Abstract
The plasmid types and serotypes of 164 Rhodococcus equi strains obtained from submaxillary lymph nodes of swine from different piggeries in 28 villages and towns located throughout the country were examined. The strains were tested by PCR for the presence of 15- to 17-kDa virulence-associated protein antigen (VapA) and 20-kDa virulence-associated protein antigen (VapB) genes. Plasmid DNAs were isolated and analyzed by digestion with restriction endonucleases to estimate size and compare their polymorphism characteristics. None of the 164 isolates contained the vapA gene, and 44 (26.8%) isolates were positive for the vapB gene, showing a product of the expected 827-bp size in the PCR amplification. The 44 isolates of intermediate virulence contained virulence plasmids that were identified as types 1 (3 isolates), 4 (1 isolate), 5 (36 isolates), 6 (1 isolate), and 7 (2 isolates) and as a new variant (1 isolate). On the basis of restriction digestion patterns of plasmid DNAs, we tentatively designated the variant as type 17. Use of the serotyping method of Prescott showed that 110 (67.1%) out of the 164 isolates were typeable and that serotype 2 predominated (83 isolates [50.6%]), followed by serotype 1 (26 strains [15.9%]). Only one isolate belonged to serotype 3. A total of 54 (32.9%) isolates were untypeable in Prescott's system. The prevalence of R. equi strains of intermediate virulence among the isolates that came from the submaxillary lymph nodes of swine in Hungary was lower than that seen with isolates obtained elsewhere.
R. equi is an aerobic, gram-positive coccobacillus which frequently causes chronic suppurative bronchopneumonia with abscesses, lymphadenitis, and ulcerative enteritis in foals less than 6 months old (3, 14). In addition to its virulence for foals, R. equi seems also to be an important pathogen to immunocompromised humans, such as organ transplant and AIDS patients (21). R. equi is also common in the submaxillary lymph nodes of pigs (2, 8, 15, 25, 27). Katsumi and others (8) isolated R. equi from 45.6% of the submaxillary lymph nodes of swine with lesions and from 9.4% of lymph nodes of swine without lesions. Takai and colleagues (19) described a 3.1% isolation rate on the basis of examination of 1,832 submaxillary lymph nodes collected from swine.
Recently, the discovery of virulence-associated antigens and virulence plasmids has allowed classification of the virulence of R. equi strains (17, 24). At least three virulence levels of R. equi have been identified: virulence, intermediate virulence, and avirulence. Virulent R. equi is characterized by the presence of virulence-associated 15- to 17-kDa antigens (VapA), virulence plasmid DNAs of 85 to 90 kb, and suppurative pneumonia in foals (murine 50% lethal dose [LD50] = 106 cells). At this moment, there are at least 11 slightly different virulence plasmids in virulent isolates (26). R. equi strains of intermediate virulence are identified by the presence of a virulence-associated 20-kDa antigen (VapB), and virulence plasmids are found in the submaxillary lymph nodes of pigs (murine LD50 = 107 cells). Intermediately virulent strains contained one of 16 distinct plasmids of 79 to 100 kb found in human and pig isolates that were associated with the expression of the 20-kDa antigen (26). In comparison, avirulent R. equi contains neither virulence-associated antigens nor plasmid DNA (murine LD50 > 108 cells) (17). The majority of R. equi isolates from patients with AIDS are virulent (VapA+) or of intermediate virulence (VapB+), whereas most isolates from immunocompromised patients without AIDS were avirulent (21).
More recently, we demonstrated that five of the seven clinical isolates of R. equi from immunocompromised patients expressed VapB and that they were of intermediate virulence and revealed that these human isolates contained a 95-kb type 5 plasmid which was also seen in the pig isolates in Hungary (10). The route of infection in human cases is not clear. The purpose of this study was to isolate virulent R. equi strains from submaxillary lymph nodes of swine in Hungary, to determine the genotypic diversity of virulence-associated plasmids found in them, and to examine the serotypes of the isolates with the aim of finding additional data to characterize the epidemiological relationship between human R. equi infections and pigs carrying R. equi in the submaxillary lymph nodes.
