Abstract
Fish is a nutritionally rich product; however, it is easily contaminated by pathogenic microorganisms, such as Salmonella spp. Therefore, this study aimed to identify the best concentration of sodium hypochlorite (NaClO), exposure time, and water temperature that allow the most effective antimicrobial effect on the viable population of Salmonella spp. Thus, Salmonella Enteritidis ATCC 13076 and Salmonella Schwarzengrund were exposed to different time frames, ranging from 5 min to 38.5 min, temperatures between 5 and 38.5 °C, and NaClO concentrations ranging from 0.36 to 6.36 ppm, through a central composite rotational design experiment (CCRD). The results demonstrated that the ATCC strain exhibited a quadratic response to sodium hypochlorite when combined with exposure time, indicating that initial contact would already be sufficient for the compound’s action to inhibit the growth of the mentioned bacteria. However, for S. Schwarzengrund (isolated directly from fish cultivated in aquaculture), both NaClO concentration and exposure time significantly influenced inactivation, following a linear pattern. This suggests that increasing the exposure time of NaClO could be an alternative to enhance Salmonella elimination rates in fish slaughterhouses. Thus, the analysis indicates that the Salmonella spp. strains used in in vitro experiments were sensitive to concentrations equal to or greater than the recommended ones, requiring a longer exposure time combined with the recommended NaClO concentration in the case of isolates from aquaculture.
Keywords: fish, Salmonella Enteritidis, Salmonella Schwarzengrund, NaClO, exposure time, water temperature, CCRD
1. Introduction
Aquaculture is considered a zootechnical activity that has been standing out as an economic alternative, both for large, medium, and small production, as it is a way to take advantage of inactive rural areas [1]. It is estimated that Brazil will register a growth of 104% in fisheries and aquaculture production by 2025 [2]. According to The Fisheries Yearbook 2022, the State of Mato Grosso has been standing out in fish production, occupying the 7th position among the 10 largest producers of fish from fish farming in Brazil, producing approximately 42,600 tons [3].
Fish and fish products represent an important source of consumption by the population, in addition to being nutritionally rich with a high content of polyunsaturated fatty acids, proteins of high biological value, and easy digestibility [4]. Thus, fish has been highly consumed around the world due to its high nutritional value and potential health benefits [5].
Fish is a highly perishable food, so it requires a lot of care in handling, ranging from the capture process to storage and marketing. These steps are important to maintain the shelf life of the food [2]. The biggest concern is in relation to its food safety, where it is necessary to avoid contamination by pathogenic microorganisms that could harm the health of consumers [6]. Several pathogens can be present in fish, including Salmonella spp. [7].
Salmonella spp. is not a biological contaminant originally reported in fish, being introduced through contaminated water or improper handling [8]. There are about 2.659 serovars in the species [9], with the Typhimurium serotype being the most prevalent in products of animal origin [10]. In the US, S. Enteritidis, S. Typhimurium, and S. Newport represent the Salmonella spp. transmitted by frequently isolated foods [11]. However, in Brazil, there are few epidemiological studies that indicate Salmonella Typhimurium in fish [12], although other serotypes such as S. Abony and S. Schwarzengrund have been found in fish [13]. There is also evidence of the circulation of the S. Typhimurium serotype in fish from aquaculture in the State of Mato Grosso [12].
Due to increased consumption of farmed fish, the contribution of fishery products to foodborne disease outbreaks has increased [12]. Salmonella spp. has been reported as one of the most important causes of foodborne disease prevalence [14]. Food safety studies conducted by the European Center for Disease Prevention and Control have shown that almost 7% of foodborne disease outbreaks in the European Union are associated with the consumption of fish and fishery products [15]. In Brazil, more than 6000 cases of food-borne outbreaks have been officially reported from 2007 to 2016, of which 94 were caused by fish and seafood [12].
The presence of this pathogen may be related to several factors, such as the high density of fish biomass in a limited area in intensive farming systems, the access of wild and domestic animals in these areas, effluents, leaching, contaminated food, and human interventions. All these factors can lead to the contamination of pathogens such as Salmonella spp. [16]. Contamination of the aquatic ecosystem by Salmonella spp. makes the environment a source of dissemination of this microorganism, as this bacterium can survive in the environment for long periods [17]. Furthermore, it can be introduced through contact with the feces of other animals and plants [18].
In fish slaughterhouses, control of Salmonella spp. is a challenge, and concerning the efficiency of the forms of its control, it is still not well known [12]. In the animal products industry, chlorine and its derivatives are widely used agents to eliminate pathogenic microorganisms [19]. Chlorinated products are used as the main form of microbiological control in the food industry. The chlorine substance contains hypochlorous acid, which is an effective antioxidant with a neutral charge, enabling easy penetration through the outer membrane of bacteria [20].
The maximum concentration recommendation of sodium hypochlorite allowed by the Ministry of Agriculture, Livestock and Food Supply of Brazil (MAPA) is up to 5 parts per million (ppm) of free residual chlorine [21]. In the fish industry, the process of washing fish with sodium hypochlorite can be considered a critical point of risk control, being the main process to control Salmonella spp. However, a study carried out in the State of Mato Grosso indicated that even after washing in chlorinated water at the authorized concentration and time, in the evisceration stages and in the pre-packaging area of a refrigerated slaughterhouse, it was possible to isolate Salmonella Ndolo, Mbandaka, and Typhimurium [12]. Other studies from around the world have already evaluated chlorine concentrations higher than those authorized by Brazil (5 ppm) and shorter exposure times for controlling Salmonella spp., for instance, in the decontamination process of Pangasius fish and in the chlorination of water used in poultry cooling systems [20,22].
