Abstract
A fatal case of atypical septicemia of pasteurellosis in veal calves is described. The causative organism was identified as a multiresistant Pasteurella multocida capsular type F isolate. The outbreak was characterized by fibrinous peritonitis and mortality, which are hitherto unreported features of P. multocida capsular type F infections.
CASE REPORTS
Male Holstein-Friesian calves (age, 2 weeks) were housed in individual wooden straw-bedded boxes. They were fed a milk replacer diet twice a day. The diet was supplemented with 1.5 g of oxytetracycline (Oxytem; Ecuphar) (80%) and 0.5 g of colistin (polymyxin E; Promycine Pulvis; VMD) (4,800 IU/mg) for the first 5 days. At the time of investigation (16 December 2003), 180 calves 5 to 6 weeks old were present in the herd. The calves had not received any vaccination since the day of arrival at the farm. Three calves had died on the night of 15 December 2003, one of which was subjected to necropsy on the farm and showed extended peritonitis. The remaining two calves were submitted within 8 h to the Department of Pathology, Bacteriology and Poultry Diseases, Faculty of Veterinary Medicine, Ghent University, for necropsy. About 15 calves in the boxes next to those of the dead calves showed nasal discharge and mild diarrhea. Every calf (weight, ca. 50 kg) of the entire herd was then orally treated (methaphylaxis) with 0.8 g of amoxicillin (Dokamox; Emdoka) (80%) twice a day for five consecutive days. The symptoms disappeared within 2 days without relapses or deaths. Routine laboratory investigation consisted of a direct identification test for antigens of rotavirus, coronavirus, Escherichia coli F5, and Cryptosporidium parvum (Digestive enzyme-linked immunosorbent assay kit; Bio-X Diagnostics) in the feces of one calf and detection of bovine viral diarrhea virus antigens by means of real-time PCR (AdiavetBVD Realtime; Adiagène) in pooled blood samples from eight calves of the same herd. All these laboratory tests were negative.
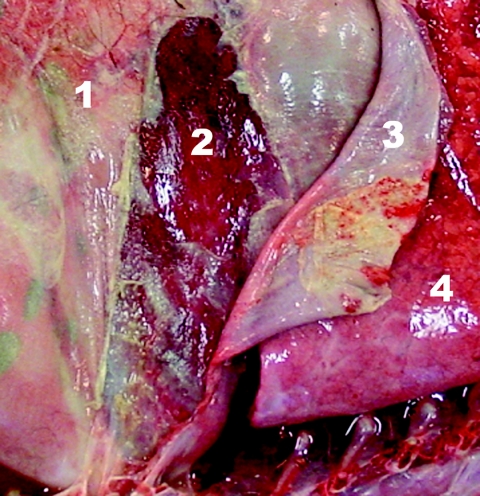
During necropsy of the two calves, samples from the cerebrum, cerebellum, brain stem, lung, mesenteric lymph nodes, synovial fluid of several joints, and omentum major were taken and processed by standard techniques for histological examination. Gross lesions were similar in both calves and consisted of an exudative fibrinous peritonitis (Fig. 1). The synovial fluid of the metacarpal, metatarsal, and elbow joint were hyperemic. The mesenteric lymph nodes were enlarged and mildly hemorrhagic. At histology, the propria of the omentum was edematous and infiltrated by moderate numbers of neutrophils. The mesothelium was covered with fibrin. The synovial fluid samples were hyperemic and edematous. A mild interstitial pneumonia was present in one calf. Lesions were not found in any of the other samples.
FIG. 1.
Opened posterior abdomen, right lateral view. Extended adhesive fibrinopurulent peritonitis can be seen. 1, omentum; 2, liver; 3, diaphragm; 4, right posterior lung.