Serotyping is a reliable method for examining R. equi strains. There are two systems for serotyping R. equi. Prescott described seven serotypes by the use of an agar gel immunodiffusion (AGID) test (13), while Nakazawa et al. differentiated 27 serotypes with a slide agglutination test (12). Beside common antigens, several different ones have been found to react in the above-named tests; the results of the two serotyping systems do not completely agree (9). When the Prescott system is used, serotype 2 is the predominating serotype of R. equi isolated from submaxillary lymph nodes of swine (8, 9, 12).
MATERIALS AND METHODS
Source of isolates.
A total of 1,173 submaxillary lymph nodes without macroscopic lesions were collected in July of 2002 in a slaughterhouse in northeast Hungary. The sampled animals were kept in different piggeries in 28 villages and towns located throughout the country. The animals were from small farms with 5 to 20 pigs that kept them in a natural environment, with the possibility of rooting through the soil. Lymph nodes were cut into two pieces with a sterile scalpel, and the cut surfaces were touched to the surface of CAZ-NB medium (7). The agar plates were incubated on 37°C for 48 h. One typical colony was subcultured onto nutrient agar plate from each culture and was identified on the basis of morphological and biochemical methods (1).
Reference strains.
Strain ATCC 33701 (equine origin, virulent strain) and 16 representative strains of intermediate virulence (human and pig origin: strain A2 [plasmid type 1]; strain S2 [plasmid type 2]; strain S3 [plasmid type 3]; strain S4 [plasmid type 4]; strain A5 [plasmid type 5]; strain S6 [plasmid type 6]; strain S7 [plasmid type 7]; strain S8 [plasmid type 8]; strain S9 [plasmid type 9]; strain A11 [plasmid type 10]; strain A43 [plasmid type 11]; strain 70 [plasmid type 12]; strain H3 [plasmid type 13]; strain H25 [plasmid type 14]; strain H43 [plasmid type 15]; strain H66 [plasmid type 16]) were used as reference strains, as some of these strains have been described previously (18, 19, 22, 26) with respect to protein profile, plasmid characteristic, and virulence level.
Isolation and examination of plasmid DNA.
Plasmid DNA was isolated from R. equi by an alkaline lysis method (5) with some modifications as described by Takai et al. (23). Plasmid DNA was digested with restriction endonucleases BamHI, EcoRI, EcoT22I, HindIII, and PvuII. DNA fragments were separated in agarose gel (0.7%) at approximately 5 V/cm for 2 h, stained with ethidium bromide, and examined under UV light conditions.
Virulence-associated gene.
The target DNAs for PCR amplification were the published sequences of the 15- to 17-kDa antigen (VapA) gene and a 20-kDa antigen (VapB) gene (GenBank database accession numbers D21236l and D44469) from R. equi strains ATCC 33701 and S5, respectively. Primer 1 (5′-GACTCTTCACAAGACGGT-3′) corresponded to the sense strand at positions 6 to 23, and primer 2 (5′-TAGGCGTTGTGCCAGCTA-3′) corresponded to the antisense strand at positions 569 to 552 in the sequence of the 15- to 17-kDa antigen gene (16). Primer 3 (5′-AACGTAGTCGCGGTGAGAA-3′) corresponded to the sense strand at positions 240 to 258, and primer 4 (5′-ACCGAGACTTGAGCGACTA-3′) corresponded to the antisense strand at positions 1066 to 1048 in the sequence of the cloned fragment containing the 20-kDa antigen gene. PCR amplification was performed with 10 ml of the DNA preparation in a 50-ml reaction mixture containing 10 mM Tris-HCl (pH 8.3 at 25°C), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM (each) deoxynucleotide triphosphates, 1 mM of each primer, and 2.5 U of Taq DNA polymerase (Takara, Tokyo, Japan), as described previously (20). The samples were subjected to 30 cycles of amplification in a GeneAmp PCR system 2400 (Perkin-Elmer, Norwalk, Conn.). The cycling conditions were as follows: denaturation for 90 s at 94°C; primer annealing for 1 min at 55°C; and extension for 2 min at 72°C (20).
