Abstract
Toxic shock syndrome (TSS) is a rare, life-threatening, toxin-mediated infectious process linked, in the vast majority of cases, to toxin-producing strains of Staphylococcus aureus or Streptococcus pyogenes. The pathophysiology, epidemiology, clinical presentation, microbiological features, management and outcome of TSS are described in this review. Bacterial superantigenic exotoxins induces unconventional polyclonal lymphocyte activation, which leads to rapid shock, multiple organ failure syndrome, and death. The main described superantigenic exotoxins are toxic shock syndrome toxin—1 (TSST-1) and enterotoxins for Staphylococcus aureus and Streptococcal pyrogenic exotoxins (SpE) A, B, and C and streptococcal superantigen A (SsA) for Streptococcus pyogenes. Staphylococcal TSS can be menstrual or nonmenstrual. Streptococcal TSS is linked to a severe group A streptococcal infection and, most frequently, to a necrotizing soft tissue infection. Management of TSS is a medical emergency and relies on early detection, immediate resuscitation, source control and eradication of toxin production, bactericidal antibiotic treatment, and protein synthesis inhibiting antibiotic administration. The interest of polyclonal intravenous immunoglobulin G administration as an adjunctive treatment for TSS requires further evaluation. Scientific literature on TSS mainly consists of observational studies, clinical cases, and in vitro data; although more data on TSS are required, additional studies will be difficult to conduct due to the low incidence of the disease.
Keywords: exotoxin, Staphylococcus aureus, Streptococcus pyogenes, toxic shock syndrome toxin 1, staphylococcal enterotoxin, streptococcal pyrogenic exotoxin
1. Introduction
Toxic shock syndrome (TSS) is a rare, life-threatening, toxin-mediated infectious process that leads to rapid and severe shock, multiple organ failure syndrome, and death. Its occurrence is linked to the toxin-producing strains of Staphylococcus aureus or Streptococcus pyogenes (group A streptococcus (GAS)) in a vast majority of cases [1]. However, clinical case reports of TSS involving other bacteria have also been reported [2,3,4,5,6,7,8,9,10].
Scientific literature on TSS mainly consists of observational studies, clinical cases, and in vitro data. The levels of evidence are low, especially when addressing TSS related to pathogens other than S. aureus or S. pyogenes.
2. Methods
This narrative review of the literature was performed using the studies on this topic found in the PubMed database. The keywords used for the manual search were “toxic shock syndrome”, “TSST-1”, “superantigen”, “severe streptococcal infection”, and “necrotizing soft tissue infection”, in the title and in all fields. Original articles, reviews and case reports were all considered. Non-English language articles were excluded.
3. Pathophysiology of Toxic Shock Syndrome
The occurrence of TSS is linked to the bacterial secretion of superantigenic exotoxins, which are bacterial virulence factors genetically encoded and secreted. Superantigenic exotoxins are able to induce unconventional activation of T cells by antigen-presenting cells (APCs).
During conventional T-cell activation, the APC absorbs foreign particles, processes protease digestion, and presents them as partially degraded in a specific binding groove in the major histocompatibility complex class II (MHC II), which is expressed on its surface. The Ag-MHC II complex binds to the surface of the T-cell receptor (TCR). This results in monoclonal activation of T cells specific to the antigen (Ag).
In the TSS, the superantigen binds the TCR and MHC II outside the Ag presentation site with high affinity. This results in nonspecific, polyclonal lymphocyte activation of 5 to 30% of the total population of T cells [11,12,13]. This simultaneous polyclonal activation results in a significant activation of NF kappa B, which plays a major role in the generation and expansion of the inflammatory response [1]. This results in a massive release of proinflammatory cytokines, with clinical signs, such as capillary leakage, arterial hypotension, organ failure, and coagulation activation, usually being reported in this setting [1].
Physiopathological specificities of staphylococcal and streptococcal TSS are detailed in the corresponding subparts.
4. Staphylococcal Toxic Shock Syndrome
4.1. Initial Reports
The first description of this syndrome was published by James Todd and colleagues in The Lancet in 1978 [14]. The authors described a pediatric case series of seven children, with clinical presentations including high fever, cephalalgia, confusion, cutaneous rash, conjunctival hyperhemia, and digestive signs. The children progressed to a state of prolonged severe shock associated with renal and hepatic failure and disseminated intravascular coagulation. Exotoxin-producing S. aureus was isolated from the foci of infection (empyema and abscess) in two patients and in mucosal swabs (nasopharyngeal, vaginal, and tracheal) in four patients but not from blood, cerebrospinal fluid (CSF), or urine. One patient died, while all the others survived and presented with desquamation of the palm of the hands or sole of the feet during recovery [14]. Staphylococcal TSS in adult patients was then described in the 1980s and predominantly involved menstruating women [15].
4.2. Diagnostic Criteria
The diagnostic criteria for staphylococcal TSS were proposed by the Centers for Disease Control and Prevention (CDC) in the 1980s and revised in 2011 [1,16]. These criteria, combining clinical and laboratory aspects, are presented in Table 1.
Table 1.
Diagnostic criteria for staphylococcal and streptococcal TSS according to the CDC recommendations [1,17].
| Staphylococcal TSS | Streptococcal TSS |
|---|---|
| Clinical criteria | |
|
|
| Laboratory criteria | |
Negative results on the following tests:
|
Isolation of group A β-hemolytic streptococci:
|
|
Case classification Probable TSS: a case which meets 4 of the 5 clinical criteria and the laboratory criteria. Confirmed TSS: a case which meets all 5 clinical criteria (including desquamation) and laboratory criteria. |
Case classification Probable TTS: a case which fulfils clinical case definition and isolation of group A β-hemolytic streptococci from a normally nonsterile site in the absence of other etiology for the illness. Definite TSS: a case which fulfils clinical case definition and isolation of group A β-hemolytic streptococci from a normally sterile site. |
TSS: toxic shock syndrome; CSF: cerebrospinal fluid; ARDS: adult respiratory distress syndrome.
However, CDC criteria only allow for a retrospective diagnosis, as they include the desquamation of the palms of the hands and soles of the feet, which occurs 8 to 21 days after the onset of the illness [1,12,17]. Moreover, a French multicentric retrospective study describing 102 cases of staphylococcal menstrual TSS (m-TSS) demonstrated that none of them met the CDC criteria for a confirmed TSS, and only half of them met the criteria for a probable TSS [18].
4.3. Epidemiology of Staphylococcal Toxic Shock Syndrome
Staphylococcal TSS is rare. According to recent studies, the annual incidence of TSS is estimated to be between 0.03 and 0.07/100,000 population [19,20] and seems to be stable. A peak in incidence (13.7/100,000 persons) was observed in the 1980s in the USA [21], linked to the use of highly absorbent tampons, but its incidence decreased after changes in tampon manufacture [22]. These features explain the differentiation in the literature of staphylococcal TSS between menstrual (m-TSS) and nonmenstrual (nm-TSS) syndromes. While m-TSS cases were largely predominant in the 1980s, compared to nm-TSS cases, the proportion of nm-TSS cases gradually increased over time [19,22]. In the UK, the incidence of m-TSS was estimated to be 0.09/100,000 and that of nm-TSS was estimated to be 0.04/100,000 persons [19]. The highest incidence of m-TSS (1.41/100,000 persons) is observed in women aged 13 to 24 years [23].
