |
| Body weight (kg) | 5.1 ± 1.1 | 5.1 ± 1.1 | 0.87 |
| Rectal temperature (ºF) | 96.4 ± 2.5 | 96.2 ± 2.6 | 0.81 |
| Affected limb lactate (mmol/l) | 10.9 ± 3.3 | 11.6 ± 3.7 | 0.62 |
| Heart rate (beats/min) | 193 ± 27 | 195 ± 37 | 0.95 |
| Respiratory rate (breaths/min) | 60 (32–120) | 60 (30–140) | 0.39 |
| Clinicopathologic variables | |||
| Sodium (mEq/l) | 152.9 ± 5.1 | 152.4 ± 5.1 | 0.76 |
| Potassium (mEq/l) | 3.9 ± 0.6 | 4.0 ± 0.8 | 0.70 |
| Creatinine (mg/dl) | 1.4 (0.9–2.3) | 1.6 (1.0–3.2) | 0.06 |
| Lactate (mmol/l) | 3.6 ± 1.2 | 3.1 ± 0.9 | 0.24 |
| Packed cell volume (%) | 42 ± 9 | 42 ± 8 | 0.89 |
| Total protein (g/dl) | 7.7 (6.4–10.6) | 8.0 (6.4–10.0) | 0.77 |
| FATE characteristics | |||
| Limb score | 10 (6–11) | 10 (6–10) | 0.31 |
| Cardiogenic FATE (%) | 95 | 90 | 1.00 |
| Congestive heart failure (%) | 85 | 60 | 0.15 |
| FATE to admission time (h) | 2.4 (0.3–5.5) | 2.0 (0.25–5.5) | 0.57 |
| Admission to needle time (h) | 1.2 (0.3–3.7) | 1.3 (0.5–4.5) | 0.91 |
| FATE to needle time (h) | 4.0 ± 1.7 | 3.8 ± 1.2 | 0.63 |
FATE was confirmed with one or more methods, including differential glucose measurements (n = 28), differential lactate measurements (n = 26), vascular ultrasound (n = 8) or lack of Doppler flow in affected limbs (n = 7). Median glucose in the affected limb was 100 mg/dl (range 26–396) vs 280 mg/dl (range 112–438) in the normal limb (P <0.0001). Mean lactate in the affected limb was 11.3 ± 3.5 mmol/l vs 3.4 ± 1.2 mmol/l in the normal limb (P <0.0001).
Confirmed or suspected heart disease using point-of-care ultrasound (POCUS) or full echocardiography at admission was present in 95% of the cats in the TPA group and 90% of the cats in the placebo group. One cat in the TPA group had neoplasia as the suspected cause of FATE and another cat had an unknown cause of FATE, due to lack of POCUS or echocardiography. Two cats in the placebo group had neoplasia as the suspected cause of FATE. Diagnosis of CHF by thoracic radiographs at admission was present in 85% of cats in the TPA group and 60% of cats in the placebo group (P = 0.015; Table 2).
Spontaneous echogenic contrast was noted by echocardiography in 25% of cats in the TPA group vs 55% in the placebo group (P = 0.30), and a left atrial or auricular thrombus was present in none of the TPA group vs 22% in the placebo group (P = 0.11).
FATE characteristics
Times from the suspected onset of FATE to admission, from admission to TPA or placebo administration, as well as the time from onset of FATE to TPA or placebo are presented in Table 2.
Treatment
Cats in the TPA group received a median of 5.15 mg (range 3.7–6.0) of TPA, with 10% of the dose given over 1 min IV as a loading dose. Cats in the placebo group received a median volume of 5.10 ml (range 2.3–6.0) NaCl 0.9%, with 10% of the dose given over 1 min IV as a loading dose (P = 0.97).
Cats in the TPA group received supportive care, including analgesia (n = 20), thromboprophylaxis with clopidogrel (n = 20), and enoxaparin (n = 11) or dalteparin (n = 9), as well as treatment for cardiac disease if applicable, including oxygen (n = 10), furosemide (n = 16), pimobendan (n = 8), dobutamine (n = 2), benazepril (n = 1), mirtazapine (n = 1) or diltiazem (n = 1). Cats in the placebo group received supportive care, including analgesia (n = 20), thromboprophylaxis with clopidogrel (n = 19), and dalteparin (n = 14) or enoxaparin (n = 6), as well as other treatments such as oxygen (n = 11), furosemide (n = 11), pimobendan (n = 10), sotalol (n = 2), dobutamine (n = 1), magnesium sulfate (n = 1), IV fluids (n = 1), tranexamic acid (n = 1), alfaxolone (n = 1), maropitant (n = 1) or gabapentin (n = 1).