Serotyping.
Serotyping was carried out according to Prescott's serotyping system (13).
Production of hyperimmune sera in rabbits.
For each serotype, two rabbits weighting approximately 3 kg were immunized intravenously with R. equi strains of serotypes 1 to 7 (ATCC 33701 to 33707). Bacteria from three nutrient agar cultures incubated at 30°C for 48 h were suspended in 40 ml of saline solution (approximately 3 × 1010 CFU/ml) containing 0.5% formaldehyde. After preparation, the suspension was allowed to stand at room temperature for 1 h, and then the bacterial cells were pelleted by centrifugation, resuspended in saline, and stored at 4°C until used. On the first occasion the rabbits were inoculated with 0.5 ml of the suspension and then with 1 ml on day 4, 2 ml on day 8, and 3 ml on each of days 11, 15, and 18. At 1 week after the last injection the rabbits were bled in ketamine-xylazine narcosis. Sera were harvested and stored in small aliquots in a refrigerator at −20°C until used.
Preparation of antigen for the AGID test.
Every R. equi strain was cultured on nutrient agar plates (30°C, 48 h), collected from the agar surface, suspended in normal saline, incubated at 30°C overnight, and then centrifuged at 3,000 × g at 4°C for 15 min. The supernatant was used as antigen. Antigens were stored in a refrigerator at −20°C until used.
AGID test.
An AGID test was carried out using 0.8% Noble agar in boric buffer (70 g of NaCl, 2 g of NaOH, and 9 g of boric acid in 1,000 ml of water, pH 8.6). The AGID test was done in petri dishes containing 15 ml of agar with eight sets of seven wells, with each set consisting of one central and six peripheral wells. The well diameter was 3 mm, and the distance between the central and peripheral wells was 4 mm. Petri dishes were incubated at room temperature for 24 h in humid chamber before the results were read.
RESULTS
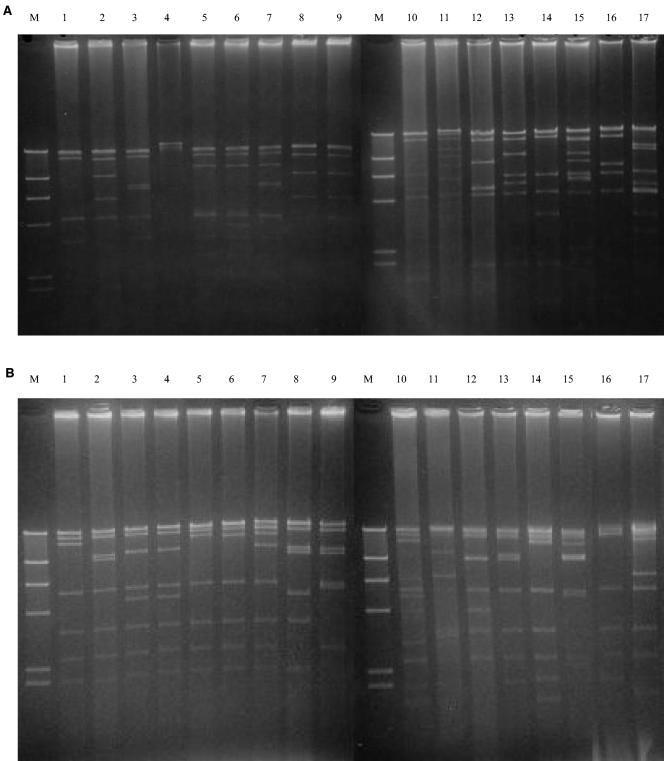
Out of 1,173 submaxillary lymph nodes collected from pigs, 164 R. equi strains were isolated from 164 R. equi-positive cultures. The rate of isolation was found to be 14.0%, but there were differences among piggeries (range, 6.1 to 20.4%). None of the 164 isolates analyzed by PCR contained vapA gene. A total of 44 (26.8%) strains were positive for the vapB gene, showing a product of the expected 827-bp size in the PCR amplification. The 44 isolates of intermediate virulence (vapB positive) were then tested for the presence of virulence plasmids and analyzed by restriction enzyme digestion with endonucleases EcoRI and EcoT22I. Of the 44 isolates of intermediate virulence, 