4.4. Staphylococcal Menstrual TSS
Menstrual toxic shock syndrome (m-TSS), which usually occurs in healthy young menstruating women [23], is linked to vaginal colonization with toxic shock syndrome toxin—1 (TSST-1)-producing S. aureus in women without neutralizing antibodies. An American study on a cohort of 262 women showed that between 2003 and 2005, 22.9% of women were vaginally colonized with S. aureus, and 4.2% were colonized with TSST-1-producing S. aureus during both menstruation and nonmenstruation [24]. The use of tampons creates a physicochemical environment favorable to S. aureus’ growth and production of TSST-1, in particular by providing oxygen in this anaerobic medium [25]. TSST-1 can bind to vaginal epithelial cells and cross the vaginal mucosa [13,25]. A large majority of m-TSS patients have undetectable levels of protective antibodies at the onset of the illness [26]. In m-TSS, blood culture results are negative in all the publications [16]. As it occurs in the absence of any staphylococcal infection, m-TSS is an exclusively toxin-mediated shock.
In a French multicentric retrospective (2005–2020) study reporting 102 cases of m-TSS, the median age was 18 (16–24) years. No previous comorbid condition was reported in 87% of the cases. Clinical presentation included tachycardia (median heart rate 128 (115–140)/min), high fever (median temperature 39.4 (38.5–40.0) °C), skin rash (87% of the cases), and mucosal involvement (50% of the cases). Digestive signs (abdominal pain, diarrhea, and vomiting) and cephalalgia were very common [18]. Vasopressor support was needed in 84% of the cases, 21% of which needed mechanical ventilation [18]. In this study, all patients were using tampons during their period [18]. However, vaginal cups and intrauterine devices have also been reported in staphylococcal m-TSS [27,28,29,30].
4.5. Staphylococcal Nonmenstrual Toxic Shock Syndrome
Nm-TSS can result from any staphylococcal infection with a toxin-producing strain of S. aureus. It is most often postoperative, even after relatively simple procedures, but can occur postpartum, after abortion, or because of nonsurgical cutaneous lesions [22]. All types of surgical procedures can precede postoperative TSS, but plastic, orthopedic, and head and neck surgery are most frequently used [12]. Postoperative TSS occurs after a median delay of 4 days after surgery [12]. Blood cultures are positive for S. aureus in 50% of cases [31]. As nm-TSS is linked to a staphylococcal infection, it is a mixed septic and toxin-mediated shock.
The clinical presentation of nm-TSS is highly comparable to that of m-TSS, although it occurs in significantly older patients (33 (0–84) years vs. 19 (10–47) years, p = 0.008) [31]. A retrospective (2003–2006) multicentric French study compared the clinical presentation of m-TSS (21 cases) and nm-TSS (34 cases). Digestive signs and mucosal involvement were less frequent (74% vs. 100%, p = 0.009, and 42% vs. 76%, p = 0.024, respectively), but neurological involvement was significantly more frequently observed (61 vs. 29%, p = 0.028). No statistically significant difference was observed between m-TSS and nm-TSS in the occurrence of other CDC clinical criteria (fever, rash, desquamation, hypotension, renal, hepatic, and hematologic failure) [31]. Another retrospective (2000–2006) American study compared m-TSS (33 cases) and nm-TSS (28 cases) and reported no difference in clinical presentation [23].
4.6. Microbiological Features
The described staphylococcal superantigenic exotoxins include TSST-1 and enterotoxins (of which approximately thirty have been described to date) [32]. TSST-1 is a 194 amino acid protein encoded by the gene tst and is responsible for 89 to 95% of m-TSSs and 50% of nm-TSSs, the other half being related to the secretion of staphylococcal enterotoxins A, B, and C [23,25,33,34]. M-TSS are nearly all linked to the USA200 clonal group [25].
S. aureus strains are, in a vast majority of cases, methicillin-susceptible strains. In a recent French multicenter retrospective study (2005–2020) describing 102 cases of m-TSS, no case of methicillin-resistant S. aureus (MRSA) was described [18]. The UK national surveillance data on 180 TSS (107 nm-TSS) between 2008 and 2012 identified only 7 (3.8%) nm-TSS cases of MRSA isolates [19]. However, 4 cases (7%) of MRSA isolates were identified in an American study (2000–2006) on 61 staphylococcal TSS—2 of them being suggestive of community-associated MRSA and 1 of them being USA400 MRSA [23]. Case reports of nonmenstrual staphylococcal TSS involving MRSA have also been published [35,36,37].
Nasal colonization with TSST-1-producing S. aureus could be a risk factor for postoperative TSS. S. aureus nasal colonization has been observed in 20 to 80% of the human population [38,39] and identified as a major risk factor for community-acquired and nosocomial infections. A recent prospective multicenter study confirmed that preoperative S. aureus carriage in nose, throat or perineum, was associated with both surgical site infections and bloodstream infections [40]. Unfortunately, this study did not provide any information on TSST-1 production by the S. aureus isolates. A recent study analyzing nasal colonization of 150 healthy volunteers in Kabul showed that 68.4% of the MRSA isolates were TSST-1 producers. TSST-1 production by MSSA isolates was not reported in this study [41]. To our knowledge, nasal colonization with TSST-1-producing S. aureus in patients with staphylococcal TSS have not been specifically assessed. Most of the published studies did not report nasal carriage of S. aureus. Celie et al. have reported positive S. aureus nasal cultures in some cases of postoperative TSS, but these samples were collected in the operating site in all cases [12]. To our knowledge, the incidence of nasal S. aureus colonization in staphylococcal TSS is unknown.
5. Streptococcal Toxic Shock Syndrome
5.1. Initial Reports
In 1987, Cone et al. described, in the New England Journal of Medicine, cases of two patients with severe GAS infection with a clinical presentation similar to staphylococcal TSS. This syndrome was named “streptococcal toxic shock-like syndrome” [42]. Two years later, Stevens et al. reported a case series of 20 patients with severe GAS infection, with clinical presentations including shock, multiorgan system involvement, and rapidly progressive local tissue destruction [43].
5.2. Diagnostic Criteria
The diagnostic criteria from the CDC for streptococcal TSS [1,44] are presented in Table 1. They include clinical signs of severity associated with the presence of GAS in a nonsterile site (throat, vagina, and sputum) or a normally sterile site (CSF, blood, peritoneal fluid, and tissue biopsy) [1].