Samples of lung tissue, peritoneal fluid, and the elbow joint were bacteriologically examined by routine standard techniques for aerobic and anaerobic bacteria as well as Mycoplasma spp. (16). In both calves, mucoid nonhemolytic gram-negative bacteria were isolated as abundant pure cultures from peritoneal fluid. In addition, morphologically similar bacteria were abundantly detected in the lung tissue and elbow joint of one calf. Phenotypic bacteriological analysis (1) of one isolate from each sample revealed that these isolates could be assigned to the species Pasteurella multocida subsp. gallicida. The species identification was confirmed by molecular biology-based techniques, including tRNA gene PCR (1), a P. multocida-specific PCR, and a multiplex PCR for the detection of capsular types (20). The protocol for the capsule multiplex PCR was slightly altered from that described previously (20): bacterial DNA samples were initially denatured at 95°C for 5 min, followed by 30 cycles of denaturation for 1 min at 95°C, annealing at 55°C for 1 min, and extension at 72°C for 1 min, with a final extension at 72°C for 7 min and cooling to 4°C. The tRNA gene PCR and the P. multocida-specific PCR confirmed assignment to the species P. multocida, whereas the capsule PCR confirmed the presence of capsular type F in all isolates (Fig. 2).
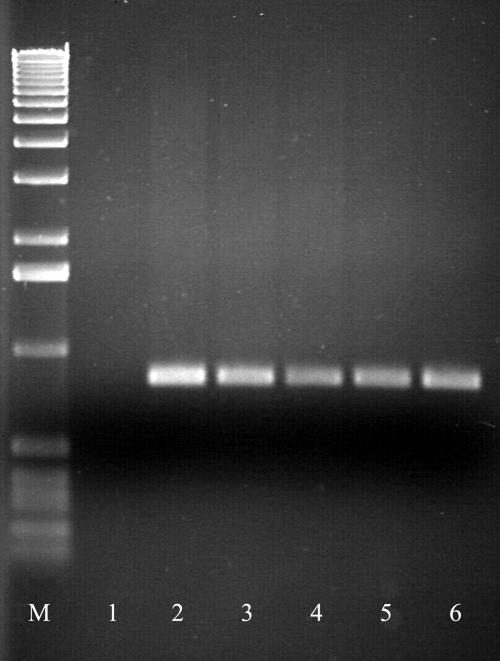
FIG. 2.
Agarose gel electrophoresis of PCR products generated for P. multocida capsular type F. Lane M, molecular size marker (1-kbp DNA ladder; Gibco-BRL-Eggenstein); lane 1, negative control; lane 2, positive control; lane 3, isolate from lung (calf 1); lane 4, isolate from elbow joint (calf 1); lane 5, isolate from abdominal fluid (calf 1); lane 6, isolate from abdominal fluid (calf 2).
In vitro susceptibility testing was performed by the agar dilution method according to NCCLS document M31-A2 (15, 18), and showed that all P. multocida capsular serotype F isolates were susceptible to florfenicol (MICs, 0.25 μg/ml), ampicillin (MICs, 0.25 μg/ml), and ceftiofur (MICs, ≤0.06 μg/ml); intermediately susceptible to enrofloxacin (MICs, 1 μg/ml); and resistant to oxytetracycline (MICs, 64 μg/ml), erythromycin (MICs, 8 μg/ml), tilmicosin (MICs, 32 μg/ml), trimethoprim-sulfamethoxazole (MICs, 6.75/128 μg/ml), gentamicin (MICs, >128 μg/ml), and spectinomycin (MICs, >128 μg/ml). Plasmid analysis revealed that the P. multocida strain of capsular type F carried a single plasmid of 5.2 kb. Electrotransformation into plasmid-free and antibiotic-susceptible P. multocida strain P4000 was conducted as described previously (14); and in vitro susceptibility testing of the corresponding transformants revealed that this plasmid mediated resistance to spectinomycin but not to sulfonamides, trimethoprim, erythromycin, tilmicosin, tetracycline, or gentamicin.