Therefore, the present study seeks to determine the best point of antimicrobial activity, considering the concentration of sodium hypochlorite recommended by Brazilian authorities [21], as well as concentrations below and above it, associated with the exposure time and the temperature of the water, to enable a significant inactivation of Salmonella Enteritidis ATCC 13076 and Salmonella Schwarzengrund in vitro.
2. Results
2.1. Central Composite Rotatable Design (CCRD)
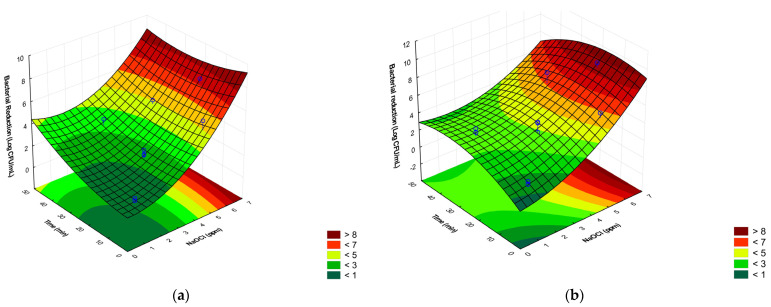
The combination of NaClO concentration, exposure time, and water temperature in relation to Salmonella Enteritidis ATCC 13076 and S. Schwarzengrund inactivation has shown statistically significant differences for both strains analyzed. For the ATCC strain, the data indicated a quadratic behavior for chlorine and a linear relationship with time. The results for the ATCC standard strain suggested that longer exposure times under the same chlorine concentration may diminish the agent’s effect (Figure 1a).
Figure 1.
Survival (Log (N0 − N) of Salmonella Enteritidis ATCC 13076 (a) and Salmonella Schwarzengrund (b) after exposure to sodium hypochlorite, time, and water temperature. Green indicates higher survival after treatments, and red indicates lower survival after treatments.
Also, it is important to note that the concentration of 5 ppm of sodium hypochlorite recommended by Brazilian legislation for 5 min and at a temperature of 5 °C was able to reduce the bacterial load from 8.3 Log10 CFU/mL to 3.1 Log10 CFU/mL, representing a reduction of 5.8 Log10 CFU/mL (point 14). As for the concentration of 5 ppm for 30 min at 5 °C, there was a reduction of 3.5 Log10 CFU/mL (point 15). It can also be seen that increasing the concentration of NaClO to 6.36 ppm had a greater influence on the reduction, reducing a population of 8.5 Log10 CFU/mL from the initial load to 7.5 Log10 CFU/mL (Table 1).
Table 1.
CCRD array for Salmonella Enteritidis ATCC 13076 and Salmonella Schwarzengrund under sodium hypochlorite concentration, exposure time, and temperature.
| Points | Chlorine (ppm) | Time (m) | Temperature (°C) | S. Enteritidis ATCC 13076 (log N0 − N) |
S. Schwarzengrund (log N0 − N) |
|---|---|---|---|---|---|
| 1 | 0.36 | 17.5 | 17.5 | 1.33 | 1.9 |
| 2 | 1 | 5 | 5 | 1.28 | 1.6 |
| 3 | 1 | 30 | 5 | 2 | 4.17 |
| 4 | 1 | 5 | 30 | 1.5 | 1.95 |
| 5 | 1 | 30 | 30 | 1.97 | 3.74 |
| 6 | 3 | 17.5 | 3.52 | 3.15 | 4.99 |
| 7 | 3 | 17.5 | 38.52 | 1.39 | 3.49 |
| 8 | 3 | 3.52 | 17.5 | 2.16 | 2.02 |
| 9 | 3 | 38.52 | 17.5 | 3.8 | 3.41 |
| 10 | 3 | 17.5 | 17.5 | 2.08 | 3.43 |
| 11 | 3 | 17.5 | 17.5 | 2.04 | 4.89 |
| 12 | 3 | 17.5 | 17.5 | 2.7 | 3.51 |
| 13 | 3 | 17.5 | 17.5 | 2.76 | 3.85 |
| 14 | 5 | 5 | 5 | 5.8 * | 4.59 |
| 15 | 5 | 30 | 5 | 3.59 | 7.28 * |
| 16 | 5 | 5 | 30 | 4.05 | 6.1 * |
| 17 | 5 | 30 | 30 | 5.19 | 5.1 |
| 18 | 6.36 | 17.5 | 17.5 | 7.52 * | 8.96 * |
* Data expressed in logarithmic scale.
Concentrations of sodium hypochlorite of ≤1 ppm were also tested with exposure times of 5 and 30 min at temperatures of 5 °C, 30 °C, and 17.5 °C (points 1–5), verifying the growth of Salmonella Enteritidis ATCC 13076 ≤ 2 Log10 CFU/mL (Table 1). The tested concentrations of 3 ppm of NaClO for this strain were maintained with a reduction of ≥2 Log10 CFU/mL.