5.3. Epidemiology of Streptococcal TSS
Streptococcal TSS is rare. Overall, 8 to 22% of patients with severe S. pyogenes infection will develop streptococcal TSS [45,46,47,48]. Approximately 40 to 50% of patients with necrotizing soft tissue infection (NSTI) will develop streptococcal TSS [46,47]. Blood cultures are positive in 60 to 86% of cases [6,49]. Common sources of infection include the vagina, pharyngeal mucosa, skin and soft tissues. Streptococcal TSS can also complicate a minor trauma without skin effraction, pneumonia, intrauterine device, septic arthritis, burn, chickenpox in children, or occur postpartum in young women [46,50]. The source of infection remains unknown in 50% of cases [49].
5.4. Clinical Presentation of Streptococcal Toxic Shock Syndrome
Streptococcal TSS mostly occurs in elderly patients between 50 and 69 years of age and in patients with comorbidities (diabetes, malignancy, hepatic disease, chronic renal impairment, and heart disease) [45,48]. Alcoholism and the use of nonsteroidal anti-inflammatory agents (NSAIDs) have also been suspected to be risk factors for streptococcal TSS [46]. A strong association between the use of NSAIDs and occurrence of necrotizing soft tissue infection has been described [51]: there is a 3-fold increased risk for streptococcal TSS [47]. However, the role of NSAIDs in streptococcal TSS remains debated. An experimental study in a murine model showed that NSAIDs’ administration resulted in a 22-fold increase in the number of GAS in an injured muscle [52]. Administration of NSAIDs could also mask the signs of severity of the infection by attenuating inflammatory signs, and delaying the diagnosis with a negative impact of the prognosis.
Clinical presentation of streptococcal TSS was described in a series of 14 cases in North Yorkshire. Hypotension was described in 100% of cases, acute kidney failure in 93%, liver failure in 57%, and disseminated intravascular coagulation in 64% [6]. Multiorgan failure syndrome was reported in 43% of the cases [6]. In this series, streptococcal TSS was associated with a necrotizing infection in 71% of the cases (predominantly NSTI and myonecrosis) [6].
5.5. Microbiological Features
The disease occurs after penetration of the exotoxin-producing S. pyogenes through a skin or mucous barrier alteration. S.pyogenes then spreads to deep tissues. The main superantigenic exotoxins described in S. pyogenes are streptococcal pyrogenic exotoxins (SpE) A, B, and C and streptococcal superantigen A (SsA). The majority of streptococcal isolates causing TSS are the emm1 (41.1% of the cases), emm3 (8.4% of the cases), emm28 (8.9% of the cases), and emm89 (9.8% of the cases) genotypes [45]. Streptococcal TSS occurs more frequently with GAS strains harboring SpeA or Spec genes (p ≤ 0.001) than those harboring Ssa genes [45]. SpeB participates in the rapid dissemination of S. pyogenes in the skin and soft tissues in combination with other streptococcal virulence factors, such as soluble M protein, which participate in the local and systemic excessive activation of T lymphocytes, APCs and neutrophils.
6. TSS Linked to Other Pathogens
Clinical case reports of TSS involving various bacteria (group B, C and G streptococci, Yersinia pseudotuberculosis, Pseudomonas fluorescens, Mycoplasma arthritidis, Clostridium, and coagulase-negative staphylococci (CNS)) have been reported [2,3,4,5,6,7,8,9,10]. The pathophysiology of these probable TSS is not yet well established. Group B and G streptococci produce pyrogenic toxins that are able to induce lethal endotoxin shock in animals [4,5]. Superantigen production has also been documented for Mycoplasma arthritidis (Mycoplasma arthritidis-derived superantigen) [53] and Yersinia pseudotuberculosis [54]. Previous studies have shown contradictory results regarding the ability of the CNS to produce superantigens [55,56,57,58]. However, stimulation of human monocytes by the killed CNS could induce a dose-dependent production of cytokines responsible for the clinical symptoms [59]. To date, only a few cases of these TSS have been reported in the literature, and additional data are therefore needed.
7. Management of Toxic Shock Syndrome
7.1. Supportive Management
First, it is essential to detect the disease early to start immediate resuscitation [1]. Organ support and symptomatic treatment are similar to any case of septic shock, with hemodynamic optimization (fluid resuscitation and early vasopressor administration), intubation, mechanical ventilation and/or renal replacement therapy being used if needed. There are currently no routine tests to detect the presence of toxins in the blood at the bedside.
7.2. Source Control and Eradication of Toxin Production
It is crucial to eradicate the source of toxin production. In the case of m-TSS, the foreign bodies (tampon, intrauterine device, menstrual cup, etc.) must be removed as soon as possible. A vaginal or cervical sample must be collected to detect S. aureus. In nm-TSS and streptococcal TSS, a surgical intervention must be urgently performed to explore an operating site, collect bacteriological samples in deep tissues, extensively debride necrotic tissues, drain abscesses, etc.
7.3. Bactericidal Antibiotic Treatment
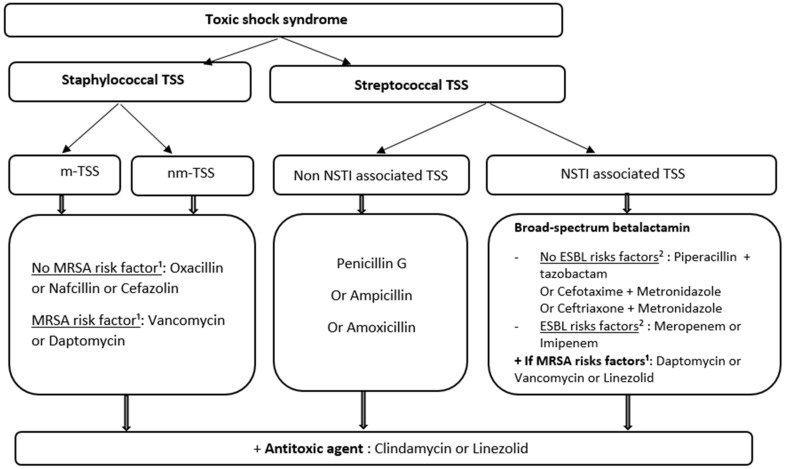
Intravenous bactericidal antibiotics must be administered as soon as possible and within the first hour following suspicion according to the Surviving Sepsis Campaign Guidelines. The steps followed in antimicrobial therapy of TSS are presented in Figure 1.
Figure 1.
Empiric antimicrobial treatment of TSS [1,60]. TSS: toxic shock syndrome; m-TSS: menstrual TSS; nm-TSS: nonmenstrual TSS; NSTI: necrotizing soft tissue infection; MRSA: methicillin-resistant Staphylococcus aureus; ESBL: extended spectrum beta lactamase. 1 High incidence of community-acquired MRSA, known carriage, household contamination, previous antibiotic therapy, recent hospital stay, and recent trip in a zone at risk. 2 Previous antibiotic treatment in the last 6 months, travel in endemic area in the last 6 months, known carriage of ESBL strain, hospital stay in the last 3 months, and long institution stay.