Primary outcomes
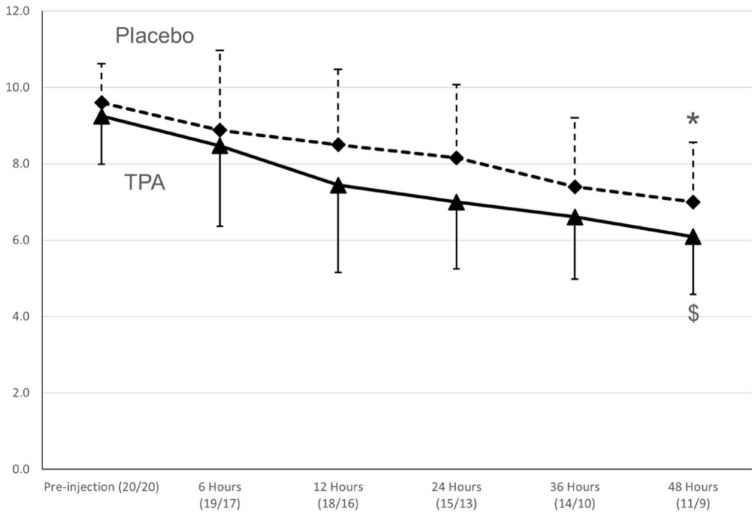
Using ANOVA for repeated measures for cats that completed the 48 h study period, there was a statistically significant improvement in limb score compared with baseline for the TPA group (n = 11; P <0.01) and the placebo group (n = 9; P <0.01), as shown in Figure 2 and Table 3. A decrease in limb score of ⩾3 was found in 35% of the cats in the TPA group vs 20% of the cats in the placebo group, but the difference was not statistically significant (P = 0.48). Clinical improvement in limb function during hospitalization was present in 60% of the TPA group and 40% of the placebo group (P = 0.21). Using a pattern-mixture model, the limb score was approximately 1 point lower in the TPA group than in the placebo group (P = 0.19; Figure 2).
Figure 2.
Mean ± SD (vertical lines) for limb scores over 48 h in 40 cats diagnosed with feline aortic thromboembolism (FATE) enrolled in a prospective study in a tissue plasminogen activator (TPA) group (n = 20) and saline placebo group (n = 20 cats). *$Limb score different over time within the same group (P <0.05). There was no significant difference between the treatments. ▲and straight line = TPA group; ♦ and dashed line = placebo group. The numbers in parenthesis below the various time points represent the number of cats in each group remaining at that time point (TPA/placebo)
Table 3.
Outcome measurements for 40 cats with feline aortic thromboembolism (FATE) enrolled in a prospective, multicenter, blinded, randomized, placebo-controlled study within 6 h of the event
| Variable | TPA group | Placebo group | P value |
|---|---|---|---|
| Limb score improvement ⩾3 points (%) | 25 | 20 | 0.48 |
| Clinical improvement (%) | 60 | 40 | 0.21 |
| 48 h survival (%) | 60 | 40 | 0.21 |
| Survival to discharge (%) | 45 | 30 | 0.33 |
| AKI at 48 h (%) | 22 | 19 | 1.00 |
| RI at 48 h (%) | 33 | 19 | 0.45 |
| AKI over hospitalization (%) | 39 | 31 | 0.73 |
| RI over hospitalization (%) | 33 | 25 | 0.71 |
As not all cats had follow-up bloodwork performed, the denominator may not be 20
TPA = tissue plasminogen activator; AKI = acute kidney injury; RI = reperfusion injury
Secondary outcomes: survival
Twenty cats completed the 48 h study: 11 in the TPA group and nine in the placebo group. The number of surviving cats decreased at each time point over the study period due to natural death or euthanasia (Figure 2). Overall, 5/6 cats that did not survive to 12 h died spontaneously, including the cat with dislodgment of the intravenous catheter. In contrast, 13/14 cats that did not survive between 12 and 48 h were euthanized based on clinician and client decisions. Survival to discharge was 45% in the TPA group vs 30% in the placebo group (P = 0.33). Geographic site of enrollment had varying 48 h survival and survival to discharge proportions (Table 4).
Table 4.