The combination of treatments for the inactivation of Salmonella Schwarzengrund is shown in Figure 1b and Table 1. The result found for this strain was similar to that of the S. Enteritidis ATCC 13076 strain, showing a significant reduction in bacterial growth at a concentration of 5 ppm of NaClO for 30 min and 5 °C, reducing a population of 8.5 Log10 CFU/mL from the initial load to 1.3 Log10 CFU/mL, representing a reduction of 7.2 Log10 CFU/mL (point 15). Still at the same concentration of NaClO and the exposure time different, adjusting the temperature to 30 °C (point 16), it could be observed through the reduction of 9.7 Log10 CFU/mL of the initial load to 3 Log10 CFU/mL that the reduction was 6.1 Log10 CFU/mL.
At a concentration of 1 ppm of NaClO at times of 5 and 30 min and at temperatures of 5 °C, 17.5 °C, and 30 °C, bacterial reduction was not achieved, maintaining ≤ 4 Log10 CFU/mL; however, even so, it showed a reduction of almost 2 Log10 CFU/mL more than when the same NaClO concentration, time, and temperature were applied to the S. Enteritidis ATCC 13076 strain. At a concentration of 1 ppm of NaClO at times of 5 and 30 min and at temperatures of 5 °C, 17.5 °C, and 30 °C, the treatment was ineffective, maintaining ≤ 4 Log10 CFU/mL; however, even so, it showed a reduction of almost 2 Log10 CFU/mL more than when the same NaClO concentration, time, and temperature were applied to the S. Enteritidis ATCC 13076 strain.
2.2. Validation of the Inactivation Model of Salmonella Enteritidis ATCC 13076 and Salmonella Schwarzengrund
The validation of the experimental model using five random points is presented in Table 2. The results for the S. Enteritidis ATCC 13076 strain indicate that in points 1 and 3, the observed values were below the values predicted using the model; values are expressed in Log10 CFU/mL. However, in points 2, 4, and 5, the predicted values were lower than the observed values. For S. Schwarzengrund, we obtained observed values higher than the values predicted using the experimental model at all points.
Table 2.
Validation of the experimental model using random points to compare observed and predicted values.
| Points | Treatments | Salmonella Enteritidis ATCC 13076 | Salmonella Schwarzengrund | ||||
|---|---|---|---|---|---|---|---|
| Chlorine ppm | Time (min) | Temperature (°C) | * Observed | * Predicted | * Observed | * Predicted | |
| 1 | 3.5 | 2.5 | 17.5° | 2.84 | 3.400821 | 4.24 | 4.08 |
| 2 | 2.5 | 8 | 17.5° | 2.579784 | 2.397442 | 3.06694679 | 2.82 |
| 3 | 4 | 3.5 | 17.5° | 3.826391 | 4.036951 | 3.903089987 | 3.74 |
| 4 | 1.5 | 25 | 17.5° | 2.253022 | 1.983209 | 3.678766618 | 3.17 |
| 5 | 3 | 19 | 17.5° | 3.051153 | 2.641538 | 4.293204658 | 3.98 |
* Observed and predicted expressed in Log10 CFU/mL. Equation S. Enteritidis ATCC 13076: Log CFU/mL = 2.1385149129786 − 0.47257539167557 × Chlorine + 0.24174445563888 × Chlorine2 + 0.014189033415319 × Time − 0.0092024974789091 × Chlorine × Time. Equation S. Schwarzengrund: Log CFU/mL = 1.6274450806394 + 0.0042166941127924 × Chlorine + 0.14263741135157 × Chlorine2 + 0.13585792619391 × Time − 0.0024777863722644 × Time − 0.62839913
2.3. Performance of the Experimental Model and Data Adjustment
To assess the quality of the fit of the models obtained and the normality of the data as well as the normality of the residuals, the performance indices are presented in Table 3. The results indicate that the data’s normality was considered acceptable, with p > 0.05, as was the case for the residuals of the model. The determination coefficient (R2adj) was deemed suitable, being >80 for the tested strains. The MSE showed low values, indicating the absence of experimental errors. Bias factor indices (Bf), calculated by averaging the observed and predicted validation data, showed values close to 1 for both strains. The same was observed for the accuracy factor (Af), which showed values near 1, indicating a minimal mean distance and data dispersion.
Table 3.
Indices of CCRD model performance and survival of Salmonella Enteritidis ATCC 13076 and Salmonella Schwarzengrund under chlorine concentration, temperature, and exposure time.
| Models | Normality of Data (p Value) * | Normality of Residuals (p Value) * | R2adj | MSE | Bf | Af | Lack of Fit (p Value) |
|---|---|---|---|---|---|---|---|
| S. Enteritidis ATCC 13076 | 0.2971 | 0.1519 | 0.82337 | 0.1265 | 1.13 | 1.42 | 0.1100 |
| S. Schwarzengrund | 0.296 | 0.4504 | 0.83952 | 0.094 | 1.1 | 1.36 | 0.3956 |
* Normally tested using the Shapiro–Wilk test.