Empirical antibiotic therapy should target Gram-positive cocci. MRSA should be considered in cases of a high incidence of community-acquired MRSA or evocative patient risk factors, including known carriage, household contamination, previous antibiotic therapy, recent hospital stay, and recent trip in a zone at risk.
In the case of NSTI-associated TSS, infection is frequently polymicrobial [61]. Broad spectrum beta-lactam therapy on Gram-positive cocci, Gram-negative bacilli, and anaerobic strains should be administered immediately without waiting for surgical exploration [60,62]. Multidrug-resistant bacteria must be targeted according to patient risk factors or local ecology [63].
De-escalation of antibiotic therapy is needed after receiving antimicrobial susceptibility testing. The duration of antibiotic treatment has not been evaluated by randomized control studies. Recent data suggest that antibiotic treatment could be safely stopped for 48 to 72 h after the final surgical resection in cases of local and systemic favorable evolution [64,65,66]. The optimal antimicrobial treatment duration in staphylococcal TTS is currently unknown. If new anti-Gram-positive antibiotics (ceftaroline and ceftobiprole) are used, antibacterial with antitoxic activity may be associated.
7.4. Adjunctive Therapies
7.4.1. Antitoxic Antibiotics
Until recently, the concept was based on in vitro and experimental data. Protein synthesis-inhibiting antibiotics (clindamycin and linezolid) have in vitro capacities for inhibiting bacterial exotoxin production through the inhibition of the transcription of exotoxin genes.
Clindamycin and linezolid, alone or in combination, have shown a significant inhibitory effect on SpE A production in vitro [67]. Some in vivo observational studies have reported an effect of clindamycin on mortality in severe GAS infections [68,69,70]. Moreover, more than 99% of the emm1 genotype GAS is susceptible to clindamycin [45]. Adjunction of clindamycin for its inhibitory capacities on protein synthesis to antibacterial treatment is recommended in GAS NSTI [63]. Recently, a retrospective single-center quasi-experimental study did not show any difference in the 30-day mortality rate of 274 NSTI patients receiving linezolid versus clindamycin plus vancomycin in association with the standard Gram-negative and anaerobic antibiotic therapy [71]. All of the studies are observational retrospective studies, and recommendations are based on low levels of evidence.
Staphylococcal sensitivity to clindamycin is variable worldwide, and clindamycin-resistant strains are frequent in some geographical areas [72]. In a case report of staphylococcal TSS, an effect of linezolid administration on TSST-1 production was also mentioned [73].
The optimal duration of antitoxic antibiotics is unknown.
7.4.2. Intravenous Immunoglobulin
In vitro, polyspecific intravenous immunoglobulins G (IVIG) have shown the ability to inhibit the superantigenic activity of streptococcal and staphylococcal exotoxins [74,75]. In vivo, some retrospective observational studies have reported an effect of polyspecific IVIG on mortality during TSS [69]. A European multicentric randomized study investigated the effect of polyspecific IVIG in streptococcal TSS [76]. Unfortunately, this study was stopped prematurely because of insufficient inclusions. A difference in mortality rates was observed (10% in the IVIG group versus 36% in the non-IVIGIV group) but did not reach statistical significance. A significant effect on SOFA score at days 2 and 3 was observed [76]. Another randomized placebo-controlled study evaluated the effect of IVIG in NSTI and did not demonstrate any effect on mortality [77]. More recently, a few meta-analyses were performed, some of them describing an effect of IVIG administration on mortality in streptococcal TSS [78,79], and some of them reporting no effect [80]. In conclusion, the interest in polyclonal IVIG administration as an adjunctive treatment for TSS requires further evaluation. Moreover, possible differences in the efficacy of polyspecific and IgG-based immunoglobulin preparations could be hypothesized but have not been studied so far.
8. Outcome of Staphylococcal and Streptococcal TSS
The mortality rate of staphylococcal TSS is estimated to be approximately 5% [19]. Mortality is rare after m-TSS. Contou et al. did not report any deaths in their 102 case series [18]. Other studies reported mortality rates between 0% and 5.7% in m-TSS [19,23]. The mortality rate seems to be significantly higher in nm-TSS, which affects an older and more comorbid population. According to the different studies, it is estimated to be between 4% and 22% [19,23,31]. In a recent review of 96 postoperative TSS (6 streptococcal TSS and the rest staphylococcal TSS), the mortality rate was 9.4%, and 24% of the patients suffered from permanent complications, including additional procedures, amputations, reduced range of motion in a joint, or death [12]. The median duration of hospitalization seems to be significantly higher in nm-TSS (11 (2–50) days versus 5 (2–25) days, p < 0.001 in m-TSS) [23].
The mortality rate of streptococcal TSS is high and estimated to be between 14 and 64% in different published series [6,45,47,81]. The lowest mortality rate (<1%) is observed in postpartum streptococcal TSS [45]. An American study describing 3974 cases of invasive GAS infections reported that streptococcal TSS during invasive GAS infection was an independent risk factor for mortality (OR 12.7 (7.5–21.3)) [82]. This observation was confirmed by another European study [47].
9. Perspectives on TSS
Two probiotics (Lactobacillus acidophilus and Lacticaseibacillus rhamnosus) could interfere with S. aureus growth and the production of TSST-1 and reduce the incidence of mucosa-associated TSS [83]. In addition, a recombinant TSST-1 variant vaccine demonstrated safety, good tolerance, and immunogenicity in a phase 1 study on 46 healthy adult volunteers [84]. Although interesting, these preliminary data demonstrate the need for extended trials.
10. Conclusions
Although TSS is an uncommon pathology, the severity of clinical symptoms, its urgent character and specificities in its management make it essential for clinicians to have good knowledge about it. The pathophysiology, clinical presentation and management of this disease have been widely described. However, most of the data in the scientific literature are provided by retrospective or in vitro studies with a low level of evidence. Further investigations are needed, including evaluation of the role of microbiota and environment in the development of TSS, and therapeutic perspectives assessing the effects of antitoxic antibiotics and their optimal duration, interest of polyclonal IVIG administration, or possible vaccination. However, prospective studies are made difficult by the low incidence of the disease.