Total number of cats treated with or without tissue plasminogen activator (TPA), and proportions of 48 h survival and survival to discharge for 40 cats with feline aortic thromboembolism (FATE) enrolled in a prospective, multicenter, blinded, randomized, placebo-controlled study by geographic site of enrollment
| Site* | No. of cats
(TPA/placebo) |
48 h survival TPA group (%) | 48 h survival placebo group (%) | Survival to discharge TPA group (%) | Survival to discharge placebo group (%) |
|---|---|---|---|---|---|
| A | 6 (3/3) | 0 | 67 | 0 | 67 |
| B | 8 (5/3) | 100 | 33 | 100 | 33 |
| C | 17 (6/11) | 50 | 27 | 33 | 18 |
| D | 9 (6/3) | 67 | 67 | 33 | 33 |
Sites have been anonymized purposefully
There was no significant treatment effect for 24 h survival (odds ratio [OR] 0.68, 95% confidence interval [CI] 0.14–3.29; P = 0.63), 48 h survival (OR 0.65, 95% CI 0.14–5.97; P = 0.93) or survival to discharge (OR 0.35, 95% CI 0.06–2.21; P = 0.27).
Results from the multiple logistic regression model for 24 h survival containing treatment and rectal temperature showed that cats with higher rectal temperature had higher 24 h survival, with an estimated OR of 1.66 (95% CI 1.14–2.43; P <0.01) per additional ºF. The ROC curve yielded an optimal cut-off that was 96.2°F, with an area under the curve (AUC) of 0.77 (95% CI 0.60–0.94). That cut-off showed a sensitivity of 75% and a specificity of 74%.
From the multiple logistic regression models for 48 h survival and survival to discharge containing treatment and affected limb lactate, cats with higher affected limb lactate at admission were found to have lower 48 h survival and survival to discharge with an estimated OR of 0.65 (95% CI 0.46–0.92; P = 0.02) and 0.70 (95% CI 0.51–0.97; P = 0.03), respectively, per additional mmol/l of lactate. The ROC curve for 48 h survival yielded an optimal cut-off of 11.5 mmol/l, with an AUC of 0.80 (95% CI 0.62–97.81). That cut-off showed a sensitivity of 67% and specificity of 86%. The ROC curve for survival to discharge using the same cut-off of 11.5 mmol/l showed an AUC of 0.77 (95% CI 0.58–0.96). That cut-off showed a sensitivity of 64% and a specificity of 92%.
Secondary outcomes: adverse events
Over the 48 h study period, 22% of cats in the TPA group were diagnosed with AKI vs 19% in the placebo group (P = 1.00). Over the 48 h study period, 33% and 19% of cats in the TPA and placebo groups, respectively, were diagnosed with RI (P = 0.45). Over the entire hospitalization stay, 39% of cats in the TPA group were diagnosed with AKI vs 31% in the placebo group (P = 0.73). Over the entire hospitalization stay, 33% and 25% of cats in the TPA and placebo groups, respectively, were diagnosed with RI (P = 0.71). One cat in the TPA group and three cats in the control group were diagnosed with both AKI and RI (P = 0.22). One cat in the TPA group had self-limiting epistaxis. Survival to discharge of cats with complications such as AKI and/or RI was 46% (TPA) and 33% (placebo; P = 0.674). No seizures were reported in any cats in our study, including in the TPA group.
In-hospital and long-term follow-up
Thirty cats had a 12 h recheck of clinical laboratory tests, with no significant differences in studied variables between groups (Table 5). However, 86.5% of cats experienced an increase in PCV or TP between admission and 12 h after admission, some of these gains were as high as 16 percentage points for PCV and 3.6 g/dl in TP.
Table 5.