P. multocida is an opportunistic pathogen present on the mucous membranes of many animal species. The bacterium predominantly causes respiratory diseases and septicemia, which have been correlated in different animal species with one of the five recognized capsular serogroups (serogroups A, B, D, E, and F) (17). Evidence is present that host predilection and pathogenesis are linked to certain capsular serogroups (4). Two well-documented bovine syndromes with high rates of morbidity and mortality are associated with P. multocida. P. multocida isolates displaying capsular type A and, to a lesser extent, capsular type D are associated with bovine enzootic bronchopneumonia (BEB) or pneumonic pasteurellosis worldwide, whereas isolates of capsular types B and E are well documented to be associated with hemorrhagic septicemia (HS) in cattle and water buffaloes in tropical regions of predominantly Asia and Africa (12, 16, 17, 19, 21). BEB is frequently encountered in Belgium and surrounding countries (2). It is characterized by depression, fever, loss of appetite, nasal discharge, and respiratory symptoms. The rate of mortality is, in general, low, but concurrent Mannheimia haemolytica infections can result in an increase in the rate of mortality (19). Gross findings are mainly a fibrinopurulent bronchopneumonia and lymphadenitis (22). In contrast, HS is a fatal septicemic disease characterized by fever and sudden death (11, 19). Gross lesions consist of edema in the head region and (less frequently) bleeding from body orifices (19). In the present study, septicemia was observed in the calves and the lesions were different from those observed in BEB and HS.
In septicemic calves, the bacteria predominantly isolated are coliforms, Clostridium perfringens type C, Salmonella spp., streptococci, Mycoplasma spp., and members of the family Pasteurellaceae (3). Pasteurella infections in cattle are recognized to be multifactorial, with the involvement of viruses (12). However, since P. multocida was isolated as a pure culture and lesions indicative of viral infections were not present at the postmortem examinations, it may be assumed that the P. multocida capsular type F isolate was the primary causative agent. The systemic manifestation might have led to endotoxemia, which could then explain the acute fatal course, as can be observed in HS (8, 11).
Molecular biology-based assay confirmation is of utmost importance in pathological studies of members of the family Pasteurellaceae. Wilson et al. (23) demonstrated that conventional serotyping is unreliable for the identification of P. multocida isolates. Therefore, in the present study the identification was based on two different molecular biology-based assays. P. multocida capsular type F isolates are predominantly retrieved from diseased poultry, in particular, turkeys (19). In Europe, this capsular type is the second most prevalent (14%) of those associated with fowl cholera and related diseases (5). Type F isolates have occasionally been reported in ruminants (10, 21), but information on their origin or the pathology involved is still missing. Recent work in the United Kingdom by Davies and colleagues demonstrated that only 1 of 153 bovine P. multocida strains (6) and 2 of 158 porcine P. multocida strains (7) could be assigned to capsular type F. While these two porcine isolates were associated with pneumonia, the single bovine strain was isolated from a calf with severe head and periocular edema that resembled conjunctivitis in poultry. Therefore, and on the basis of the unique genotype of the latter organism and epidemiological considerations (indirect contact with turkeys), an avian origin was attributed to this bovine isolate. Evidence of contact with turkeys was not found in the present study; however, ostriches were also housed on the farm. Virulent P. multocida strains (the capsular types of which were not determined) have been reported in ostriches (9). Therefore, it cannot be ruled out that the bovine P. multocida capsular type F strains were of avian origin. This is further suggested by the subspecies of the P. multocida strain, namely P. multocida subsp. gallicida, which is a typical avian subspecies (16).
Vaccination against P. multocida can be achieved with whole-cell bacterins. However, efficacy is limited and restricted to the homologous serotype (5, 12, 13, 19). If cases of P. multocida capsular type F-associated septicemia further emerge, the presence of serotype F as a virulent contributor should be taken into account during the development of bovine pasteurellosis vaccines. The close relationship between capsular serotypes A and F (20), possibly related to similar immunogenic structures like outer membrane proteins (5), may result in a certain degree of cross-protection between these serotypes.