36 isolates contained a type 5 plasmid 95 kb in size, 3 contained a type 1 plasmid, 2 contained a type 7 plasmid, 1 contained a type 4 plasmid, and 1 contained a type 6 plasmid. The remaining one had unique restriction cleavage patterns and did not match any of the 16 previously reported EcoRI and EcoT22I digestion patterns for R. equi. Lanes 1 to 16 in Fig. 1 represent the 16 previously reported EcoRI and EcoT22I digestion patterns, and lane 17 represents the plasmid type newly identified in this study. Plasmid DNAs of the 16 representative types and of the 1 new type digested with EcoT22I were examined by Southern analysis with PCR probes. The PCR products labeled with digoxigenin-11-dUTP hybridized with one of the fragments of each plasmid DNA (data not shown). From these results, we tentatively designated the new plasmid type as type 17.
FIG. 1.
EcoRI (A) and EcoT22I (B) restriction fragments of the 17 plasmid types of R. equi isolates of intermediate virulence. Lanes 1, strain A2 (plasmid type 1); lanes 2, strain S2 (plasmid type 2); lanes 3, strain S3 (plasmid type 3); lanes 4, strain S4 (plasmid type 4); lanes 5, strain A5 (plasmid type 5); lanes 6, strain S6 (plasmid type 6); lanes 7, strain S7 (plasmid type 7); lanes 8, strain S8 (plasmid type 8); lanes 9, strain S9 (plasmid type 9); lanes 10, strain A11 (plasmid type 10); lanes 11, strain A43 (plasmid type 11); lanes 12, strain 70 (plasmid type 12); lanes 13, strain H3 (plasmid type 13); lanes 14, strain H25 (plasmid type 14); lanes 15, strain H43 (plasmid type 15); lanes 16, strain H66 (plasmid type 16); lanes 17, strain 316 (new plasmid type 17). The markers (lanes M) are HindIII digestion products of bacteriophage lambda DNA.
When the method of Prescott was used, 28 (63.6%) of the 44 intermediately virulent isolates and 82 (68.3%) of the 120 avirulent isolates were typeable. By the same method, 54.5% of the intermediately virulent and 49.2% of the avirulent isolates belonged to serotype 2 and 15.9% of the strains were of serotype 1. Only one isolate belonged to serotype 3. A total of 54 (32.9%) strains were not typeable in Prescott's system (Table 1).
TABLE 1.
Serotypes of 164 R. equi isolates from swine in Hungary
| Pig isolate | No. of isolates | No. of isolates that werea:
|
|||
|---|---|---|---|---|---|
| Serotype 1 | Serotype 2 | Serotype 3 | NT | ||
| Intermediate virulent | 44 | 3 | 24 | 1 | 16 |
| Avirulent | 120 | 23 | 59 | 0 | 38 |
| Total | 164 | 26 (15.9) | 83 (50.6) | 1 (0.6) | 54 (33.9) |
Values in parentheses are percentages of the total number of isolates. NT, nontypeable.