Abbreviations
| Ag | antigen |
| APC | antigen-presenting cell |
| CDC | Centers for Disease Control |
| CNS | coagulase-negative staphylococci |
| CSF | cerebrospinal fluid |
| GAS | group A Streptococcus |
| IVIG | intravenous immunoglobulin G |
| nm-TSS | nonmenstrual toxic shock syndrome |
| NSTI | necrotizing soft tissue infection |
| MHC II | class II major histocompatibility complex |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MSSA | methicillin-sensitive Staphylococcus aureus |
| m-TSS | menstrual toxic shock syndrome |
| SsA | streptococcal super antigen |
| SpE | streptococcal pyrogenic exotoxin |
| TCR | T-cell receptor |
| TSS | toxic shock syndrome |
| TSST-1 | toxic shock syndrome 1 |
Author Contributions
E.A.: writing—original draft preparation; C.D.T., N.G., S.T. and P.M.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
P.M. received consultation fees, payment or honoraria for presentations or educational events from Viatris, Menarini, Pfizer, Univero, Mundipharma, MSD. E.A., C.D.T., N.G. and S.T. have no conflict of interest to declare.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lappin E., Ferguson A.J. Gram-positive toxic shock syndromes. Lancet Infect. Dis. 2009;9:281–290. doi: 10.1016/S1473-3099(09)70066-0. [DOI] [PubMed] [Google Scholar]
- 2.Armeftis C., Ioannou A., Lazarou T., Giannopoulos A., Dimitriadou E., Makrides K., Pana Z.D. Staphylococcus epidermidis induced toxic shock syndrome (TSS) secondary to influenza infection. BMC Infect. Dis. 2023;23:583. doi: 10.1186/s12879-023-08487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assimacopoulos A.P., Stoehr J.A., Schlievert P.M. Mitogenic factors from group G streptococci associated with scarlet fever and streptococcal toxic shock syndrome. Adv. Exp. Med. Biol. 1997;418:109–114. doi: 10.1007/978-1-4899-1825-3_27. [DOI] [PubMed] [Google Scholar]
- 4.Schlievert P.M., Gocke J.E., Deringer J.R. Group B streptococcal toxic shock-like syndrome: Report of a case and purification of an associated pyrogenic toxin. Clin. Infect. Dis. 1993;17:26–31. doi: 10.1093/clinids/17.1.26. [DOI] [PubMed] [Google Scholar]
- 5.Wagner J.G., Schlievert P.M., Assimacopoulos A.P., Stoehr J.A., Carson P.J., Komadina K. Acute group G streptococcal myositis associated with streptococcal toxic shock syndrome: Case report and review. Clin. Infect. Dis. 1996;23:1159–1161. doi: 10.1093/clinids/23.5.1159. [DOI] [PubMed] [Google Scholar]
- 6.Barnham M., Weightman N., Anderson A., Tanna A. Streptococcal toxic shock syndrome: A description of 14 cases from North Yorkshire, UK. Clin. Microbiol. Infect. 2002;8:174–181. doi: 10.1046/j.1469-0691.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- 7.Pomputius W.F., Kilgore S.H., Schlievert P.M. Probable enterotoxin-associated toxic shock syndrome caused by Staphylococcus epidermidis. BMC Pediatr. 2023;23:108. doi: 10.1186/s12887-023-03914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goda K., Kenzaka T., Hoshijima M., Yachie A., Akita H. Toxic shock syndrome with a cytokine storm caused by Staphylococcus simulans: A case report. BMC Infect. Dis. 2021;21:19. doi: 10.1186/s12879-020-05731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pence S., Joshi R., Shweikeh F., Mouchli M., Shrestha K. Clostridium sordellii: A Cause of Toxic Shock Syndrome after a Breach in the GI Tract. Cureus. 2023;15:e44604. doi: 10.7759/cureus.44604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covin B.D., Chapa H., Pham N. Clostridium perfringens of unclear origin causing pelvic inflammatory disease and toxic shock syndrome in a previously healthy young woman. BMJ Case Rep. 2021;14:e242492. doi: 10.1136/bcr-2021-242492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grumann D., Nübel U., Bröker B.M. Staphylococcus aureus toxins—Their functions and genetics. Infect. Genet. Evol. 2014;21:583–592. doi: 10.1016/j.meegid.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Celie K.-B., Colen D.L., Kovach S.J.I. Toxic Shock Syndrome after Surgery: Case Presentation and Systematic Review of the Literature. Plast. Reconstr. Surg.-Glob. Open. 2020;8:e2499. doi: 10.1097/gox.0000000000002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinges M.M., Orwin P.M., Schlievert P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000;13:16–34. doi: 10.1128/CMR.13.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Todd J., Fishaut M., Kapral F., Welch T. Toxic-shock syndrome associated with phage-group-I Staphylococci. Lancet. 1978;2:1116–1118. doi: 10.1016/S0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- 15.Davis J.P., Chesney P.J., Wand P.J., LaVenture M. Toxic-shock syndrome: Epidemiologic features, recurrence, risk factors, and prevention. N. Engl. J. Med. 1980;303:1429–1435. doi: 10.1056/NEJM198012183032501. [DOI] [PubMed] [Google Scholar]
- 16.Berger S., Kunerl A., Wasmuth S., Tierno P., Wagner K., Brügger J. Menstrual toxic shock syndrome: Case report and systematic review of the literature. Lancet Infect. Dis. 2019;19:e313–e321. doi: 10.1016/S1473-3099(19)30041-6. [DOI] [PubMed] [Google Scholar]
- 17.Wilkins A.L., Steer A.C., Smeesters P.R., Curtis N. Toxic shock syndrome—The seven Rs of management and treatment. J. Infect. 2017;74((Suppl. S1)):S147–S152. doi: 10.1016/S0163-4453(17)30206-2. [DOI] [PubMed] [Google Scholar]
- 18.Contou D., Colin G., Travert B., Jochmans S., Conrad M., Lascarrou J.-B., Painvin B., Ferré A., Schnell D., La Combe B., et al. Menstrual Toxic Shock Syndrome: A French Nationwide Multicenter Retrospective Study. Clin. Infect. Dis. 2022;74:246–253. doi: 10.1093/cid/ciab378. [DOI] [PubMed] [Google Scholar]
- 19.Sharma H., Smith D., Turner C.E., Game L., Pichon B., Hope R., Hill R., Kearns A., Sriskandan S. Clinical and Molecular Epidemiology of Staphylococcal Toxic Shock Syndrome in the United Kingdom. Emerg. Infect. Dis. 2018;24:258–266. doi: 10.3201/eid2402.170606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams D.A., Thomas K.R., Jajosky R.A., Foster L., Sharp P., Onweh D.H., Schley A.W., Anderson W.J. Summary of Notifiable Infectious Diseases and Conditions–United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016;63:1–152. doi: 10.15585/mmwr.mm6354a1. [DOI] [PubMed] [Google Scholar]
