Abstract
We characterized the effects of Leishmania infection on activation-induced translocation of protein kinase C (PKC) isoforms in murine bone marrow-derived macrophages. Although PKC-dependent gene expression was attenuated by infection, the distribution and translocation of PKC isoforms were unaffected. However, subsequent dissociation from membranes was substantially delayed for some isoforms, particularly PKCβ.
Infection of host macrophages by the protozoan Leishmania has multiple effects that might contribute to intracellular survival, including inhibition of both induction of inflammatory mediators (6, 10, 27, 28) and expression of class I and class II major histocompatibility complex genes (25, 26), impairment of the oxidative burst (3, 5, 10, 22), and inhibition of apoptosis (19). Isoforms of protein kinase C (PKC) have been implicated in these pathways (11, 13, 24, 30), and a number of PKC-mediated functions, including chemotaxis (10) and c-fos expression (7), have been observed to be inhibited in Leishmania-infected cells. Recent studies have attributed at least some of these activities to the major surface lipophosphoglycan (LPG) (8–10, 18). A systematic study of the PKC isoforms in murine macrophages that might be the targets of Leishmania or LPG has not been performed.
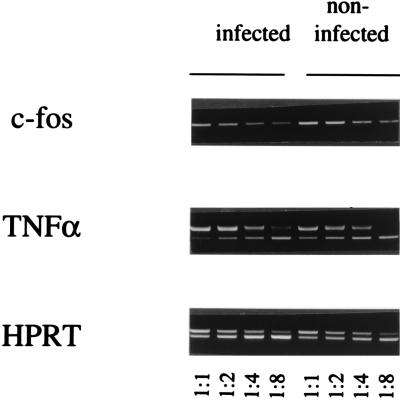
The effects of Leishmania major infection on PKC-dependent induction of c-fos and tumor necrosis factor alpha (TNF-α) gene expression were investigated by using bone marrow-derived macrophages (BMM) prepared from BALB/c mice as described previously (9). BMM monolayers (4 × 106 cells) were left uninfected or were infected with L. major metacyclic promastigotes for 16 h by using 20 parasites/cell. After the monolayers were washed, cellular PKC was activated for 30 min with 100 nM phorbol 12-myristate 12-acetate (PMA). Total RNA was extracted, reverse transcribed, and used as a template for a semiquantitative PCR assay in the presence of twofold serial dilutions of the cytokine polycompetitor plasmid pQRS, as described previously (28). After being standardized to the levels of the constitutively expressed hypoxanthine guanine phosphoribosyltransferase (HPRT) gene, transcripts for c-fos and the TNF-α gene were reduced two- to fourfold in infected cells compared to uninfected cells (Fig. 1). Thus, as with Leishmania donovani (7), L. major inhibits PKC-mediated gene expression in macrophages.
FIG. 1.
Inhibition of c-fos and TNF-α gene expression by L. major. Total RNA from infected or noninfected BMM monolayers stimulated with PMA was used to prepare cDNA for PCR in the presence of twofold serial dilutions of a competitor containing pseudotemplates for the TNF-α gene and the gene for HPRT, which is constitutively expressed. Shown here is an ethidium bromide-stained gel in which upper bands in the HPRT and TNF-α lanes represent amplification of the competitor and lower bands represent amplification from the authentic cDNAs. Expression of c-fos was analyzed with the same standardized cDNA dilutions. Primers for the HPRT and TNF-α genes and PCR conditions were as described previously (28). Primers for c-fos were selected to span introns 2 and 3 as follows: 5′-AGAGCGCAGAGCATCGGCAGA-3′ and 5′-GCTTGGGCTCAGGGTCGTTGA-3′. Neither TNF-α gene nor c-fos expression occurred in BMM that had not been stimulated with PMA.