DISCUSSION
The present study demonstrated that the presence of R. equi is widespread (on average, in 14% of pigs) in the submaxillary lymph nodes of healthy pigs in Hungary. R. equi strains were isolated from 164 submaxillary lymph nodes of pigs of different piggeries in 28 villages and towns located throughout the country, and the majority (73.2%) of R. equi strains from pigs were avirulent (i.e., did not carry virulence plasmids), 26.8% of the isolates were intermediately virulent, and none of the isolates represented virulent (vapA-positive) R. equi. The finding of the prevalence of R. equi of intermediate virulence in Hungarian pigs was quite different from our previous finding that 368 (93.9%) of 392 isolates from the submaxillary lymph nodes of Japanese pigs were intermediately virulent, 2 (0.5%) of the isolates were virulent, and the remaining 22 (5.6%) were avirulent; however, the isolation rate in that study was lower (19). A difference between the methods of breeding pigs in Hungary (pigs were kept in natural environment) and in Japan (pigs were kept in isolated large-scale farms) may be the reason for the differences in the isolation rates and in the levels of prevalence of R. equi of intermediate virulence in pig isolates between Hungary and Japan. It might be interesting to investigate the prevalence of virulent R. equi in soil isolates from environment of piggeries in Hungary. The pathological significance of R. equi strains in the submaxillary lymph nodes of pigs cannot be judged, since the strains were isolated from lymph nodes without lesions.
R. equi is an emerging pathogen of humans, particularly for those with a compromised immune system (14, 21). Our previous study showed that five of the seven clinical isolates of R. equi from immunocompromised patients expressed VapB which contained a 95-kb type 5 plasmid (10). In the present study, the same plasmid type was found in the majority (81.9%) of the 44 pig isolates and the same serotype (serotype 2) was also found in 54.5% (24 of 44) of the intermediately virulent isolates. These results confirm the presumption that there is an epidemiological relationship between human R. equi infections and the presence of R. equi of intermediate virulence in submaxillary lymph nodes of pigs (19, 26).
There are at least 16 distinct plasmids of 79 to 100 kb in human and pig isolates that are associated with the expression of the 20-kDa antigen (VapB), as shown by restriction enzyme digestion patterns of virulence plasmids determined using EcoRI and EcoT22I (6, 19, 21, 26). In the present study, 43 of 44 VapB-positive isolates contained virulence plasmids that have been isolated from humans or pigs; 3 isolates contained a 79-kb plasmid (designated type 1), 1 isolate contained an 88-kb plasmid (designated type 4), 36 isolates contained a 95-kb plasmid (designated type 5), 1 isolate contained a 79-kb plasmid (designated type 6), and 2 isolates contained an 88.5-kb plasmid (designated type 7). However, the one remaining isolate contained a different large plasmid, which differed from the previously described 16 representative patterns. This new plasmid type (type 17) was found in pig isolates in Hungary for the first time. Of the 16 representative plasmid types, nine have been found in Japanese pig isolates (6, 19, 26). The type 5 plasmid has been found in 80% or more of pig isolates in Hungary, but 2.9 to 9.9% of pig isolates from three regions of Japan contained this plasmid type (6). Further surveillance studies will reveal the geographical difference or similarity in the distribution of virulence plasmids in pig isolates from Hungary and Japan.
The dominance of serotype 2 among the R. equi strains isolated from the submaxillary lymph nodes was shown in our examination. These results agree with the data in the literature (8, 9, 11); however, Prescott found serotype 1 to be the predominating serotype among isolates from swine (13). A similar dominance of serotype 2 among R. equi strains isolated from humans was described by several authors (4, 9) and shows an epidemiological connection between pigs and humans.
Acknowledgments
This study was supported by Hungarian Scientific Research Fund grant OTKA F26597, by the Hungarian Ministry of Education (FKFP 0096/2000), by a grant-in-aid from the Equine Research Institute, Japan Racing Association, and by grants-in-aid for general scientific research (12876071, 14656124, and 14405031) from the Ministry of Education, Science, Sports and Culture of Japan.
Our thanks are due to A. Drótos, who helped us in collecting the samples.