Twelve-hour recheck clinicopathologic data for 40 cats with feline aortic thromboembolism enrolled in a prospective, multicenter, blinded, randomized, placebo-controlled study
| TPA | Placebo | P value | |
|---|---|---|---|
| 12 h sodium (mEq/l) | 151.0 ± 5.6 | 148.8 ± 6.7 | 0.30 |
| 12 h potassium (mEq/l) | 4.5 (3.3–11.6) | 4.5 (3.2–10.1) | 0.74 |
| 12 h creatinine (mg/dl) | 1.6 (0.8–5.3) | 1.9 (0.9–6.3) | 0.31 |
| 12 h lactate (mmol/l) | 2.1 (0.6–11.6) | 1.6 (0.2–4.3) | 0.31 |
| 12 h PCV (%) | 43.4 ± 5.9 | 44.6 ± 9.7 | 0.70 |
| 12 h TP (mg/dl) | 9.0 ± 1.4 | 8.4 ± 1.3 | 0.23 |
| Delta PCV (%) | 3.0 ± 4.7 | 2.2 ± 6.3 | 0.73 |
| Delta TP (mg/dl) | 1.2 ± 1.2 | 0.3 ± 1.5 | 0.10 |
Delta is calculated by the formula: 12 h datum minus admission datum
TPA = tissue plasminogen activator; PCV = packed cell volume; TP = total proteins
Follow-up information was obtained for all 20 cats discharged from the hospital. Median follow-up time was 45 days (range 3–649) for the TPA group and 59 days (range 1–156) for the placebo group (P = 0.44). At the time of last follow-up in the TPA group, two cats were alive, five were euthanized and two had died. At the time of last follow-up in the placebo group, one cat was alive, three had died and two were euthanized. One-year survival was 33% and 0% for the TPA and the placebo groups, respectively (P = 0.23).
Autopsy was performed in 12 cats: three in the TPA group and nine in the placebo group. Abnormalities excluding primary diseases (ie, cardiomyopathy or neoplasia) and the aortic embolus included chronic renal infarcts without renal artery thrombosis (n = 8), gastrointestinal thrombosis (n = 3), unilateral artery thrombosis on the right (n = 2) or left renal artery (n = 1), multiple pulmonary thrombi (n = 3), acute renal infarction (n = 2), gastrointestinal hemorrhages (n = 2), left atrial infarction (n = 1) and acute liver, pericardial and abdominal hemorrhage (n = 1). None of the cats with hemorrhages found on necropsy were in the TPA group.
Discussion
This prospective, randomized, placebo-controlled study did not show a statistically significant difference in primary and secondary outcomes between the TPA and the control groups. No differences in the proportion of adverse side effects between the groups was seen. However, with a 50% study mortality rate within the 48 h study period, this 40-cat study became underpowered to detect a positive impact of thrombolysis on limb scores in this patient population. Although in-hospital mortality was anticipated, data from the largest retrospective study available at the time of study design indicated that natural death at 24 h represented 7.2% of the treated cats. 3 Therefore, we anticipated that decisions regarding euthanasia – thus secondary outcomes – would be made at the completion of the 48 h study. Undoubtedly, perceptions of medical progress – or its absence – influenced the decision to euthanize many of these cats prior to the 48 h measurement period. Euthanasia involves multiple decision factors that can include clinician and client perspectives, comorbidities (such as CHF) and financial considerations (although the study covered the cost of the first 48 h of treatment). Many of these cats presented during emergency hours and therefore various primary clinicians provided care, even within the same institution. This situation might not foster uniform veterinary care beyond the trial protocol, but it is realistic in terms of current practices.
Our study focused on cats with ⩾2 limbs affected, which represents 74–80% of cats with the clinical syndrome; these cats may have a worse prognosis and therefore the highest potential for improvement.2,5 Confirmation of FATE was an entry criterion and this was achieved preferentially using paired glucose or lactate measurements. Based on the lack of overlap in lactate between affected and non-affected limbs, the lactate differential might be the confirmatory test of choice. The demographics and admission characteristics were similar to those of previously published studies. Male castrated cats represented 75% of cats – marginally higher than previous studies, with some exceptions.2,4–6,12 Cats in our study had a mean age of 8.5–10.5 years, consistent with previous reports.1,3,5,6 Our study showed that 90–95% of cats suffered from cardiomyopathy, which is higher than the 23–76.4% range reported in previous studies.3,5,6,22 Similarly, >70% of cats presented with confirmed CHF, which is at the high end of the range published in the literature.2,4,5,12 This could have affected the overall survival proportion. 6
As our study focused on early treatment, the median FATE to admission time in our study was <2.5 h. This short time frame indicates that FATE is easily identifiable by clients, and treatment can be initiated quickly in the case of a witnessed event. It is also consistent with the fact that approximately 50% of cats presented within 2 h of a FATE event in a recent study. 3 Recent advances in diagnosis, such as thermal imaging or lactate differential, may speed up the definitive diagnosis, especially in challenging cases. 15
