Abstract
Outer membrane porin genes of Salmonella typhimurium, including ompC, ompF, and tppB, are regulated by the products of ompB, a two-component regulatory locus encoding OmpR and EnvZ. S. typhimurium ompR mutants are attenuated in mice, but to date no one has studied the intracellular trafficking of S. typhimurium porin-deficient mutants. In this study, isogenic transposon mutants of S. typhimurium with insertions in ompR, envZ, ompF, ompC, ompD, osmZ, and tppB were compared with wild-type SL1344 for trafficking in the human epithelial cell line HeLa. We found that ompR and envZ mutants were reduced or completely inhibited for the formation of Salmonella-induced filaments (Sifs). This result was confirmed with an ompB deletion mutant. Sifs are tubular structures containing lysosomal glycoprotein which are induced specifically by intracellular Salmonella. Genetic analysis showed that the ompR mutation could be complemented in trans by cloned ompR to restore its ability to induce Sifs. In contrast, mutations in the known ompR-regulated genes ompF, ompC, and tppB (as well as the ompR-independent porin gene, ompD) had no effect on Sif formation relative to that of wild-type SL1344, thus indicating that OmpR does not exert its role on these genes to induce Sif formation. The omp mutants studied were able to invade and replicate in HeLa cells at levels comparable to those in wild-type SL1344. We conclude that OmpR and EnvZ appear to regulate Sif formation triggered by intracellular S. typhimurium.
Salmonella spp. are facultative intracellular pathogens which cause a variety of diseases in humans, ranging from acute gastroenteritis (Salmonella typhimurium) to enteric fever (Salmonella typhi) (20). S. typhimurium causes self-limiting illnesses in humans, such as gastroenteritis (food poisoning), but in mice it causes fatal enteric fever resembling human typhoid fever. Therefore, mice provide a useful animal model in which to study enteric fever. After ingestion, Salmonella organisms colonize the lower intestine and invade Peyer’s patches to gain access to the lamina propria; from there, they can be disseminated systemically. The first cellular barriers that Salmonella faces in the body are epithelial cells and M cells of Peyer’s patches (19). The mechanism by which S. typhimurium invades epithelial cells has been well studied and shown to involve many genes encoding a type III secretion system and specific secreted proteins (7, 8, 16, 18). At the site of bacterial contact with the host cell surface, S. typhimurium induces massive host membrane ruffling (6), capping of specific plasma membrane proteins (12), and macropinocytosis (11). After invasion, the host plasma membrane normalizes and the internalized bacteria reside within a host membrane-derived vacuole, where they are able to survive and replicate. The vacuolar membrane enclosing S. typhimurium acquires and maintains the host lysosomal marker, lysosomal glycoprotein (lgp), within 30 min after invasion (13). Similar mechanisms for invasion and intracellular trafficking have been reported for S. typhi (9, 25).
After cell invasion, there is a lag period in S. typhimurium growth, during which time the bacteria presumably acclimate to their new intracellular surroundings (for example, altered pH and osmolarity). Within 4 to 6 h after invasion, S. typhimurium organisms begin to replicate, and by 10 to 16 h postinvasion the bacteria fill the cell, resulting in lysis. Intracellular replication is an essential S. typhimurium virulence trait, since prototrophic nonreplicating mutants are attenuated in mice (22). Coincident with the onset of S. typhimurium replication within cultured epithelial cells is the formation of lgp-containing tubular structures that appear to connect multiple Salmonella-containing vacuoles within the cell (10). These tubular structures are induced specifically by Salmonella spp., and thus far no other known invasive bacterial pathogen, including Yersinia, Shigella, enteropathogenic Escherichia coli, and Listeria, has been shown to induce these structures (10, 32a). It is thought that these tubular structures, termed Sifs (Salmonella-induced filaments), are somehow involved in Salmonella’s ability to acquire nutrients and replicate intracellularly, since all intracellular nonreplicating mutants of S. typhimurium tested thus far are unable to induce Sifs (22).