REFERENCES
- 1.Barrow, G. I., and R. K. A. Feltham. 1993. Cowan and Steel's manual for the identification of medical bacteria. Cambridge University Press, Cambridge, United Kingdom.
- 2.Barton, M. D., and K. L. Hughes. 1980. Corynebacterium equi: a review. Vet. Bull. 50:65-80. [Google Scholar]
- 3.Bell, K. S., J. C. Philp, D. W. J. Aw, and N. Christofi. 1998. The genus Rhodococcus. A review. J. Appl. Microbiol. 85:195-210. [DOI] [PubMed] [Google Scholar]
- 4.Bern, D., and C. Lämmler. 1994. Biochemical and serological characteristics of Rhodococcus equi isolates from animals and humans. Zentbl. Vet. Med. B. 41:161-165. [DOI] [PubMed] [Google Scholar]
- 5.Birnboim, H., C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acid Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukunaga, N., T. Okoda, M. Katsumi, and S. Takai. 1999. Restriction cleavage patterns of plasmid DNA of intermediately virulent Rhodococcus equi isolates from the mandibular lymph nodes of pigs in Kagoshima, Aomori and Miyagi prefectures and the environment of pig-breeding farms. J. Jpn. Vet. Med. Assoc. 52:789-792. [Google Scholar]
- 7.Graevenitz, A., and V. Pünter-Streit. 1995. Development of a new selective plating medium for Rhodococcus equi. Microbiol. Immunol. 39:283-284. [DOI] [PubMed] [Google Scholar]
- 8.Katsumi, M., N. Kodama, Y. Miki, T. Hiramune, N. Kikuchi, R. Yanagawa, and M. Nakazawa. 1991. Typing of Rhodococcus equi isolated from submaxillary lymph nodes of pigs in Japan. Zentbl. Veterinärmed. B. 38:299-302. [DOI] [PubMed] [Google Scholar]
- 9.Lämmler, C., B. Zimmermann, and C. Fuhrmann. 1997. Serological properties of Rhodococcus equi isolates of various origins determined with two typing systems. Med. Sci. Res. 25:187-189. [Google Scholar]
- 10.Makrai, L., S. Takai, M. Tamura, A. Tsukamoto, R. Sekimoto, Y. Sasaki, T. Kakuda, S. Tsubaki, J. Varga, L. Fodor, N. Solymosi, and A. Major. 2002. Characterization of virulence plasmid types in Rhodococcus equi isolates from foals, pigs, humans and soil in Hungary. Vet. Microbiol. 88:377-384. [DOI] [PubMed] [Google Scholar]
- 11.Mutimer, M. D., J. F. Prescott, and J. B. Woolcock. 1982. Capsular serotypes of Rhodococcus equi. Aust. Vet. J. 58:67-69. [DOI] [PubMed] [Google Scholar]
- 12.Nakazawa, M., M. Kubo, C. Sugimoto, and Y. Isayama. 1983. Serogrouping of Rhodococcus equi. Microbiol. Immunol. 27:837-846. [DOI] [PubMed] [Google Scholar]
- 13.Prescott, J. F. 1981. Capsular serotypes of Corynebacterium equi. Can. J. Comp. Med. 45:130-134. [PMC free article] [PubMed] [Google Scholar]
- 14.Prescott, J. F. 1991. Rhodococcus equi: an animal and a human pathogen. Clin. Microbiol. Rev. 4:20-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao, M. S., S. Zaki, B. S. Keshavamurthy, and K. C. Singh. 1982. An outbreak of acute Corynebacterium equi infection in piglets. Indian Vet. J. 59:787-788. [Google Scholar]
- 16.Sekizaki, T., S. Takai, Y. Egawa, T. Ikeda, H. Ito, and S. Tsubaki. 1995. Sequence of the Rhodococcus equi gene encoding the virulence-associated 15-17-kDa antigens. Gene 155:135-136. [DOI] [PubMed] [Google Scholar]