3. Discussion
Chlorine and its various forms are the compounds commonly used for disinfection in the food and food service industries [23]. According to Brazilian legislation, NaClO is one of the chlorinated products authorized for washing fish in the processing stages [21]. The recommended concentration is 5 ppm, as specified by MAPA and adopted by Brazilian slaughterhouses, despite the cases of isolated Salmonella strains in the processing stages in fish slaughterhouses in Brazil [12]. The use of a chemical disinfectant agent such as chlorine must be able to reduce pathogenic bacteria like Salmonella by at least 5 Log10 CFU/mL [24]. Our results support this information, as the use of 5 ppm of NaClO, an exposure time of 5 min, and a water temperature of 5 °C proved to be effective, resulting in the inactivation of 5.8 Log10 CFU/mL in Salmonella Enteritidis ATCC 13076. However, for this strain, based on statistical analyses, only the NaClO concentration was significantly affected. A significant difference was observed with a p value < 0.05, which was not the case for the variables of temperature and exposure time.
One of the factors highlighted [25] is that the effectiveness of chlorine-based disinfectants is influenced by temperature, concentration, and contact time. For the Salmonella Schwarzengrund strain, the results were similar to those of S. Enteritidis ATCC 13076. At 5 ppm, it was able to inactivate bacterial growth within 30 min at a temperature of 5 °C, reducing the microbial load by more than 7 Log10 CFU/mL. However, raising the water temperature to 30 °C with an exposure time of 5 min resulted in a reduction of 6.1 Log10 CFU/mL, indicating that higher water temperatures negatively impact the action of NaClO. Thermal resistance mechanisms are directly associated with cellular permeability, involving changes in the lipid composition of the membrane [26].
In a previous study [13], the presence of Salmonella Schwarzengrund was identified in fish processing environments that utilize NaClO. In the current study, this strain demonstrated resistance to variations in NaClO concentration. Through statistical analysis, it was determined that p < 0.05 for both the NaClO concentration and time variables, indicating that its microbial load did not decrease. The resistance of pathogens to widely used disinfectants in food companies and industries may contribute to the involvement of specific microorganisms in foodborne outbreaks [23].
Chlorinated compounds do not leave flavors in products as long as they are used in appropriate concentrations [27]. Therefore, a concentration of 6.36 ppm, higher than the recommended level, was chosen to test the hypothesis that a greater concentration results in more effective inactivation of the tested strains. Precisely because this concentration exceeds the limit, it becomes impractical for industrial use. However, our results showed a greater reduction in the microbial load at this concentration; both strains studied were reduced by more than 7 Log10 CFU/mL. It is expected that the higher the concentration of NaClO, the greater the inactivation of the microorganism. The National Health Surveillance Agency (ANVISA) approves the use of sanitizers at a concentration of 5 ppm, as this concentration is sufficient to achieve a satisfactory effect without compromising the authenticity and quality of the food, being the only sanitizer permitted to come into direct contact with the fish [21].
A study [20] demonstrated the inefficiency of chlorine used in the quality control of Pangafish (Pangasius hypophthalmus) in the fish industry. This was observed through the application of 10, 20, 50, and 120 ppm for durations of 10, 20, and 240 s, with one of the main reasons being the concentration used in the study. Therefore, in this study, we tested varying concentrations of NaClO at varying times and temperatures to optimize the use of this substance in refrigerated slaughterhouses.
Chlorinated compounds do not impart flavors to products, provided they are used in appropriate concentrations [27]. Therefore, the concentration of 6.36 ppm used in our study is unfeasible for industries as it exceeds the recommended level. Although NaClO is a widely used chemical compound, there are no documented reports in the literature about its potential risks to human health. It is important to highlight, however, that the correct use of NaClO is essential in all stages of processing fish and other food preparations, as well as in cleaning processes such as washing the inside of refrigerators. Your inappropriate use of NaClO can lead to changes in the odor or flavor of food.
Adaptations to sanitizers, which can lead to pathogenic bacteria developing resistance, should be avoided by not using them below the recommended concentrations [28]. Prudent use of NaClO is essential to maintaining its effectiveness against microorganisms [29]. We observed that concentrations of 1 ppm, 3 ppm, and 0.36 ppm were not sufficient for effective inactivation, resulting in only a 1 Log10 CFU/mL reduction (Table 2).
There is limited information regarding the effect of free chlorine concentrations on bacterial survival in fish [20], highlighting the importance and relevance of this study due to the scarcity of literature on this topic. Adequate NaClO concentration and exposure time are crucial to eliminating pathogens present on the surface of the fish, as reported in previous studies [23,30]. Disinfectant contact time and concentration can significantly affect the effectiveness of the disinfection process.
In the present study, it was verified that the Salmonella Enteritidis ATCC 13076 strain was not influenced by the contact time or temperature but by the NaClO concentration, while the Salmonella Schwarzengrund strain was influenced by the NaClO concentration and exposure time but not by the temperature. The fish washing step aims to eliminate bacteria from the processing environment, and the mucus present on the surface of the fish, where the glycoproteins released by the skin glands are located, creates a favorable environment for the growth and proliferation of undesirable microorganisms [12]. The correct washing with NaClO is a preventive factor for the control of pathogenic microorganisms in order to avoid problems in the health of the consumer who purchased this product [31].