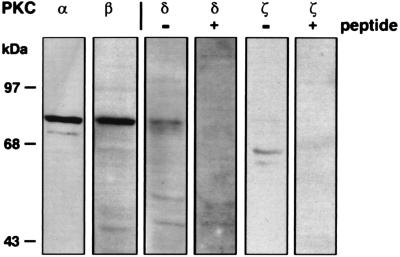
To assess the specific PKC isoforms that might be affected by infection, monolayers were lysed and analyzed for the presence of PKC isoforms by Western blotting after sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (Fig. 2). Two members of the classical PKC isoform family, PKCα and PKCβ, migrated at the appropriate 76-kDa molecular sizes. One of the novel PKCs, PKCδ, and one of the atypical PKCs, PKCζ, were also identified. Antisera against PKCδ recognized a doublet of proteins at 70 and 76-kDa, whereas antisera against PKCζ recognized a doublet of proteins at 60 and 67 kDa. Recognition could be specifically blocked by incubation with the respective peptides against which the antibodies had been produced (Fig. 2). No other PKC isoforms, including the classical PKCγ and the novel members PKCɛ, PKCη, PKCθ, and PKCμ (which is also known as PKD), were revealed with the reagents used (data not shown). Polyclonal antisera against some of the isoforms reacted with L. major-derived proteins after Western blotting of promastigote lysates (2 × 107 cells), although these were readily distinguished from the macrophage proteins (data not shown).
FIG. 2.
PKC isoforms in BMM. Western blotting was performed with the following antibodies: anti-PKCα (clone M6; Upstate Biotechnology Inc., Lake Placid, N.Y.); anti-PKCβ (clone MC-2a; Seikagaku, Rockville, Md.); anti-PKCδ (affinity-purified rabbit polyclonal antisera raised against a synthetic peptide corresponding to amino acids 657 to 673; Santa Cruz Biotechnology, Santa Cruz, Calif.); and anti-PKCζ (affinity-purified rabbit polyclonal antisera raised against a synthetic peptide corresponding to amino acids 480 to 492; Research and Diagnostic Antibodies, Berkeley, Calif.). The detection of PKCδ and PKCζ (−peptide) was blocked by preincubation (2 h, room temperature) of the antisera with a 10-fold excess of the respective peptides (+ peptide). Additional antibodies used in the screening included anti-PKCγ, -ɛ, -η, and -θ (Research and Diagnostic Antibodies), anti-PKCμ (Santa Cruz Biotechnology), and anti-PKCɛ (Gibco BRL Life Technologies, Gaithersburg, Md.), but no corresponding PKC isoforms were detectable. After incubation with horseradish peroxidase-conjugated secondary antibodies, the blots were developed with an ECL kit (Amersham Life Science, Cleveland, Ohio).
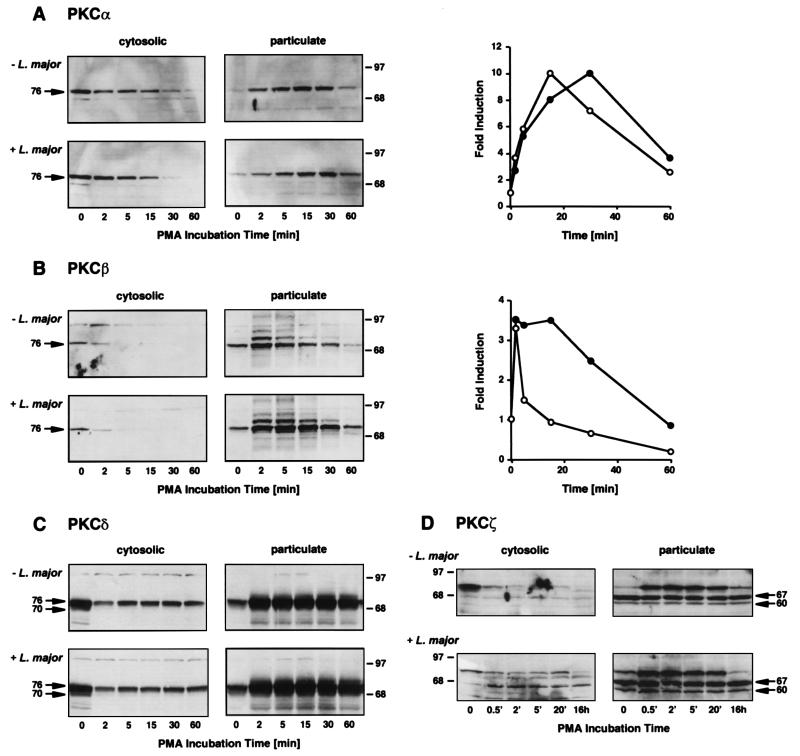