- 21.Osterholm M.T., Forfang J.C. Toxic-shock syndrome in minnesota: Results of an active-passive surveillance system. J. Infect. Dis. 1982;145:458–464. doi: 10.1093/infdis/145.4.458. [DOI] [PubMed] [Google Scholar]
- 22.Hajjeh R.A., Reingold A., Weil A., Shutt K., Schuchat A., Perkins B.A. Toxic Shock Syndrome in the United States: Surveillance Update, 1979–1996. Emerg. Infect. Dis. 1999;5:807–810. doi: 10.3201/eid0506.990611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeVries A.S., Lesher L., Schlievert P.M., Rogers T., Villaume L.G., Danila R., Lynfield R. Staphylococcal toxic shock syndrome 2000–2006: Epidemiology, clinical features, and molecular characteristics. PLoS ONE. 2011;6:e22997. doi: 10.1371/journal.pone.0022997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlievert P.M., Case L.C., Strandberg K.L., Tripp T.J., Lin Y.C., Peterson M.L. Vaginal Staphylococcus aureus superantigen profile shift from 1980 and 1981 to 2003, 2004, and 2005. J. Clin. Microbiol. 2007;45:2704–2707. doi: 10.1128/JCM.02295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlievert P.M., Davis C.C. Device-Associated Menstrual Toxic Shock Syndrome. Clin. Microbiol. Rev. 2020;33:e00032-19. doi: 10.1128/CMR.00032-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stolz S.J., Davis J.P., Vergeront J.M., Crass B.A., Chesney P.J., Wand P.J., Bergdoll M.S. Development of Serum Antibody to Toxic Shock Toxin among Individuals with Toxic Shock Syndrome in Wisconsin. J. Infect. Dis. 1985;151:883–889. doi: 10.1093/infdis/151.5.883. [DOI] [PubMed] [Google Scholar]
- 27.El Soufi H., El Soufi Y., Al-Nuaimi S., Bagheri F. Toxic shock syndrome associated with menstrual cup use. IDCases. 2021;25:e01171. doi: 10.1016/j.idcr.2021.e01171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann C., Kaiser R., Bauer J. Menstrual Cup-Associated Toxic Shock Syndrome. Eur. J. Case Rep. Intern. Med. 2020;7:001825. doi: 10.12890/2020_001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell M.A., Bisch S., Arntfield S., Hosseini-Moghaddam S.M. A confirmed case of toxic shock syndrome associated with the use of a menstrual cup. Can. J. Infect. Dis. Med. Microbiol. 2015;26:218–220. doi: 10.1155/2015/560959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klug C.D., Keay C.R., Ginde A.A. Fatal Toxic Shock Syndrome from an Intrauterine Device. Ann. Emerg. Med. 2009;54:701–703. doi: 10.1016/j.annemergmed.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 31.Descloux E., Perpoint T., Ferry T., Lina G., Bes M., Vandenesch F., Mohammedi I., Etienne J. One in five mortality in non-menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur. J. Clin. Microbiol. Infect. Dis. 2008;27:37–43. doi: 10.1007/s10096-007-0405-2. [DOI] [PubMed] [Google Scholar]
- 32.Truant S.N., Redolfi D.M., Sarratea M.B., Malchiodi E.L., Fernández M.M. Superantigens, a Paradox of the Immune Response. Toxins. 2022;14:800. doi: 10.3390/toxins14110800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohach G.A., Fast D.J., Nelson R.D., Schlievert P.M. Staphylococcal and Streptococcal Pyrogenic Toxins Involved in Toxic Shock Syndrome and Related Illnesses. Crit. Rev. Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 34.Whiting J.L., Rosten P.M., Chow A.W. Determination by western blot (immunoblot) of seroconversions to toxic shock syndrome (TSS) toxin 1 and enterotoxin A, B, or C during infection with TSS- and non-TSS-associated Staphylococcus aureus. Infect. Immun. 1989;57:231–234. doi: 10.1128/iai.57.1.231-234.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deguchi Y., Horiuchi Y., Shojima K., Iwahashi N., Ikejima M., Ino K., Furukawa K. Postpartum Methicillin-Resistant Staphylococcus aureus Toxic Shock Syndrome Caused by a Perineal Infection. Case Rep. Obstet. Gynecol. 2018;2018:2670179. doi: 10.1155/2018/2670179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrews J.I., Shamshirsaz A.A., Diekema D.J. Nonmenstrual Toxic Shock Syndrome Due to Methicillin-Resistant Staphylococcus aureus. Obstet. Gynecol. 2008;112:933–938. doi: 10.1097/AOG.0b013e3181866468. [DOI] [PubMed] [Google Scholar]
- 37.Collet C., Petsaris O., Lafforgue N., Poulain P., Gautier P., Michelet C., Donnio P.-Y. Postpartum toxic shock syndrome due to methicillin-resistant Staphylococcus aureus epidemic in community. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;144:184–185. doi: 10.1016/j.ejogrb.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 38.Brown A.F., Leech J.M., Rogers T.R., McLoughlin R.M. Staphylococcus aureus Colonization: Modulation of Host Immune Response and Impact on Human Vaccine Design. Front. Immunol. 2014;4:507. doi: 10.3389/fimmu.2013.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakr A., Brégeon F., Mège J.-L., Rolain J.-M., Blin O. Staphylococcus aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections. Front. Microbiol. 2018;9:2419. doi: 10.3389/fmicb.2018.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troeman D.P.R., Hazard D., Timbermont L., Malhotra-Kumar S., van Werkhoven C.H., Wolkewitz M., Ruzin A., Goossens H., Bonten M.J.M., Harbarth S., et al. Postoperative Staphylococcus aureus Infections in Patients with and without Preoperative Colonization. JAMA Netw. Open. 2023;6:e2339793. doi: 10.1001/jamanetworkopen.2023.39793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naimi H.M., Tristan A., Bes M., Vandenesch F., Nazari Q.A., Laurent F., Dupieux C. Molecular characterization and antimicrobial resistance of nasal Staphylococcus aureus in the community of Kabul. J. Glob. Antimicrob. Resist. 2023;34:18–22. doi: 10.1016/j.jgar.2023.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Cone L.A., Woodard D.R., Schlievert P.M., Tomory G.S. Clinical and Bacteriologic Observations of a Toxic Shock–like Syndrome Due to Streptococcus pyogenes. N. Engl. J. Med. 1987;317:146–149. doi: 10.1056/NEJM198707163170305. [DOI] [PubMed] [Google Scholar]
- 43.Stevens D.L., Tanner M.H., Winship J., Swarts R., Ries K.M., Schlievert P.M., Kaplan E. Severe Group A Streptococcal Infections Associated with a Toxic Shock-like Syndrome and Scarlet Fever Toxin A. N. Engl. J. Med. 1989;321:1–7. doi: 10.1056/nejm198907063210101. [DOI] [PubMed] [Google Scholar]
- 44.Breiman R.F., Davis J.P., Facklam R.R., Gray B.M., Hoge C.W., Kaplan E.L., Mortimer E.A., Schlievert P.M., Schwartz B., Stevens D.L., et al. Defining the Group A Streptococcal Toxic Shock Syndrome: Rationale and Consensus Definition. JAMA. 1993;269:390–391. doi: 10.1001/jama.1993.03500030088038. [DOI] [PubMed] [Google Scholar]