The survival data of the Salmonella Enteritidis ATCC 13076 and Salmonella Schwarzengrund strains tested were normal (p > 0.05), as well as for the model residues, as shown in Table 2. The adjusted coefficient determination (R2adj) for both strains was considered adequate (>80). The mean square error model (MSE) indicated low values, meaning that there were no experimental errors, since MSE values symbolize possible variability and errors in the experiment [32], being able to measure the reliability of the data obtained [26].
Bias factor indices (Bf) measuring the mean agreement between predicted and observed data [33] were calculated through validation experiments with five random points that were not used in the experimental model, with results close to 1. The precision factor (Af) indicates the average distance and dispersion of the data in relation to the measure of equivalence [34]. The expected range for Af in the variables is between 0.10 and 0.15 [35]. In current experimental models, a range of 1.2 to 1.4 is considered acceptable. Our results corroborate these standards in current experimental models. The lack of fit provides an estimate of the fit of the experimental model. The lack of fit provides an estimate of how well the experimental model fits the data, determining whether the model is adequate. In the present study, this was found to be not significant for the models, with a p-value greater than 0.05, as expressed in Table 4. Thus, R2adj, MSE, Bf, Af, and lack of fit demonstrate that the experimental model was adequate to measure the survival of S. Enteritidis ATCC 13076 and S. Schwazengrund submitted to selected sodium hypochlorite concentrations during varying exposure times and at different temperatures.
Table 4.
Variables used in CCRD. Factorial design points (−1; +1), smallest and largest values used (−1.44; +1.44), and center point (0).
| Factors | Variables | ||
|---|---|---|---|
| Chlorine (ppm) | Time (m) | Temperature (°C) | |
| −1.44 | 0.36 | 3.52 | 3.52 |
| −1 | 1 | 5 | 5 |
| 0 | 3 | 17.5 | 17.5 |
| 1 | 5 | 30 | 30 |
| 1.44 | 6.36 | 38.5 | 38.5 |
In view of this, the relevance of this study is highlighted with regard to the adoption of effective procedures for the control of Salmonella spp. in fish processing environments in refrigerated slaughterhouses, because in the absence of these procedures, fish become an environment conducive to the proliferation of pathogenic microorganisms. For example, more efficient exposure times within industries combined with the use of NaClO concentrations of 5 ppm.
4. Materials and Methods
4.1. Experimental Design
To carry out this study, strains of Salmonella Enteritidis ATCC 13076 and Salmonella Schwarzengrund previously isolated [13] and stored at −80 °C were revitalized from the bacteriotheque of the Molecular Microbiology of Food Laboratory—LabMMA. A total of 18 points were performed according to the central composite rotatable design (CCRD), followed by validation of the results with 5 additional randomized experiments, followed by analysis of the results (Figure 2). All experimental steps were carried out at the Federal University of Mato Grosso—UFMT, at the Food Molecular Microbiology Laboratory (LabMMA).
Figure 2.
Schematic flowchart of the experimental design.
4.2. Preparation of the Strains
Salmonella Enteritidis ATCC 13076 and Salmonella Schwarzengrund, previously isolated from eviscerated and frozen tambaqui (Colossoma macropomum) [13], were used in the present study. Both were preserved in brain heart infusion (BHI) broth (KASVI®, São José dos Pinhais, Brazil) + 15% (v/m) glycerol (Labsynth®, São Paulo, Brazil) and stored at −80 °C.
The strains were revitalized in 9 ml of BHI broth (KASVI®, Spain) and subsequently incubated at 37 °C for 24 h. Then, the tubes containing the growing bacteria were centrifuged at 2000 rpm for 10 min. The supernatant was discarded, and the bacterial pellet was resuspended in 9 ml of saline solution (0.85%). The concentration of the initial population (N0) was quantified before the development of the experiment through surface plating.
4.3. Central Composite Rotatable Design (CCRD)
The CCRD was used under an arrangement of factors and variables, conducted at 18 points in order to evaluate the combined effect of sodium hypochlorite concentration (ppm), exposure time (minutes), and temperature (Celsius) on the survival of Salmonella spp. in fish. The central combination of the parameters, defined as the central point (3 ppm, 17.5 °C, and 17:50 min), was performed in 4 repetitions to measure possible experimental errors and lack of fit of the model (Table 1).
The quantification of the initial bacterial load was performed with inoculum at 7 log10 CFU/mL. For each CCRD experiment, 300 mL of sterile water was used to dilute 12% NaClO (Chloro MT®, Cuiabá, Brazil) at the desired concentration and at the target temperature of the experiment. NaClO concentrations were measured using the Exact® Micro7+ equipment (Rio Grande do Sul, Brazil), using the Micro7+/Micro20 free chlorine test strip (AKSO®, São José dos Pinhais, Brazil) for testing.
Then, 100 µL of bacterial suspension was added to a 9 mL test tube containing sterile water with the desired NaClO concentration, timing the time of exposure to chlorine. After counting the time, 10% thiosulfate (Labsynth®, São Paulo, Brazil) was used to neutralize the action of chlorine, and serial dilutions from 10−1 to 10−5 were performed, followed by surface plating with 100 µL of bacterial suspension in plates of glass. Petri dishes were coated with nutrient agar (KASVI®, São José dos Pinhais, Brazil) and incubated at 37 °C for 24 h. After this period, colonies were counted using an electronic plate counter (Edulab, Curitiba, Brazil).