Activation of PKC isoforms is associated with their translocation from soluble to particulate fractions within the cell. The kinetics of translocation were assessed after activation with PMA. Infected or uninfected BMM (1.5 × 106 cells) were stimulated with 100 nM PMA for designated times, scraped into ice-cold homogenization buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 1 mM EGTA, 100 μM phenylmethylsulfonyl fluoride, and 5 μg each of soybean trypsin inhibitor, aprotinin, and leupeptin per ml), and centrifuged at 100,000 × g to create particulate and soluble fractions for analysis by Western blotting and densitometric quantitation (Fig. 3). These data can be summarized as follows. (i) PKCα translocated from the cytosol to the particulate compartment after stimulation, with a peak accumulation by 15 min before subsequent degradation. Similar distribution and translocation kinetics occurred in infected macrophages, although the disappearance of PKCα from the particulate fraction was consistently delayed compared to that in uninfected cells. (ii) PKCβ was comparably distributed between cytosolic and particulate fractions in resting infected and uninfected macrophages and rapidly translocated after stimulation with PMA in both groups of cells. Degradation of PKCβ began sooner after translocation than that of PKCα, and this disappearance from the particulate fraction was even more affected by infection with L. major: even after 30 min, by which time 80% of the particulate-associated enzyme had disappeared in uninfected cells, over 70% of the kinase remained localized to the particulate fraction in infected cells. (iii) PKCδ was predominantly in the cytosolic fraction, translocated rapidly after stimulation, and demonstrated partial recovery in the cytosol after 5 min but was subsequently maintained at a stable level. Infection had little effect on the distribution, translocation, or degradation of PKCδ. (iv) PKCζ was predominantly membrane associated, and its distribution was unaffected by L. major infection. In agreement with prior studies, PKCζ did not translocate in response to PMA (20). None of the isoforms translocated in response to attachment or infection in the absence of PMA as assessed by analyses from 5 min to 4 h after addition of organisms, by which time 90% of the BMM were infected with three to seven parasites.
FIG. 3.
Translocation of PKC isoforms in uninfected and Leishmania-infected macrophages. BMM monolayers were left uninfected (− L. major) or were infected with L. major metacyclic promastigotes (+ L. major) (29) before activation with 100 nM PMA. After the designated times, cytosolic and particulate fractions were prepared and analyzed by Western blotting for PKCα, PKCβ, PKCδ, and PKCζ as described in the legend to Fig. 2. The numbers on the sides of the gels are molecular masses, in kilodaltons. The densities of translocated PKCα (graph in panel A) and PKCβ (graph in panel B) were assessed with ImageQuant version 3.3 (Molecular Dynamics, Sunnyvale, Calif.), and induction was calculated by comparing each density with that of the control in the particulate fraction. Closed circles, L. major-infected BMM; open circles, uninfected BMM.