- 45.Plainvert C., Doloy A., Loubinoux J., Lepoutre A., Collobert G., Touak G., Trieu-Cuot P., Bouvet A., Poyart C. Invasive group A streptococcal infections in adults, France (2006–2010) Clin. Microbiol. Infect. 2012;18:702–710. doi: 10.1111/j.1469-0691.2011.03624.x. [DOI] [PubMed] [Google Scholar]
- 46.Lamagni T., Neal S., Keshishian C., Hope V., George R., Duckworth G., Vuopio-Varkila J., Efstratiou A. Severe Streptococcus pyogenes Infections, United Kingdom, 2003–2004. Emerg. Infect. Dis. 2008;14:202–209. doi: 10.3201/eid1402.070888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamagni T.L., Darenberg J., Luca-Harari B., Siljander T., Efstratiou A., Henriques-Normark B., Vuopio-Varkila J., Bouvet A., Creti R., Ekelund K., et al. Epidemiology of Severe Streptococcus pyogenes Disease in Europe. J. Clin. Microbiol. 2008;46:2359–2367. doi: 10.1128/jcm.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lepoutre A., Doloy A., Bidet P., Leblond A., Perrocheau A., Bingen E., Trieu-Cuot P., Bouvet A., Poyart C., Lévy-Bruhl D., et al. Epidemiology of InvasiveStreptococcus pyogenesInfections in France in 2007. J. Clin. Microbiol. 2011;49:4094–4100. doi: 10.1128/jcm.00070-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevens D.L. Streptococcal toxic shock syndrome. Clin. Microbiol. Infect. 2002;8:133–136. doi: 10.1046/j.1469-0691.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 50.Gruttadauria M., Pollard J., Kim S., Lian X. Streptococcal toxic shock syndrome in the setting of recent gynecologic surgery: A case report. Case Rep. Women’s Health. 2021;32:e00352. doi: 10.1016/j.crwh.2021.e00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Souyri C., Olivier P., Grolleau S., Lapeyre-Mestre M., French Network of Pharmacovigilance Centres Severe necrotizing soft-tissue infections and nonsteroidal anti-inflammatory drugs. Clin. Exp. Dermatol. 2008;33:249–255. doi: 10.1111/j.1365-2230.2007.02652.x. [DOI] [PubMed] [Google Scholar]
- 52.Hamilton S.M., Bayer C.R., Stevens D.L., Lieber R.L., Bryant A.E. Muscle injury, vimentin expression, and nonsteroidal anti-inflammatory drugs predispose to cryptic group A streptococcal necrotizing infection. J. Infect. Dis. 2008;198:1692–1698. doi: 10.1086/593016. [DOI] [PubMed] [Google Scholar]
- 53.Rink L., Kruse A., Nicklas W., Hoyer J., Kirchner H. Induction of cytokines in human peripheral blood and spleen cells by the Mycoplasma arthritidis-derived superantigen. Lymphokine Cytokine Res. 1992;11:105–108. [PubMed] [Google Scholar]
- 54.Dlaske H., Karaüzüm H., Monzon-Casanova E., Rudolf R., Starick L., Müller I., Wildner G., Diedrichs-Möhring M., Koch N., Miyoshi-Akiyama T., et al. Superantigen-presentation by rat major histocompatibility complex class II molecules RT1.Bl and RT1.Dl. Immunology. 2009;128((Suppl. S1)):e572–e581. doi: 10.1111/j.1365-2567.2008.03033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kreiswirth B.N., Schlievert P.M., Novick R.P. Evaluation of coagulase-negative staphylococci for ability to produce toxic shock syndrome toxin 1. J. Clin. Microbiol. 1987;25:2028–2029. doi: 10.1128/jcm.25.10.2028-2029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madhusoodanan J., Seo K.S., Remortel B., Park J.Y., Hwang S.Y., Fox L.K., Park Y.H., Deobald C.F., Wang D., Liu S., et al. An Enterotoxin-Bearing Pathogenicity Island in Staphylococcus epidermidis. J. Bacteriol. 2011;193:1854–1862. doi: 10.1128/JB.00162-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stach C.S., Vu B.G., Schlievert P.M. Determining the Presence of Superantigens in Coagulase Negative Staphylococci from Humans. PLoS ONE. 2015;10:e0143341. doi: 10.1371/journal.pone.0143341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crass B.A., Bergdoll M.S. Involvement of coagulase-negative staphylococci in toxic shock syndrome. J. Clin. Microbiol. 1986;23:43–45. doi: 10.1128/jcm.23.1.43-45.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lina G., Fleer A., Etienne J., Greenland T.B., Vandenesch F. Coagulase-negative staphylococci isolated from two cases of toxic shock syndrome lack superantigenic activity, but induce cytokine production. FEMS Immunol. Med. Microbiol. 1996;13:81–86. doi: 10.1111/j.1574-695X.1996.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 60.Sartelli M., Coccolini F., Kluger Y., Agastra E., Abu-Zidan F.M., Abbas A.E.S., Ansaloni L., Adesunkanmi A.K., Augustin G., Bala M., et al. WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections. World J. Emerg. Surg. 2022;17:3. doi: 10.1186/s13017-022-00406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thy M., Tanaka S., Tran-Dinh A., Ribeiro L., Lortat-Jacob B., Donadio J., Zappella N., Ben-Rehouma M., Tashk P., Snauwaert A., et al. Dynamic Changes in Microbial Composition During Necrotizing Soft-Tissue Infections in ICU Patients. Front. Med. 2020;7:609497. doi: 10.3389/fmed.2020.609497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka S., Thy M., Tashk P., Ribeiro L., Lortat-Jacob B., Hermieu J.-F., Zappella N., Rozencwajg S., Snauwaert A., Atchade E., et al. Impact of prior antibiotic therapy on severe necrotizing soft-tissue infections in ICU patients: Results from a French retrospective and observational study. Eur. J. Clin. Microbiol. Infect. Dis. 2022;41:109–117. doi: 10.1007/s10096-021-04354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stevens D.L., Bisno A.L., Chambers H.F., Dellinger E.P., Goldstein E.J., Gorbach S.L., Hirschmann J.V., Kaplan S.L., Montoya J.G., Wade J.C. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014;59:e10–e52. doi: 10.1093/cid/ciu296. [DOI] [PubMed] [Google Scholar]
- 64.Kenneally A.M., Warriner Z., VanHoose J.D., Ali D., McCleary E.J., Davenport D.L., Parli S.E. Evaluation of Antibiotic Duration after Surgical Debridement of Necrotizing Soft Tissue Infection. Surg. Infect. 2022;23:357–363. doi: 10.1089/sur.2021.256. [DOI] [PubMed] [Google Scholar]
- 65.Terzian W.H., Nunn A.M., Call E.B., Bliss S.E., Swinarska J.T., Rigdon J., Avery M.D., Hoth J.J., Miller P.R., III Duration of Antibiotic Therapy in Necrotizing Soft Tissue Infections: Shorter is Safe. Surg. Infect. 2022;23:430–435. doi: 10.1089/sur.2022.011. [DOI] [PubMed] [Google Scholar]