4.4. Model of Inactivation and Validation of Salmonella Enteritidis ATCC 13076 and Salmonella Schwarzengrund
To validate the experimental model, 5 random points were analyzed to compare the values observed and predicted using the model in both strains, where NaClO concentrations and exposure time varied, being 1.5 ppm in 25 min, 2.5 ppm in 8 min, 3 ppm in 19 min, 3.5 ppm in 2 and a half minutes, and 4 ppm in 3 and a half minutes. The temperature was the same for the 5 points, being 17.5 °C. After reaching the time required for each experiment, we neutralized the action of NaClO with 10% thiosulfate (Labsynth®, Brazil), and within 24 h, we observed the surviving colonies for counting. The results were analyzed using multiple regression analysis [36].
4.5. Performance of the Experimental Model and Data Adjustment
To determine the bacterial survival (SB), the initial count (N0) was subtracted from the final count (N) in log (SB = N0 − N), and the results obtained through the variables used in this study were analyzed through the response surface methodology.
The data obtained were fitted to a polynomial model for each strain of Salmonella spp., in which the statistically significant terms (p < 0.05) of the model were maintained. The formality of model adjustment was established through the adjusted coefficient of determination (R2adj). The model mean square error (MSE) [32], performance bias factor (Bf), and accuracy factor (Af) indices [34] were calculated using the following equations:
where are the values predicted using the model, are the observed values, and is the number of experiments performed in validation [26].
4.6. Data Analysis
The experimental data from the CCRD and all other data obtained were analyzed using the Statistica 10.0 software (StartSoft Power Solutions, Tulsa, OK, USA). Data normality, residuals, and lack of fit were checked using the Shapiro–Wilk test.
5. Conclusions
It was observed that the inactivation pattern caused by sodium hypochlorite was quadratic for the ATCC strain and linear for the wild strain (S. Schwarzengrund). In this way, the results suggest that the strain originating from the aquaculture system (S. Schwarzengrund) may perhaps be combated by increasing the exposure time to NaClO, as even with the prolonged exposure, the elimination showed a linear profile (as both chlorine concentration and exposure time increase, the effectiveness of Salmonella elimination also grows).
Furthermore, the isolated strains were evaluated following the model obtained using CCRD, which demonstrated good values in quality controls (Af, Bf, and MSE). Therefore, this study suggests that increasing the fish exposure time to NaClO in the industry (specifically 30 min) could be a promising strategy for combating this pathogen in the final product. It is important to highlight that the standardization developed in this study, which involves using a combination of exposure time and NaClO concentration to reduce populations of contaminating microorganisms (pathogens), was not carried out in vivo (on fish).
Acknowledgments
To the State of Mato Grosso Research Support Foundation (FAPEMAT) for granting the scholarship to Nathaly Barros Nunes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13010068/s1, Table S1: Analysis of variance (ANOVA) of Salmonella Enteritidis ATCC 13076. This strain was not influenced by exposure time or temperature. NaClO concentration was the only variable significantly affected, with p < 0.05. Results indicated in red; Table S2: Analysis of variance (ANOVA) of Salmonella Schwarzengrund. This strain was influenced by NaClO concentration and exposure time, presenting p < 0.05. Results indicated in red.
Author Contributions
Conceptualization, N.B.N., J.O.d.R., V.S.C., M.A.M.M., A.d.C.-N. and E.E.d.S.F.; methodology, N.B.N. and J.O.d.R.; software, V.S.C.; validation, N.B.N. and J.O.d.R.; formal analysis, N.B.N. and J.O.d.R.; resources, E.E.d.S.F.; data curation, N.B.N., V.S.C. and E.E.d.S.F.; writing—preparation of the original draft, N.B.N. and E.E.d.S.F.; writing—proofreading and editing, N.B.N., V.S.C., A.d.C.-N., M.A.M.M. and E.E.d.S.F.; supervision, V.S.C. and E.E.d.S.F.; project management, E.E.d.S.F.; financing acquisition, E.E.d.S.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
Government of Mato Grosso (SEDEC-MT), Fundação de Apoio a Pesquisa de Mato Grosso (FAPEMAT), Conselho Nacional de Desenvolvimento Tecnológico e Científico—CNPq/Brazil (310181/2021-6).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Racki A.S. Master’s Thesis. Universidade Tecnológica Federal do Paraná; Paraná, Brazil: 2018. Riscos Ambientais em um Frigorífico De Peixes. Monografia de Especialização. [Google Scholar]