The median admission to needle (door to needle) time for treatment was 1.2 h, but this was, at times, delayed up to 4.5 h, which represents an opportunity for improvement and point-of-care action. In general, it is recommended that thrombolysis be attempted as soon as a thrombus is diagnosed, if appropriate. 23 In people who have suffered an acute ischemic stroke (AIS), patients who were treated with TPA and had door to needle times of >45 mins had significantly higher all-cause mortality, higher all-cause readmission and higher all-cause mortality or readmission compared with those treated within 45 mins.24–26 Current AIS guidelines recommend IV TPA in people for whom treatment can be initiated within 3 h of symptom onset, and downgrade their recommendations to a suggestion (not a recommendation) between 3 and 4.5 h after symptom onset. 27 Rapid cardiac POCUS or N-terminal probrain natriuretic peptide tests might be helpful in shortening the admission to needle time if needed for confirmation of heart disease or the staging of comorbidities.28–30 Our study design included cats enrolled within 6 h of a known event, which differs from previous reports of TPA use in FATE, where TPA-treated cats had a median length of clinical signs associated with thromboembolism of 17 h (range 5–29) or were enrolled within 12 h of the onset of clinical signs.7,31
The TPA regimen tested in our study is similar to that previously reported in cats and people with AIS; 1 mg/kg was given over 1 h with 10% as a bolus without redosing.1,10 This differs from previous reports where TPA was used at 0.25–1 mg/kg/h IV constant rate infusion (CRI) for a total of 1–10 mg/kg or where 1 mg/kg was administered as a front-loaded accelerated protocol or over 4 h, and could be redosed.7,31 The ideal TPA regimen is unknown, and it may depend on thrombus location, comorbidities, risk of bleeding and clinical signs. In infants and children, who are more prone to develop aortic thrombosis or thromboembolism than adults, a CRI regimen of 0.1 mg/kg/h is recommended with close monitoring of clot resolution, with a dose range between 0.1 and 0.5 mg/kg/h. 32 In a study of 12 infants and children with aortic thrombosis, seven had complete clot resolution with that regimen over 2 h to 3 days. 32
The change over time of a previously published limb score was the primary outcome in the present study. We showed that the change in limb score was approximately 1 point lower in the TPA group than in the placebo group, although this difference did not achieve statistical significance. This could be a real lack of therapeutic benefit, or a type II statistical error, as only 20 cats completed the study. As noted previously, power analysis predicted a sample size analysis requiring 40 cats. Limb scores improved over the study period for both the TPA and the placebo groups, which is not currently reported in the literature, where improvement has been described to happen over 5–15 days.6,14 This study showed that improvement was usually noted within 24–48 h of hospitalization in the cats that survived.
Forty-five percent of the TPA treatment group were discharged, similar to the 44% released in a recent retrospective study that compared TPA with treatment without thrombolysis. 1 The 45% discharge proportion for the TPA group is 50% higher than the 30% in the placebo group; however, this difference was not statistically significant. As noted earlier, this could indicate a lack of treatment benefit beyond the conventional therapy of pain control and thromboprophylaxis, or a lack of statistical power. Survival to discharge in the placebo group was 30%, which is similar to the 29% reported in the previously cited retrospective study. 1 This led to an overall 37.5% discharge rate in our study, which should be considered similar to or higher than that reported in the current literature.3,6,7 Survival to discharge for the cats suffering from ⩾2 limbs affected was 35% in a large retrospective study. 6 In a study that investigated the use of TPA within 12 h of FATE, only 20% of cats with ⩾2 limbs affected survived to discharge. 7
AKI, RI, bleeding and seizures have been described in cats receiving TPA.1,7,33 AKI and RI were consequences of treatment at a similar proportion to our study, similar to the 2018 retrospective study. 1 Specifically, AKI was diagnosed in approximately 35% of cats, similar to the 30% in the same retrospective study, and RI was diagnosed in 30% of cats in our study vs 50% in the same retrospective study. It is important to note that approximately 40% of cats that received treatment for their complications survived to discharge.
AKI seems to be the dominating complication while treating FATE, which has been previously documented, although the risks of RI dominated the literature in the 1980s.1,2,7,14 Necropsy results shed some light on the cause of AKI. Traditionally, thrombosis of the renal artery or arteries has been discussed as the major cause of AKI in FATE. 34 However, bilateral renal thrombosis was not seen in any of the necropsied cats in our study, where chronic renal infarction was the most common renal abnormality at necropsy. This information, coupled with the relatively high PCV/TP at admission that worsened, overall, at 12 h, raises the question of pre-renal azotemia, or exacerbation of chronic renal disease, as the main cause of AKI in FATE. Therapeutic actions such as judicious fluid therapy, venodilators such as cilostazol or drugs such as pentoxifylline may require consideration, although their use was beyond the scope of our study and should be studied further.