Sif formation in epithelial cells requires the Salmonella-specific gene sifA (33). sifA encodes a single protein and is found specifically on the Salmonella chromosome located within the pot operon at approximately 59 min. It contains inverted repeats on either end to suggest horizontal transfer via transposition and shows no clear homology to genes thus far identified. sifA mutants display several features that distinguish them from the parental strain. They are unable to induce Sifs in epithelial cell lines, they replicate at a faster rate than the wild-type parent in these cells, and they are attenuated in mice (33). sifA is the only Salmonella-specific gene shown thus far to influence intracellular trafficking in HeLa cells.
Biosyntheses of some S. typhimurium (and E. coli) outer membrane porin proteins such as OmpF, OmpC, and TppB are regulated by the ompB locus (26, 29). The ompB locus encodes OmpR-EnvZ, a two-component regulatory system in which EnvZ, a transmembrane sensory protein with histidine kinase activity, controls the activity of OmpR, a transcriptional regulator, in response to changes in external environmental factors such as osmolarity, temperature, and pH. These environmental changes that regulate the activity of OmpR-EnvZ are likely encountered by Salmonella during its adaptation to the intracellular environment after invasion, and thus S. typhimurium omp mutants may be affected in their intracellular interactions in epithelial cells. It has been previously reported that ompR mutants of S. typhimurium are avirulent in a mouse model (3, 5). In addition, OmpR-EnvZ has been shown to play an important role in the virulence of Shigella flexneri (2). Given these in vivo data, we looked at several isogenic S. typhimurium strains with mutations in various porin biosynthesis genes (ompC, ompF, ompD, and tppB) and regulatory genes (osmZ, ompR, and envZ) influencing porin gene expression to determine their influence on intracellular trafficking of S. typhimurium in HeLa cells, as determined by Sif formation.
Isogenic transposon mutants affected in outer membrane protein biosynthesis in S. typhimurium SL1344, which is virulent in mice, were either obtained from the various sources listed in Table 1 or constructed by P22 transduction into wild-type strain SL1344 with P22 HT105/1 (30) as previously described (34). Outer membranes were prepared from these strains as described previously (31) and analyzed by sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis to verify the mutations as described previously (references 5 and 21 and data not shown). Lack of OmpC in mutants containing insertions in ompR, envZ, or ompC was further supported by Western blotting with monoclonal mouse anti-OmpC antibody CM 95.3 (reference 32 and data not shown). The tppB mutants were confirmed by their resistance to the antibiotic peptide alafosfalin relative to the situation for wild-type SL1344, as in the procedure of Gibson et al. (15).
TABLE 1.
Strains of S. typhimurium and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| SL1344 | Wild type | 17 |
| CJD359 | SL1344 ompR::Tn10 | 5 |
| SR-11 | Wild type | 8 |
| SWL350 | SR-11 ompR276::Mu dJ-lacZ | 23 |
| ARD3 | SL1344 ompR276::Mu dJ-lacZ-P22 from SWL350 | A. Richter-Dalfours (unpublished) |
| CJD372 | SL1344 ompC396::Tn10, ompF1006::Mu d1-8 | 3 |
| BRD455 | SL1344 ompD159::Tn10 | 3 |
| BRD454 | SL1344 ompC396::Tn10 | 3 |
| J1-3 | SL1344 sifA::Tn10 dCm | 33 |
| CJD408 | SL1344 tppB83::Mu dJ | 3 |
| CH1082 | LT2 supD zeb609::Tn10, ompF1004::Mu dJ | G. Dougan and C. F. Higgins |
| SDM1082 | SL1344 ompF1004::Mu dJ − P22 from CH1082 | This study |
| CJD409 | SL1344 ompC396::Tn10, ompF1006::Mu d1-8, tppB83::Mu dJ | 3 |
| CH1118 | LT2 envZ1005::Mu dJ | 14 |
| TT15265 | LT2 envZ1005::Mu dP | 1 |
| SDM1118 | SL1344 envZ1005::Mu dJ − P22 from CH1118 | This study |
| SDM15265 | SL1344 envZ1005::Mu dP − P22 from TT15265 | This study |
| Plasmids | ||
| pWSK29 | Cloning vector | 35 |
| pSWLOMP | pWSK29 containing ompR-envZ operon from SR-11 | 23 |