- 66.Valadez M.G., Patel N., Chong V., Putnam B.A., Moazzez A., Saltzman D., Kim D.Y. Short Courses of Antibiotics Are Safe in Necrotizing Soft Tissue Infections. Am. Surg. 2021;87:1666–1671. doi: 10.1177/00031348211051700. [DOI] [PubMed] [Google Scholar]
- 67.Coyle E.A., Cha R., Rybak M.J. Influences of Linezolid, Penicillin, and Clindamycin, Alone and in Combination, on Streptococcal Pyrogenic Exotoxin A Release. Antimicrob. Agents Chemother. 2003;47:1752–1755. doi: 10.1128/AAC.47.5.1752-1755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carapetis J.R., Jacoby P., Carville K., Ang S.J.J., Curtis N., Andrews R. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin. Infect. Dis. 2014;59:358–365. doi: 10.1093/cid/ciu304. [DOI] [PubMed] [Google Scholar]
- 69.Linner A., Darenberg J., Sjolin J., Henriques-Normark B., Norrby-Teglund A. Clinical Efficacy of Polyspecific Intravenous Immunoglobulin Therapy in Patients with Streptococcal Toxic Shock Syndrome: A Comparative Observational Study. Clin. Infect. Dis. 2014;59:851–857. doi: 10.1093/cid/ciu449. [DOI] [PubMed] [Google Scholar]
- 70.Babiker A., Li X., Lai Y.L., Strich J.R., Warner S., Sarzynski S., Dekker J.P., Danner R.L., Kadri S.S. Effectiveness of adjunctive clindamycin in β-lactam antibiotic-treated patients with invasive β-haemolytic streptococcal infections in US hospitals: A retrospective multicentre cohort study. Lancet Infect. Dis. 2021;21:697–710. doi: 10.1016/S1473-3099(20)30523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dorazio J., Chiappelli A.L., Shields R.K., Tsai Y.V., Skinker P., Nabozny M.J., Bauza G., Forsythe R., Rosengart M.R., Gunn S.R., et al. Clindamycin plus Vancomycin versus Linezolid for Treatment of Necrotizing Soft Tissue Infection. Open Forum Infect. Dis. 2023;10:ofad258. doi: 10.1093/ofid/ofad258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Helmy H.A., AbdElhamed M.R., Youssef M.I., El Zamek H.M.F., Kamal A., Abdelfattah A., Shabana H., Abuamer A., Aboufarrag G.A., Elshormilisy A.A., et al. A Multicenter Experience of Inducible Clindamycin Resistance in Staphylococcus aureus Infection among 800 Egyptian Patients with or without Diabetes Mellitus. Am. J. Trop. Med. Hyg. 2023;109:350–355. doi: 10.4269/ajtmh.22-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stevens D.L., Wallace R.J., Hamilton S.M., Bryant A.E. Successful Treatment of Staphylococcal Toxic Shock Syndrome with Linezolid: A Case Report and In Vitro Evaluation of the Production of Toxic Shock Syndrome Toxin Type 1 in the Presence of Antibiotics. Clin. Infect. Dis. 2006;42:729–730. doi: 10.1086/500265. [DOI] [PubMed] [Google Scholar]
- 74.Takei S., Arora Y.K., Walker S.M. Intravenous immunoglobulin contains specific antibodies inhibitory to activation of T cells by staphylococcal toxin superantigens [see comment] J. Clin. Investig. 1993;91:602–607. doi: 10.1172/JCI116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Norrby-Teglund A., Kaul R., Low D.E., McGeer A., Newton D.W., Andersson J., Andersson U., Kotb M. Plasma from patients with severe invasive group A streptococcal infections treated with normal polyspecific IgG inhibits streptococcal superantigen-induced T cell proliferation and cytokine production. J. Immunol. 1996;156:3057–3064. doi: 10.4049/jimmunol.156.8.3057. [DOI] [PubMed] [Google Scholar]
- 76.Darenberg J., Ihendyane N., Sjölin J., Aufwerber E., Haidl S., Follin P., Andersson J., Norrby-Teglund A. The Streptlg Study Group Intravenous Immunoglobulin G Therapy in Streptococcal Toxic Shock Syndrome: A European Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2003;37:333–340. doi: 10.1086/376630. [DOI] [PubMed] [Google Scholar]
- 77.Madsen M.B., Hjortrup P.B., Hansen M.B., Lange T., Norrby-Teglund A., Hyldegaard O., Perner A. Immunoglobulin G for patients with necrotising soft tissue infection (INSTINCT): A randomised, blinded, placebo-controlled trial. Intensiv. Care Med. 2017;43:1585–1593. doi: 10.1007/s00134-017-4786-0. [DOI] [PubMed] [Google Scholar]
- 78.Parks T., Wilson C., Curtis N., Norrby-Teglund A., Sriskandan S. Polyspecific Intravenous Immunoglobulin in Clindamycin-treated Patients with Streptococcal Toxic Shock Syndrome: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2018;67:1434–1436. doi: 10.1093/cid/ciy401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartoszko J.J., Elias Z., Rudziak P., Lo C.K.L., Thabane L., Mertz D., Loeb M. Prognostic factors for streptococcal toxic shock syndrome: Systematic review and meta-analysis. BMJ Open. 2022;12:e063023. doi: 10.1136/bmjopen-2022-063023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Senda A., Endo A., Fushimi K., Otomo Y. Effectiveness of intravenous immunoglobulin therapy for invasive group A Streptococcus infection: A Japanese nationwide observational study. Int. J. Infect. Dis. 2023;135:84–90. doi: 10.1016/j.ijid.2023.08.011. [DOI] [PubMed] [Google Scholar]
- 81.Davies H.D., McGeer A., Schwartz B., Green K., Cann D., Simor A.E., Low D.E., Ontario Group A Streptococcal Study Group Invasive group A streptococcal infections in Ontario, Canada. Ontario Group A Streptococcal Study Group. N. Engl. J. Med. 1996;335:547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- 82.O’Loughlin R.E., Roberson A., Cieslak P.R., Lynfield R., Gershman K., Craig A., Albanese B.A., Farley M.M., Barrett N.L., Spina N.L., et al. The Epidemiology of Invasive Group A Streptococcal Infection and Potential Vaccine Implications: United States, 2000–2004. Clin. Infect. Dis. 2007;45:853–862. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- 83.Schlievert P.M., Gaitán A.V., Kilgore S.H., Roe A.L., Maukonen J., Lehtoranta L., Leung D.Y.M., Marsman D.S. Inhibition of Toxic Shock Syndrome-Associated Staphylococcus aureus by Probiotic Lactobacilli. Microbiol. Spectr. 2023;11:e0173523. doi: 10.1128/spectrum.01735-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwameis M., Roppenser B., Firbas C., Gruener C.S., Model N., Stich N., Roetzer A., Buchtele N., Jilma B., Eibl M.M. Safety, tolerability, and immunogenicity of a recombinant toxic shock syndrome toxin (rTSST)-1 variant vaccine: A randomised, double-blind, adjuvant-controlled, dose escalation first-in-man trial. Lancet Infect. Dis. 2016;16:1036–1044. doi: 10.1016/S1473-3099(16)30115-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.