A sufficiently high curative dose of antimicrobial drugs is recommended in cases of bovine septicemia (3). Concentrations in plasma must be appropriate in order to obtain inhibitory concentrations of a certain antimicrobial drug in the case of septicemia. In agreement with the results of in vitro susceptibility testing, successful therapy in the present study consisted of in-feed medication with amoxicillin. If no acquired resistance is present, good alternatives may be in-feed administration of tetracycline, the 16-ring macrolide tylosin, or the combination of trimethoprim and sulfonamides. Systemic administration of newer molecules like expanded-spectrum cephalosporins (ceftiofur, cefquinome) or florfenicol, a fluorinated derivative of chloramphenicol, is also indicated (2, 12). The multiresistant nature of the P. multocida isolates is worrisome. According to the farmer, the ostriches housed on the same farm had not received any antimicrobial treatment. The multiresistance might therefore be a reflection of the high selection pressure exerted in the Belgian veal calf industry by means of starter rations and in-feed medication. The plausible horizontal transfer of a multiresistance plasmid could, however, not be confirmed by the transformation experiments applied. The locations of the remaining resistance genes, except the one encoding spectinomycin resistance, are therefore likely to be chromosomal.
In conclusion, this is the first report describing a case of septicemia in calves caused by an uncommon multiresistant P. multocida capsular type F isolate.
Acknowledgments
This study was supported by the Institute for the Promotion of Innovation by Science & Technology Flanders (grant IWT/SB/13134). J. Mollet and A. Van de Kerckhove are acknowledged for excellent technical assistance.
REFERENCES
- 1.Catry, B., M. Baele, G. Opsomer, A. de Kruif, A. Decostere, and F. Haesebrouck. 2004. tRNA-intergenic spacer PCR for the identification of Pasteurella and Mannheimia spp. Vet. Microbiol. 98:251-260. [DOI] [PubMed] [Google Scholar]
- 2.Catry, B., J. L. J. Govaere, L. Devriese, H. Laevens, F. Haesebrouck, and A. de Kruif. 2002. Bovine enzootic bronchopneumonia: prevalence of pathogens and their antimicrobial susceptibility. Vlaams Diergen. Tijds. 71:348-354. [Google Scholar]
- 3.Catry, B., G. Opsomer, A. Decostere, B. Feyen, A. de Kruif, and F. Haesebrouck. 2004. Fatal meningitis in a calf caused by Mannheimia varigena. Res. Vet. Sci. 77:187-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung, J. Y., I. Wilkie, J. D. Boyce, K. M. Townsend, A. J. Frost, M. Ghoddusi, and B. Adler. 2001. Role of capsule in the pathogenesis of fowl cholera caused by Pasteurella multocida serogroups A. Infect. Immun. 69:2487-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies, R. L., R. MacCorquodale, and B. Caffrey. 2003. Diversity of avian Pasteurella multocida strains based on capsular PCR typing and variation of the OmpA and OmpH outer membrane proteins. Vet. Microbiol. 91:169-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies, R. L., R. MacCorquodale, and B. Caffrey. 2004. Characterisation of bovine strains of Pasteurella multocida and comparison with isolates of avian, ovine and porcine origin. Vet. Microbiol. 99:145-158. [DOI] [PubMed] [Google Scholar]
- 7.Davies, R. L., R. MacCorquodale, S. Baillie, and B. Caffrey. 2003. Characterization and comparison of Pasteurella multocida strains associated with porcine pneumonia and atrophic rhinitis. J. Med. Microbiol. 52:59-67. [DOI] [PubMed] [Google Scholar]
- 8.De Alwis, M. C. L. 1995. Haemorrhagic septicaemia (Pasteurella multocida serotype B:2 and E:2 infection) in cattle and buffaloes, p. 9-24. In W. Donachie, F. A. Lainson, and J. C. Hodgson (ed.), Haemophilus, Actinobacillus and Pasteurella. Plenum Press, London, United Kingdom.