- 2.FAO, editor. The State of World Fisheries and Aquaculture. FAO; Rome, Italy: 2016. Contributing to Food Security and Nutrition for All. [Google Scholar]
- 3.Albuquerque A.C., Lopes A.L., Pedrini B., Dellova D., França D., Souza F., Almeida G., Nascimento G., Borielo G., Dias I., et al. Veículo Oficial da Associação Brasileira da Piscicultura. 2022. [(accessed on 10 September 2022)]. Available online: https://www.peixebr.com.br/anuario2022/
- 4.de Oliveira Sartori A.G., Amancio R.D. Pescado: Importância nutricional e consumo no Brasil. Segur. Aliment. Nutr. 2012;19:83–93. doi: 10.20396/san.v19i2.8634613. [DOI] [Google Scholar]
- 5.Li N., Wu X., Zhuang W., Xia L., Chen Y., Wu C., Rao Z., Du L., Zhao R., Yi M., et al. Fish Consumption and Multiple Health Outcomes: Umbrella Review. Trends Food Sci. Technol. 2020;99:273–283. doi: 10.1016/j.tifs.2020.02.033. [DOI] [Google Scholar]
- 6.de Araujo L.P., Ferreira L.P., Freitas M.F.U., Nunes C.d.R. Análise Microbiologica de Alimentos Minimamente Processados Comercializados em Campos Dos Goytacazes—RJ. Rev. Interdiscip. Pensamento Cient. 2020;6:187–262. doi: 10.20951/2446-6778/v6n1a15. [DOI] [Google Scholar]
- 7.Heinitz M.L., Ruble R.D., Wagner D.E., Tatini S.R. Incidence of Salmonella in Fish and Seafood. J. Food Prot. 2000;63:579–592. doi: 10.4315/0362-028X-63.5.579. [DOI] [PubMed] [Google Scholar]
- 8.de Souza Sant’Ana A. Introduction to the Special Issue: Salmonella in Foods: Evolution, Strategies and Challenges. Food Res. Int. 2012;45:451–454. doi: 10.1016/j.foodres.2012.01.003. [DOI] [Google Scholar]
- 9.Monte D.F.M., Sellera F.P., Lopes R., Keelara S., Landgraf M., Greene S., Fedorka-Cray P.J., Thakur S. Class 1 integron-borne cassettes harboring blaCARB-2 gene in multidrug-resistant and virulent Salmonella Typhimurium ST19 strains recovered from clinical human stool samples, United States. PLoS ONE. 2020;15:e0240978. doi: 10.1371/journal.pone.0240978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari R.G., Rosario D.K.A., Cunha Neto A., Mano S.B., Figueiredo E.E.S., Conte-Junior C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-Analysis. Appl. Environ. Microbiol. 2019;85:e00591-19. doi: 10.1128/AEM.00591-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown A.C., Grass J.E., Richardson L.C., Nisler A.L., Bicknese A.S., Gould L.H. Antimicrobial Resistance in Salmonella That Caused Foodborne Disease Outbreaks: United States, 2003–2012. Epidemiol. Infect. 2017;145:766–774. doi: 10.1017/S0950268816002867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes D.V.G.S., Carvalho R.C.T., Castro V.S., Cunha Neto A., Muller B., Carvalho F.T., dos Prazeres Rodrigues D., Vieira B.S., de Souza Figueiredo E.E. Salmonella in the Processing Line of Farmed Tambatinga (Colossoma Macropomum x Piaractus Brachypomus) in Mato Grosso, Brazil: Serotypes of Occurrence and Antimicrobial Profile. Trop. Anim. Health Prod. 2021;53:146. doi: 10.1007/s11250-021-02584-8. [DOI] [PubMed] [Google Scholar]
- 13.Cunha Neto A., Panzenhagen P., Carvalho L., Rodrigues D., Conte Junior C., Figueiredo E. Occurrence and Antimicrobial Resistance profile of Salmonella Isolated from Native Fish Slaughtered and Commercialised in Brazil. Arch. Leb. 2019;70:94–98. doi: 10.2376/0003-925X-70-94. [DOI] [Google Scholar]
- 14.European Food Safety Authority (EFSA) Data dictionaries—Guidelines for reporting data on zoonoses, antimicrobial resistance and food-borne outbreaks using the EFSA data models for the Data Collection Framework (DCF) EFSA Support. Publ. 2017;2:14. doi: 10.2903/sp.efsa.2017.EN-1178. [DOI] [Google Scholar]
- 15.European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 2015;12:13. doi: 10.2903/j.efsa.2015.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.dos Santos R.R., Xavier R.G.C., de Oliveira T.F., Leite R.C., Figueiredo H.C.P., Leal C.A.G. Occurrence, Genetic Diversity, and Control of Salmonella Enterica in Native Brazilian Farmed Fish. Aquaculture. 2019;501:304–312. doi: 10.1016/j.aquaculture.2018.11.034. [DOI] [Google Scholar]
- 17.Pui C., Wong W., Chai L., Tunung R., Jeyaletchumi P., Hidayah N., Ubong A., Farinazleen M., Cheah Y., Son R. Salmonella: A Foodborne Pathogen. Int. Food Res. J. 2011;18:465–473. [Google Scholar]