The median follow-up time after release for the cats in our study was particularly low – around 50 days – although it included cats still alive when lost to follow-up. The median survival time for FATE is reported in retrospective studies to be between 117 and 345 days.6,22,35 In the prospective FATCAT study, the median time to FATE recurrence was 443 days in the clopidogrel group, although cats needed to survive for at least 1 month to be enrolled in that study. 16 The reasons for the discrepancy are unclear, but differences in case selection is possible, as well as the high incidence of CHF in our study.
Despite the multicenter, randomized, placebo-controlled design of this clinical trial, there were study limitations. We elected to analyze our data set using an intention-to-treat principle, as it is the recommended method in superiority trials to avoid any bias compared with the per-protocol analysis.36,37 It is unlikely that a per-protocol analysis would have modified the outcomes in our study. Our study also became underpowered for the primary outcome, as the 48 h survival rate was 50%.
The mortality rate within the first 48 h might be explained by euthanasia bias. It has been shown that up to 90% of FATE patients may be euthanized at admission, without any treatment attempted.3,4 In our study, only six cats (15%) spontaneously died during our study period, including one shortly after admission, and mainly within the first 12 h. The remaining cats that did not survive the 48 h study period were euthanized. The decision for euthanasia could be related to clinician bias over lack of improvement and possible complications, or client perceptions, although many cases treated for their complications survived to discharge. Perhaps this explains why the two recruiting sites with the most experience using TPA for FATE reported a combined 48 h survival rate of 82% for the TPA group and 50% for the placebo group, although this is conjecture. Results from these two hospitals were similar to the projected outcomes used for calculation of sample sizes for the secondary study outcome. This is consistent with the findings of a previous study, which showed a 73% survival rate for cats with FATE treated in the last year of the study vs a 0% survival rate in the early years of the study. 6 Clinician education regarding FATE is important. Although FATE is a devastating disease with a poor long-term prognosis, some cats may live for >1 year when treated, as described in this study and by others. 35
The present study also suffers from survival bias, as cats that survived or were not euthanized were probably cats that improved the most or did not suffer complications, regardless of their group allocation. This is shown by the improvement of the limb score in both groups. However, this underscores the limitations of the published limb score.1,7 A large number of cats in our study regained motor function without regaining obvious femoral pulses, which is consistent with the development of collateral circulation shown in one experimental study. 38 Therefore, cats with strong motor activity but no pulse may have been scored 1, 2, 3 or 4 by the individual scorer, not allowing for the limb score to represent motor function. We do not recommend the use of this limb score in future studies. Survival bias also means that complications were not recorded in the cats that died, which may be particularly important at the 12 h checkpoint, when twice as many cats in the placebo group were not alive vs the TPA group cats. Also, despite the prospective study design, the reader can appreciate the difference in cat numbers for some measured variables. Indeed, of the 12 cats alive in the TPA group at the 48 h mark, only 11 had a limb score available. Similarly, one cat in the placebo group that was euthanized at the 48 h mark had a limb score performed prior to euthanasia.
Our study evaluated a relatively small sample size, and there were apparent differences in outcomes based on geographic site of enrollment. Thus, the generalizability of our results to the larger FATE population can be questioned. Lastly, the TPA regimen used in our study and others are based on recommendations for people suffering from AIS.1,7 Both the benefit (ie, reperfusion of the brain vs reperfusion of pelvic limbs) and the risks (ie, brain bleed vs reperfusion of a large amount of ischemic tissues) are different between AIS and FATE. Regarding timing, we targeted FATE cats able to receive TPA within 6 h of events, but that time frame may need to be more restrictive, as it is in people with AIS. 23 Regarding the regimen, and for practical reasons, the maximum dose was 6 mg. However, three cats in the TPA group and one cat in the placebo group weighed >6 kg and received the maximum dose of 6 mg total. As the veterinary community becomes more comfortable treating cats with FATE, and armed with the knowledge of complication proportions and types, cardiac and thoracic POCUS, infrared thermal imaging and therapeutic options, the TPA regimen can be modified to include only FATE cats thrombolyzed within 3 h of the event, or use of a low-dose CRI with careful monitoring of complications and recanalization, as carried out in infants and children. 32
Conclusions
Although bilateral FATE is a devastating disease, treatment with or without thrombolysis is associated with a 37.5% discharge rate, with a calculated 95% CI of 22.5–52.5%, and with some cats experiencing >1 year survival. Based on the results of the present clinical investigation, the administration of TPA cannot be advanced as a current standard of care. However, the administration of TPA did not appear to worsen the prognosis or cause higher bleeding risks during concomitant thromboprophylaxis. Although it is conceivable that cats with specific characteristics of FATE might benefit from this treatment, such a recommendation would require definitive clinical trial evidence. Large-scale studies should be performed to further investigate the risks and benefits of thrombolysis in FATE, and our study can be used for sample size calculations and to identify appropriate funding sources. Additionally, any future studies of TPA in this disorder should consider alternative dosing regimens, time from event to treatment and the likelihood of survival based on cardiac and non-cardiac comorbidities, as well as other objective clinical findings.