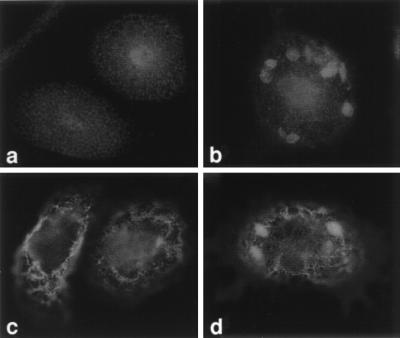
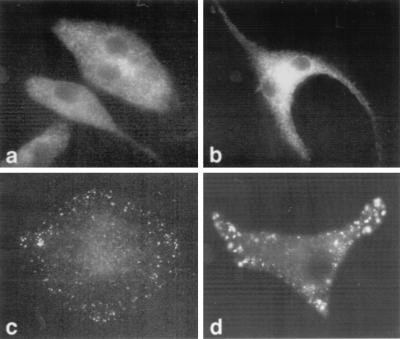
Because individual PKC isoforms translocate to distinct intracellular sites (15, 21), we used immunofluorescence to examine activation-induced trafficking. In resting, uninfected BMM, PKCδ was located predominantly in the cytosol and in the nucleus; infection with L. major did not change this localization (Fig. 4a and b). After PMA activation, PKCδ translocated to circular, filamentous structures located well within the peripheral aspects of the cells and having the appearance of cytoskeletal elements (Fig. 4c). In agreement with the Western blot experiments, translocation of PKCδ was unimpeded in infected cells (Fig. 4d). The presence of the parasites was evident because they could be readily stained with the polyclonal anti-PKCδ antiserum, presumably reflecting cross-reactivity of the antibodies with parasite antigen (Fig. 4d).
FIG. 4.
Localization of PKCδ in macrophages. BMM were differentiated on coverslips and were left uninfected (a and c) or were infected for 16 h with 105 L. major metacyclic promastigotes (b and d) (29). The cells were left untreated (a and b) or were treated with 100 nM PMA for 15 min at 37°C (c and d). The cells were fixed for immunofluorescence microscopy using a pH shift protocol (1), stained with anti-PKCδ antibodies (Research and Diagnostic Antibodies) followed by fluorescein-conjugated goat anti-rabbit immunoglobulin G antibodies (Gibco BRL Life Technologies), and examined with a Zeiss Axioskop microscope by using a 100× oil immersion objective.
PKCβ was localized diffusely in the cytosol in resting, uninfected BMM and was concentrated in areas between the nuclei of dividing cells (Fig. 5a and b). After PMA activation, PKCβ translocated to punctate areas at the ends of the cells (Fig. 5c). Infection affected neither the distribution nor the translocation pattern (Fig. 4d).
FIG. 5.
Localization of PKCβ in macrophages. Uninfected (a and c) or L. major-infected (b and d) BMM were left untreated (a and b) or were treated with 100 nM PMA for 15 min at 37°C (c and d). The cells were prepared for immunofluorescence microscopy with anti-PKCβ antibodies (Seikagaku) and fluorescein-conjugated goat anti-mouse immunoglobulin G antibodies (Gibco BRL Life Technologies) as described in the legend to Fig. 4.
Like PKCδ, PKCζ was present in the cytosol and nucleus in BMM, and its distribution was unaffected by either PMA stimulation, in agreement with the biochemical analysis, or infection with L. major (data not shown). Attempts to immunolocalize PKCα were unsatisfactory with both the monoclonal anti-PKCα antibody used in the Western blots and various polyclonal antisera raised against PKCα-specific peptides.
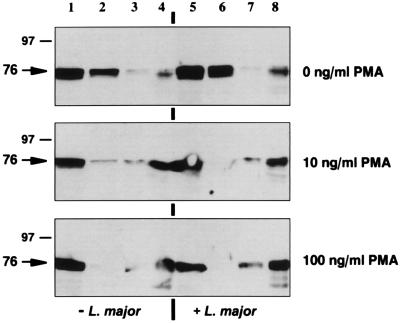
The filamentous structures revealed by translocated PKCδ and the peripheral punctate structures revealed by translocated PKCβ suggested their association with cytoskeletal elements. Most soluble intracellular components, together with the plasma and intracellular organelle membranes, can be extracted with Triton X-100, leaving behind only stable cytoskeletal components (31). Uninfected or infected BMM (2 × 107 cells) were left untreated or were incubated with 10 or 100 ng of PMA per ml for 15 min, lysed in ice-cold homogenization buffer to create whole-cell lysates (crude extracts), and ultracentrifuged (100,000 × g) to create soluble cytosolic extracts. The membrane pellets were resuspended for 60 min at 4°C in homogenization buffer containing 1% (vol/vol) Triton X-100 and then recentrifuged to separate Triton-soluble and -insoluble constituents. The crude extracts, soluble cytosolic extracts, Triton-soluble membranes, and Triton-stable cytoskeleton were analyzed (106 cell equivalents) by Western blotting to localize PKCδ and PKCβ (Fig. 6). Activation with PMA resulted in the dose-dependent translocation of PKC from a predominantly cytosolic compartment to a Triton-insoluble compartment, consistent with movement to a cytoskeletally associated form. Comparable results were obtained with these isoforms, and infection of BMM with L. major did not affect translocation (Fig. 6).