- 17.Takai, S. 1997. Epidemiology of Rhodococcus equi infections: a review. Vet. Microbiol. 56:167-176. [DOI] [PubMed] [Google Scholar]
- 18.Takai, S., M. Shoda, Y. Sasaki, S. Tsubaki, G. Fortier, S. Pronost, T. Becu, A. Begg, G. Browning, V. M. Nicholson, and J. F. Prescott. 1999. Genetic analysis of virulent Rhodococcus equi based on restriction fragment length polymorphism of virulence plasmids: a molecular approach for epidemiology of virulent R. equi in the world. J. Clin. Microbiol. 37:3417-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takai, S., and S. Tsubaki. 1985. The incidence of Rhodococcus (Corynebacterium) equi in domestic animals and soil. J. Vet. Med. Sci. 47:1291-1293. [DOI] [PubMed] [Google Scholar]
- 20.Takai, S., M. K. Chaffin, N. D. Cohen, M. Hara, N. Nakamura, T. Kakuda, Y. Sasaki, S. Tsubaki, and R. J. Martens. 2001. Prevalence of virulent Rhodococcus equi in soil from five R. equi-endemic horse-breeding farms and restriction fragment length polymorphisms of virulence plasmids in isolates from soil and infected foals in Texas. J. Vet. Diagn. Investig. 13:489-494. [DOI] [PubMed] [Google Scholar]
- 21.Takai, S., N. Fukunaga, S. Ochiai, Y. Imai, Y. Sasaki, S. Tsubaki, and T. Sekizaki. 1996. Identification of intermediately virulent Rhodococcus equi isolates from pigs. J. Clin. Microbiol. 34:1034-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takai, S., N. Murata, R. Kudo, N. Narematsu, T. Kakuda, Y. Sasaki, and S. Tsubaki. 2001. Two new variants of the Rhodococcus equi virulence plasmid, 90 kb type III and type IV, recovered from a foal in Japan. Vet. Microbiol. 82:373-381. [DOI] [PubMed] [Google Scholar]
- 23.Takai, S., P. Tharavichitkul, P. Takarn, B. Khantawa, M. Tamura, A. Tsukamoto, S. Takayama, N. Yamatoda, A. Kimura, Y. Sasaki, T. Kakuda, S. Tsubaki, N. Maneekarn, T. Sirisanthana, and T. Kirikae. 2003. Molecular epidemiology of Rhodococcus equi of intermediate virulence isolated from patients with and without AIDS in Chiang Mai, Thailand. J. Infect. Dis. 188:1717-1723. [DOI] [PubMed] [Google Scholar]
- 24.Takai, S., T. Ikeda, Y. Sasaki, Y. Watanabe, T. Ozawa, S. Tsubaki, and T. Sekizaki. 1995. Identification of virulent Rhodococcus equi by amplification of gene coding for 15- to 17-kilodalton antigens. J. Clin. Microbiol. 33:1624-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takai, S., T. Sekizaki, T. Ozawa, T. Sugawara, Y. Watanabe, and S. Tsubaki. 1991. Association between a large plasmid and 15- to 17-kilodalton antigens in virulent Rhodococcus equi. Infect. Immun. 59:4056-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takai, S., T. Takeuchi, and S. Tsubaki. 1986. Isolation of Rhodococcus (Corynebacterium) equi and atypical mycobacteria from the lymph nodes of healthy pigs. J. Vet. Med. Sci. 48:445-448. [DOI] [PubMed] [Google Scholar]
- 27.Takai, S., Y. Imai, N. Fukunaga, Y. Uchida, K. Kamisawa, Y. Sasaki, S. Tsubaki, and T. Sekizaki. 1995. Identification of virulence-associated antigens and plasmids in Rhodococcus equi from patients with AIDS. J. Infect. Dis. 172:1306-1311. [DOI] [PubMed] [Google Scholar]