Footnotes
Accepted: 7 October 2022
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was support by a grant from Morris Animal Foundation (First Award Grant D15FE-304)
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals and procedures that differed from established internationally recognized high standards (‘best practice’) of veterinary clinical care for the individual patient. The study therefore had prior ethical approval from an established (or ad hoc) committee as stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Julien Guillaumin  https://orcid.org/0000-0001-8622-4387
https://orcid.org/0000-0001-8622-4387
References
- 1. Guillaumin J, Gibson RM, Goy-Thollot I, et al. Thrombolysis with tissue plasminogen activator (TPA) in feline acute aortic thromboembolism: a retrospective study of 16 cases. J Feline Med Surg 2019; 21: 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pion PD. Feline aortic thromboemboli. Vet Clin North Am Small Anim Pract 1988; 18: 260–262. [PubMed] [Google Scholar]
- 3. Borgeat K, Wright J, Garrod O, et al. Arterial thromboembolism in 250 cats in general practice: 2004–2012. J Vet Intern Med 2014; 28: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schoeman JP. Feline distal aortic thromboembolism: a review of 44 cases (1990–1998). J Feline Med Surg 1999; 1: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laste NJ, Harpster NK. A retrospective study of 100 cases of feline distal aortic thromboembolism: 1977–1993. J Am Anim Hosp Assoc 1995; 31: 492–500. [DOI] [PubMed] [Google Scholar]
- 6. Smith SA, Tobias AH, Jacob KA, et al. Arterial thromboembolism in cats: acute crisis in 127 cases (1992–2001) and long-term management with low-dose aspirin in 24 cases. J Vet Intern Med 2003; 17: 73–83. [DOI] [PubMed] [Google Scholar]
- 7. Welch KM, Rozanski EA, Freeman LM, et al. Prospective evaluation of tissue plasminogen activator in 11 cats with arterial thromboembolism. J Feline Med Surg 2010; 12: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adams HP, Jr, Brott TG, Furlan AJ, et al. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke. Circulation 1996; 94: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 9. Royal College of Physicians. National clinical guideline for stroke. https://www.strokeaudit.org/SupportFiles/Documents/Guidelines/2016-National-Clinical-Guideline-for-Stroke-5t-(1).aspx (2016, accessed 18 October 2022) [Google Scholar]
- 10. IST-3 collaborative group. Effect of thrombolysis with alteplase within 6 h of acute ischaemic stroke on long-term outcomes (the third International Stroke Trial [IST-3]): 18-month follow-up of a randomised controlled trial. Lancet Neurol 2013; 12: 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. DOI: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 12. Abastado M, et al. Thrombolysis by streptokinase (experimental and clinical study). Arch Mal Coeur Vaiss 1969; 62: 700–719. [PubMed] [Google Scholar]
- 13. Killingsworth CR, Eyster GE, Adams T, et al. Streptokinase treatment of cats with experimentally induced aortic thrombosis. Am J Vet Res 1986; 47: 1351–1359. [PubMed] [Google Scholar]
- 14. Pion PD. Feline aortic thromboemboli and the potential utility of thrombolytic therapy with tissue plasminogen activator. Vet Clin North Am Small Anim Pract 1988; 18: 79–86. [DOI] [PubMed] [Google Scholar]
- 15. Pouzot-Nevoret C, Barthelemy A, Goy-Thollot I, et al. Infrared thermography: a rapid and accurate technique to detect feline aortic thromboembolism. J Feline Med Surg 2018; 20: 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hogan DF, Fox PR, Jacob K, et al. Secondary prevention of cardiogenic arterial thromboembolism in the cat: the double-blind, randomized, positive-controlled feline arterial thromboembolism; clopidogrel vs. aspirin trial (FAT CAT). J Vet Cardiol 2015; 17 Suppl 1: S306–S317. [DOI] [PubMed] [Google Scholar]
- 17. Sharp CR, et al. Clinical application of the American College of Veterinary Emergency and Critical Care (ACVECC) consensus on the rational use of antithrombotics in veterinary critical care (CURATIVE) guidelines to small animal cases. J Vet Emerg Crit Care (San Antonio) 2019; 29: 121–131. [DOI] [PubMed] [Google Scholar]