FIG. 6.
PKCδ translocates to Triton-stable cytoskeletal components. Uninfected (−L. major) or infected (+ L. major) BMM were left untreated or were incubated with 10 or 100 ng of PMA per ml for 15 min at 37°C. The cells were lysed to obtain whole-cell lysates (lanes 1 and 5) and cytosolic fractions (lanes 2 and 6) prepared after centrifugation at 100,000 × g as described in the legend to Fig. 3. The membrane pellets were solubilized in homogenization buffer containing 1% (vol/vol) Triton X-100 and then recentrifuged to separate Triton-extractable membranes (lanes 3 and 7) from unstable constituents (lanes 4 and 8). The resultant preparations (106 cell equivalents) were analyzed by Western blotting with anti-PKCδ antibodies (Santa Cruz Biotechnology). The numbers on the left are molecular masses, in kilodaltons.
In summary, we have established the inhibitory effect of L. major on PKC-mediated activation of BMM. As with L. donovani (7), c-fos gene expression was impaired in infected cells after stimulation with PMA. Additionally, TNF-α gene expression, previously shown to be reduced after incubation of infected cells with lipopolysaccharide (6), was linked to impairment of PKC-mediated signalling. Further, we identified four PKC isoforms—PKCα, PKCβ, PKCδ, and PKCζ—in BMM and characterized their translocation after PMA activation in the presence or absence of L. major infection. We could discern no differences in the amounts or distributions of these enzymes after infection, nor did infection affect the initial translocation in the cell. Although the associations of PKCα and PKCβ with membranes occurred with similar kinetics, the subsequent disappearance from the particulate fraction was substantially delayed in infected macrophages compared to that in uninfected macrophages. The ability of LPG to inhibit membrane-associated PKCα in an in vitro assay using unilamellar vesicles may be relevant to this finding (12). LPG was inhibitory even when applied to the opposite side of the monolayer to which PKC was bound, even though the amounts of bound enzyme remained unaffected (12). Prior studies using L. donovani have been conflicting; attenuation of PMA-induced translocation (22), a lack of substantial effects on translocation (9), and attenuated activity despite normal translocation (3) have been found with different assays. However, none of these studies examined specific PKC isoforms. The prolonged association of PKCα and PKCβ found in this study may ensure inhibition of the membrane-associated enzymes. As shown here and elsewhere (16), a number of isoforms of PKC exist in macrophages. These may differ among discrete macrophage populations (11, 16, 24, 32, 33), and infection may differentially affect specific isoforms.
As shown here, PKCβ and PKCδ translocated to compartments associated with the macrophage cytoskeleton. The regulation of membrane-cytoskeleton interactions constitutes an important aspect of PKC signalling (14). The cytoskeletal proteins that are the major targets of PKC isoforms, however, can be cell specific. PKCδ associated with newly formed focal adhesion points in 3T3 fibroblasts (2) and with vimentin in HL60 cells which have differentiated into monocytes/macrophages (23). The punctate structures seen in BMM with activated PKCβ resembled actin dots described as adhesion structures in close contact with the substratum in murine peritoneal macrophages (17). Recently, PKCβ was reported to specifically bind to and be activated by F-actin (4). Additional studies will be needed to correlate PKC-mediated cellular events with individual PKC isoforms and to analyze cytoskeletal proteins as potential enzyme substrates. As shown here, infection with Leishmania affects only a portion of the PKC isoforms present in phagocytic cells, although the functional consequences remain to be determined.
Acknowledgments
We thank members of the laboratory for helpful comments.