- 18.Dróżdż M., Małaszczuk M., Paluch E., Pawlak A. Zoonotic Potential and Prevalence of Salmonella Serovars Isolated from Pets. Infect. Ecol. Epidemiol. 2021;11:1975530. doi: 10.1080/20008686.2021.1975530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FAO, editor. The State of World Fisheries and Aquaculture. FAO; Rome, Italy: 2018. Meeting the Sustainable Development Goals. [Google Scholar]
- 20.Tong Thi A.N., Sampers I., Van Haute S., Samapundo S., Ly Nguyen B., Heyndrickx M., Devlieghere F. Decontamination of Pangasius Fish (Pangasius Hypophthalmus) with Chlorine or Peracetic Acid in the Laboratory and in a Vietnamese Processing Company. Int. J. Food Microbiol. 2015;208:93–101. doi: 10.1016/j.ijfoodmicro.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Brasil Ministério da Agricultura, Pecuária e Abastecimento . Manual de Procedimentos para Implantação de Estabelecimento Industrial de Pescado: Produtos Frescos e Congelados. Ministério da Agricultura, Pecuária e Abastecimento; Brasília, Brazil: 2007. [Google Scholar]
- 22.Tsai L.-S., Schade J.E., Molyneux B.T. Chlorination of Poultry Chiller Water: Chlorine Demand and Disinfection Efficiency. Poult. Sci. 1992;71:188–196. doi: 10.3382/ps.0710188. [DOI] [Google Scholar]
- 23.Machado T.R.M., Malheiros P.S., Brandelli A., Tondo E.C. Avaliação da resistência de Salmonella à ação de desinfetantes ácido peracético, quaternário de amônio e hipoclorito de sódio. Rev. Inst. Adolfo Lutz. 2010;69:475–481. doi: 10.53393/rial.2010.v69.32604. [DOI] [Google Scholar]
- 24.Venkitanarayanan K.S., Lin C.M., Bailey H., Doyle M.P. Inactivation of Escherichia coli O157:H7, Salmonella enteritidis, and Listeria monocytogenes on apples, oranges, and tomatoes by lactic acid with hydrogen peroxide. J. Food Prot. 2002;65:100–105. doi: 10.4315/0362-028X-65.1.100. [DOI] [PubMed] [Google Scholar]
- 25.Byun K.-H., Han S.H., Yoon J., Park S.H., Ha S.-D. Efficacy of Chlorine-Based Disinfectants (Sodium Hypochlorite and Chlorine Dioxide) on Salmonella Enteritidis Planktonic Cells, Biofilms on Food Contact Surfaces and Chicken Skin. Food Control. 2021;123:107838. doi: 10.1016/j.foodcont.2020.107838. [DOI] [Google Scholar]
- 26.Castro V.S., Rosario D.K.A., Mutz Y.S., Paletta A.C.C., Figueiredo E.E.S., Conte-Junior C.A. Modelling Inactivation of Wild-Type and Clinical Escherichia coli O26 Strains Using UV-C and Thermal Treatment and Subsequent Persistence in Simulated Gastric Fluid. J. Appl. Microbiol. 2019;127:1564–1575. doi: 10.1111/jam.14397. [DOI] [PubMed] [Google Scholar]
- 27.Saraiva A.A.M. Master’s Thesis. Universidade de Aveiro; Aveiro, Portugal: 2011. Avaliação da Eficácia de Desinfectantes da Indústria Agro-Alimentar. [Google Scholar]
- 28.Meyer B., Cookson B. Does Microbial Resistance or Adaptation to Biocides Create a Hazard in Infection Prevention and Control. J. Hosp. Infect. 2010;76:200–205. doi: 10.1016/j.jhin.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Riazi S., Matthews K.R. Failure of Foodborne Pathogens to Develop Resistance to Sanitizers Following Repeated Exposure to Common Sanitizers. Int. Biodeterior. Biodegrad. 2011;65:374–378. doi: 10.1016/j.ibiod.2010.12.001. [DOI] [Google Scholar]
- 30.Menegaro A., Flores A.F., Simer P., Silva F.I., Sbardelotto P.R.R., Pinto E.P. Sanitizantes: Concentrações e Aplicabilidade na Indústria de Alimentos. Sci. Agrar. Parana. 2016;15:171–174. doi: 10.18188/1983-1471/sap.v15n2p171-174. [DOI] [Google Scholar]
- 31.Leite S.B.P., de Arruda Sucasas L.F., Oetterer M. Resíduos da Comercialização de Pescado Marinho—Volume De Descarte E Aspectos Microbiológicos. Rev. Bras. Tecnol. Agroind. 2016;10:2112–2125. doi: 10.3895/rbta.v10n1.2692. [DOI] [Google Scholar]
- 32.te Giffel M.C., Zwietering M.H. Validation of Predictive Models Describing the Growth of Listeria Monocytogenes. Int. J. Food Microbiol. 1999;46:135–149. doi: 10.1016/S0168-1605(98)00189-5. [DOI] [PubMed] [Google Scholar]
- 33.Ross T. Indices for Performance Evaluation of Predictive Models in Food Microbiology. J. Appl. Bacteriol. 1996;81:501–508. doi: 10.1111/j.1365-2672.1996.tb03539.x. [DOI] [PubMed] [Google Scholar]
- 34.Baranyi J., Pin C., Ross T. Validating and Comparing Predictive Models. Int. J. Food Microbiol. 1999;48:159–166. doi: 10.1016/S0168-1605(99)00035-5. [DOI] [PubMed] [Google Scholar]
- 35.Ross T. Predictive Modelling of the Growth and Survival of Listeria in Fishery Products. Int. J. Food Microbiol. 2000;62:231–245. doi: 10.1016/S0168-1605(00)00340-8. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt A.F., Finan C. Linear Regression and the Normality Assumption. J. Clin. Epidemiol. 2018;98:146–151. doi: 10.1016/j.jclinepi.2017.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.