- 18. Zeller FP, Spinler SA. Alteplase: a tissue plasminogen activator for acute myocardial infarction. Drug Intell Clin Pharm 1988; 22: 6–14. [DOI] [PubMed] [Google Scholar]
- 19. Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail 2007; 13: 599–608. [DOI] [PubMed] [Google Scholar]
- 20. Salah K, Kok WE, Eurlings LW, et al. Competing risk of cardiac status and renal function during hospitalization for acute decompensated heart failure. JACC Heart Fail 2015; 3: 751–761. [DOI] [PubMed] [Google Scholar]
- 21. Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods 1997; 2: 64–78. [Google Scholar]
- 22. Moore KE, Morris N, Dhupa N, et al. Retrospective study of streptokinase administration in 46 cats with arterial thromboembolism. J Vet Emerg Crit Care 2000; 10: 245–257. [Google Scholar]
- 23. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2017; 135: e1159–e1195. DOI: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 24. Man S, Xian Y, Holmes DN, et al. Association between thrombolytic door-to-needle time and 1-year mortality and readmission in patients with acute ischemic stroke. JAMA 2020; 323: 2170–2184. DOI: 10.1001/jama.2020.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fonarow GC, Smith EE, Saver JL, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation 2011; 123: 750–758. [DOI] [PubMed] [Google Scholar]
- 26. Bahnasy WS, Ragab OAA, Elhassanien ME. Stroke onset to needle delay: where these golden hours are lost? An Egyptian center experience. eNeurologicalSci 2019; 14: 68–71. DOI: 10.1016/j.ensci.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest physicians evidence-based clinical practice guidelines. Chest 2012; 141: e601S–636S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janson CO, Hezzell MJ, Oyama MA, et al. Focused cardiac ultrasound and point-of-care NT-proBNP assay in the emergency room for differentiation of cardiac and noncardiac causes of respiratory distress in cats. J Vet Emerg Crit Care (San Antonio) 2020; 30: 376–383. [DOI] [PubMed] [Google Scholar]
- 29. Ward JL, Lisciandro GR, Ware WA, et al. Evaluation of point-of-care thoracic ultrasound and NT-proBNP for the diagnosis of congestive heart failure in cats with respiratory distress. J Vet Intern Med 2018; 32: 1530–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loughran KA, Rush JE, Rozanski EA, et al. The use of focused cardiac ultrasound to screen for occult heart disease in asymptomatic cats. J Vet Intern Med 2019; 33: 1892–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pion PD, Kittleson MD, Peterson S, et al. Thrombolysis of aortic thromboembolism in cats using tissue plasminogen activator: clinical data. Proceedings of the American College of Veterinary Internal Medicine Annual Forum; 925; American College of Veterinary Internal Medicine, 1987. [Google Scholar]
- 32. Levy M, Benson LN, Burrows PE, et al. Tissue plasminogen activator for the treatment of thromboembolism in infants and children. J Pediatr 1991; 118: 467–472. [DOI] [PubMed] [Google Scholar]
- 33. Oh Y-I, et al. Comparison between a continuous rate infusion protocol and an accelerated dosing protocol using tissue plasminogen activator for thrombolysis in cats and dogs. Int J Appl Res Vet Med 2018; 16: 149–155. [Google Scholar]
- 34. dvm360. How to handle feline aortic thromboembolism. https://www.dvm360.com/view/how-handle-felineaortic-thromboembolism (2010, accessed 14 December 2021).
- 35. Rush JE, Freeman LM, Fenollosa NK, et al. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc 2002; 220: 202–207. [DOI] [PubMed] [Google Scholar]
- 36. Shah PB. Intention-to-treat and per-protocol analysis. CMAJ 2011; 183: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tripepi G, Chesnaye NC, Dekker FW, et al. Intention to treat and per protocol analysis in clinical trials. Nephrology 2020; 25: 513–517. [DOI] [PubMed] [Google Scholar]
- 38. Butler HC. An investigation into the relationship of an aortic embolus to posterior paralysis in the cat. J Small Anim Pract 1971; 12: 141–158. [DOI] [PubMed] [Google Scholar]