This work was supported by NIH grant AI26918.
REFERENCES
- 1.Bacallao R, Stelzer E H K. Preservation of biological specimens for observation in a confocal fluorescence microscope and operational principles of confocal fluorescence microscopy. Methods Cell Biol. 1989;31:437–452. doi: 10.1016/s0091-679x(08)61621-0. [DOI] [PubMed] [Google Scholar]
- 2.Barry S T, Critchley D R. The RhoA-dependent assembly of focal adhesions in Swiss 3T3 cells is associated with increased tyrosine phosphorylation and the recruitment of both pp125FAK and protein kinase C δ to focal adhesions. J Cell Sci. 1994;107:2033–2045. doi: 10.1242/jcs.107.7.2033. [DOI] [PubMed] [Google Scholar]
- 3.Bhunia A K, Sarkar D, Das P K. Leishmania donovani attachment stimulates PKC-mediated oxidative events in bone marrow-derived macrophages. J Eukaryot Microbiol. 1996;43:373–379. doi: 10.1111/j.1550-7408.1996.tb05046.x. [DOI] [PubMed] [Google Scholar]
- 4.Blobe G C, Stribling D S, Fabbro D, Stabel S, Hannun Y A. Protein kinase C βII specifically binds to and is activated by F-actin. J Biol Chem. 1996;271:15823–15830. doi: 10.1074/jbc.271.26.15823. [DOI] [PubMed] [Google Scholar]
- 5.Buchmüller-Rouiller Y, Mauël J. Impairment of the oxidative metabolism of mouse peritoneal macrophages by intracellular Leishmania spp. Infect Immun. 1987;55:587–593. doi: 10.1128/iai.55.3.587-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrera L, Gazzinelli R T, Badolato R, Hieny S, Müller W, Kühn R, Sacks D L. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med. 1996;183:515–526. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Descoteaux A, Matlashewski G. c-fos and tumor necrosis factor gene expression in Leishmania donovani-infected macrophages. Mol Cell Biol. 1989;9:5223–5227. doi: 10.1128/mcb.9.11.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Descoteaux A, Turco S J, Sacks D L, Matlashewski G. Leishmania donovani lipophosphoglycan selectively inhibits signal transduction in macrophages. J Immunol. 1991;146:2747–2753. [PubMed] [Google Scholar]
- 9.Descoteaux A, Matlashewski G, Turco S J. Inhibition of macrophage protein kinase C-mediated protein phosphorylation by Leishmania donovani lipophosphoglycan. J Immunol. 1992;149:3008–3015. [PubMed] [Google Scholar]
- 10.Frankenburg S, Leibovici V, Mansbach N, Turco S J, Rosen G. Effect of glycolipids of Leishmania parasites on human monocyte activity. J Immunol. 1990;145:4284–4289. [PubMed] [Google Scholar]
- 11.Fujihara M, Connolly N, Ito N, Suzuki T. Properties of protein kinase C isoforms (βII, ɛ, and ζ) in a macrophage cell line (J774) and their roles in LPS-induced nitric oxide production. J Immunol. 1994;152:1898–1906. [PubMed] [Google Scholar]
- 12.Giorgione J R, Turco S J, Epand R M. Transbilayer inhibition of protein kinase C by the lipophosphoglycan from Leishmania donovani. Proc Natl Acad Sci USA. 1996;93:11634–11639. doi: 10.1073/pnas.93.21.11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hug H, Sarre T F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaken S. Protein kinase C isozymes and substrates. Curr Opin Cell Biol. 1996;8:168–173. doi: 10.1016/s0955-0674(96)80062-7. [DOI] [PubMed] [Google Scholar]
- 15.Kiley S C, Jaken S, Whelan R, Parker P J. Intracellular targeting of protein kinase C isoenzymes: functional implications. Biochem Soc Trans. 1995;23:601–605. doi: 10.1042/bst0230601. [DOI] [PubMed] [Google Scholar]
- 16.Lake F R, Dempsey E C, Spahn J D, Riches D W H. Involvement of protein kinase C in macrophage activation by poly(I · C) Am J Physiol. 1994;266:C134–C142. doi: 10.1152/ajpcell.1994.266.1.C134. [DOI] [PubMed] [Google Scholar]