- 9.Elfaki, M. G., B. Abbas, O. M. Mahmoud, E. M. Haroun, and E. M. Abdel-Magied. 2002. Septicaemic pasteurellosis in ostriches (Struthio camelus) in central Saudi Arabia. Vet. J. 163:218-221. [DOI] [PubMed] [Google Scholar]
- 10.Gautam, R., A. A. Kumar, V. P. Singh, V. P. Singh, T. K. Dutta, and S. B. Shivachandra. 2004. Specific identification of Pasteurella multocida serogroup A isolates by PCR assay. Res. Vet. Sci. 76:179-185. [DOI] [PubMed] [Google Scholar]
- 11.Horadagoda, N. U., J. C. Hodgson, G. M. Moon, T. G. Wijewardana, and P. D. Eckersall. 2001. Role of endotoxin in the pathogenesis of haemorrhagic septicaemia in the buffalo. Microb. Pathog. 30:171-178. [DOI] [PubMed] [Google Scholar]
- 12.Kehrenberg, C., G. Schulze-Tanzil, J. L. Martel, E. Chaslus-Dancla, and S. Schwarz. 2001. Antimicrobial resistance in Pasteurella and Mannheimia: epidemiology and genetic basis. Vet. Res. 32:323-339. [DOI] [PubMed] [Google Scholar]
- 13.Marchart, J., G. Dropmann, S. Lechleitner, T. Schlapp, G. Wanner, M. P. Szostak, and W. Lubitz. 2003. Pasteurella multocida- and Pasteurella haemolytica-ghosts: new vaccine candidates. Vaccine 21:3988-3997. [DOI] [PubMed] [Google Scholar]
- 14.Miranda, C. D., C. Kehrenberg, C. Ulep, S. Schwarz, and M. C. Roberts. 2003. Diversity of tetracycline resistance genes from bacteria isolated from Chilean salmon farms. Antimicrob. Agents Chemother. 47:883-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard, 2nd ed. Document M31-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 16.Quinn, P. J., M. E. Carter, B. K. Markey, and G. R. Carter. 1994. Clinical veterinary microbiology. Mosby-Year Book Europe Limited, London, United Kingdom.
- 17.Rimler, R. B., and K. R. Rhoades. 1989. Pasteurella multocida, p. 37-73. In C. Adlam and J. M. Rutter (ed.), Pasteurella and pasteurellosis. Academic Press Limited, London, United Kingdom.
- 18.Schwarz, S., C. Kehrenberg, S.A. Salmon, and J. L. Watts. 2004. In vitro activities of spectinomycin and comparator agents against Pasteurella multocida and Mannheimia haemolytica from respiratory tract infections of cattle. J. Antimicrob. Chemother. 53:379-382. [DOI] [PubMed] [Google Scholar]
- 19.Shewen, P. E., and J. A. Rice Conlon. 1993. Pasteurella, p. 216-225. In C. L. Gyles and C. O. Thoen (ed.), Pathogenesis of bacterial infections in animals, 2nd ed. Iowa State University Press, Ames.
- 20.Townsend, K. M., J. D. Boyce, J. Y. Chung, A. J. Frost, and B. Adler. 2001. Genetic organization of Pasteurella multocida cap loci and development of a multiplex capsular PCR typing system. J. Clin. Microbiol. 39:924-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Townsend, K. M., A. J. Frost, C. W. Lee, J. M. Papadimitriou, and H. J. Dawkins. 1998. Development of PCR assays for species- and type-specific identification of Pasteurella multocida isolates. J. Clin. Microbiol. 36:1096-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wikse, S. E., and J. C. Baker. 1996. The bronchopneumonias. .In G. P. Smith (ed.), Large animal internal medicine: diseases of horses, cattle, sheep, and goats, 2nd ed. Mosby-Year Book, Inc., St. Louis, Mo.
- 23.Wilson, M. A., M. J. Morgan, and R. E. Barger. 1993. Comparison of DNA fingerprinting and serotyping for identification of avian Pasteurella multocida isolates. J. Clin. Microbiol. 31:255-259. [DOI] [PMC free article] [PubMed] [Google Scholar]