- 17.Marchisio P C, Cirillo D, Teti A, Zambonin-Zallone A, Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interaction. Exp Cell Res. 1987;169:202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- 18.McNeely T B, Turco S J. Requirement of lipophosphoglycan for intracellular survival of Leishmania donovani within human monocytes. J Immunol. 1990;144:2745–2750. [PubMed] [Google Scholar]
- 19.Moore K J, Matlashewski G. Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J Immunol. 1994;152:2930–2937. [PubMed] [Google Scholar]
- 20.Nakanishi H, Exton J H. Purification and characterization of the ζ isoform of protein kinase C from bovine kidney. J Biol Chem. 1992;267:16347–16354. [PubMed] [Google Scholar]
- 21.Newton A C. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 22.Olivier M, Brownsey R W, Reiner N E. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc Natl Acad Sci USA. 1992;89:7481–7485. doi: 10.1073/pnas.89.16.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen P J, Johnson G D, Lord J M. Protein kinase C-δ associates with vimentin intermediate filaments in differentiated HL60 cells. Exp Cell Res. 1996;225:366–373. doi: 10.1006/excr.1996.0187. [DOI] [PubMed] [Google Scholar]
- 24.Paul A, Doherty K, Plevin R. Differential regulation by protein kinase C isoforms of nitric oxide synthase induction in RAW 264.7 macrophages and rat aortic smooth muscle cells. Br J Pharmacol. 1997;120:940–946. doi: 10.1038/sj.bjp.0700976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiner N E, Ng W, McMaster W R. Parasite-accessory cell interactions in murine leishmaniasis: Leishmania donovani suppresses macrophage expression of class I and class II major histocompatibility complex gene products. J Immunol. 1987;138:1926–1932. [PubMed] [Google Scholar]
- 26.Reiner N E, Ng W, Ma T, McMaster W R. Kinetics of γ interferon binding and induction of major histocompatibility class II mRNA in Leishmania-infected macrophages. Proc Natl Acad Sci USA. 1988;85:4330–4334. doi: 10.1073/pnas.85.12.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiner N E, Ng W, Wilson C B, McMaster W R, Burchett S K. Modulation of in vitro monocyte cytokine responses to Leishmania donovani. J Clin Invest. 1990;85:1914–1924. doi: 10.1172/JCI114654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiner S L, Zheng S, Corry D B, Locksley R M. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 29.Reiner S L, Zheng S, Wang Z-E, Stowring L, Locksley R M. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawai H, Okazaki T, Takeda Y, Tashima M, Sawada H, Okuma M, Kishi S, Umehara H, Domae N. Ceramide-induced translocation of protein kinase C-δ and -ɛ to the cytosol: implications in apoptosis. J Biol Chem. 1997;272:2452–2458. doi: 10.1074/jbc.272.4.2452. [DOI] [PubMed] [Google Scholar]
- 31.Schliwa M, vanBlerkom J, Porter K R. Stabilization of the cytoplasmic ground substance in detergent-opened cells and a structural and biochemical analysis of its composition. Proc Natl Acad Sci USA. 1981;78:4329–4333. doi: 10.1073/pnas.78.7.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinji H, Akagawa K S, Yoshida T. LPS induces selective translocation of protein kinase C β in LPS-responsive mouse macrophages, but not in LPS-nonresponsive mouse macrophages. J Immunol. 1994;153:5760–5771. [PubMed] [Google Scholar]
- 33.Tachado S D, Gerold P, Schwarz R, Novakovic S, McConville M, Schofield L. Signal transduction in macrophages by glycosylphosphatidylinositols of Plasmodium, Trypanosoma, and Leishmania: activation of protein tyrosine kinases and protein kinase C by inositolglycan and diacylglycerol moieties. Proc Natl Acad Sci USA. 1997;94:4022–4027. doi: 10.1073/pnas.94.8.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]