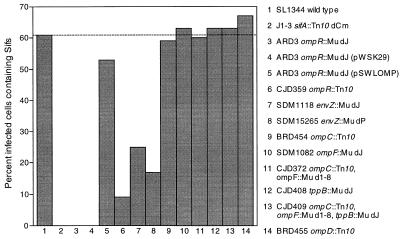
Porin-deficient S. typhimurium mutants (Table 1) were then evaluated for their abilities to induce Sifs in HeLa cells (ATCC CCL2) at 6 h postinvasion relative to the abilities of wild-type SL1344 and J1-3 (sifA::Tn10 dCm). J1-3 has previously been shown to be defective for Sif formation due to inactivation of sifA (33). HeLa cells were infected and processed for detection of Sifs by epifluorescence microscopy as previously described (25). Sif formation was assessed as the percentage of infected cells that contained Sifs, as stained by anti-lgp antibody. One hundred infected cells were counted per treatment, and each experiment was performed a minimum of three times. The results from these experiments showed that mutations in ompR and envZ (the ompB regulatory locus) render these strains completely defective or highly reduced for the induction of Sif formation (Fig. 1). Further, genetic trans complementation of ARD3 (ompR::Mu dJ) with a cloned ompB locus from S. typhimurium (pSWLOMP) (Table 1) demonstrated that the ability of this mutant to induce Sifs could be restored to wild-type levels, while the vector alone (pWSK29) had no effect on the ability of ARD3 to induce Sifs (Fig. 1). The cloned ompB locus (pSWLOMP) also restored the ability of ARD3 (ompR::Mu dJ) to produce OmpC, as shown by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (data not shown). In contrast, mutations in the genes known to be regulated by the ompB locus (ompC, ompF, and tppB) had no effect on the induction of Sifs (Fig. 1). BRD455 (ompD::Tn10) also induced Sif formation comparable to that of wild-type SL1344, as was expected, since ompD is expressed independent of the ompB locus. These results indicate that ompR and envZ are required to induce wild-type Sif formation, while the porin-deficient mutants ompC, ompF, ompD, and tppB (and multiple mutants thereof) play no apparent role in Sif formation.
FIG. 1.
ompB (ompR and envZ) mutants are defective or highly reduced for Sif formation. Induction of Sif formation in HeLa cells by the Salmonella strains listed in Table 1 was determined at 6 h postinvasion. This graph represents results from one of four experiments where cells (100 for each strain) infected by each S. typhimurium strain tested were evaluated for Sif formation at 6 h postinvasion. Values are given as the percentage of infected cells containing Sifs. The dashed line provides a reference for Sif induction by wild-type SL1344.
A S. typhimurium ompB deletion mutant was constructed in strain SL1344 to confirm the results obtained with the ompR and envZ transposon insertion mutants. S. typhimurium SL1344 ompB was PCR amplified with oligonucleotides derived from the S. typhimurium ompR and envZ genes (accession no. X12374). Oligonucleotide 327U19 (5′-CTGCGGGCGCTACTGGAAC-3′), corresponding to positions 327 to 345 in the ompR sequence, and oligonucleotide 2308L19, corresponding to positions 2308 to 2326 in the envZ sequence (5′-GACGCGAGCCACAGGAACC-3′), were used to amplify ompB from heat-disrupted S. typhimurium as described previously (33). The PCR product was cloned into pCR2.1-TOPO using the TOPO TA cloning kit (Invitrogen). An internal portion of the cloned ompB locus was deleted from bases 710 to 1649 by digestion with PmeI and SmaI and subsequent religation, introducing a stop codon at the 10th and 20th codons after the PmeI-SmaI junction. The ΔompB was cloned, by using the pCR2.1-TOPO polylinker-encoded SacI and XbaI sites, into the positive allelic exchange vector pCVD442 linearized by digestion with XbaI and SacI. Allelic exchange and selection were performed as described previously (33). The ompB lesion was confirmed by PCR, and this ompB deletion strain (MS123) was characterized for Sif formation in HeLa cells at hours 6 and 8 postinvasion. No Sif formation was observed for the ompB deletion mutant in two separate experiments when the latter was compared with wild-type SL1344. These results were comparable to the results obtained with the SL1344 ompR::Mu dJ and ompR::Tn10 strains.
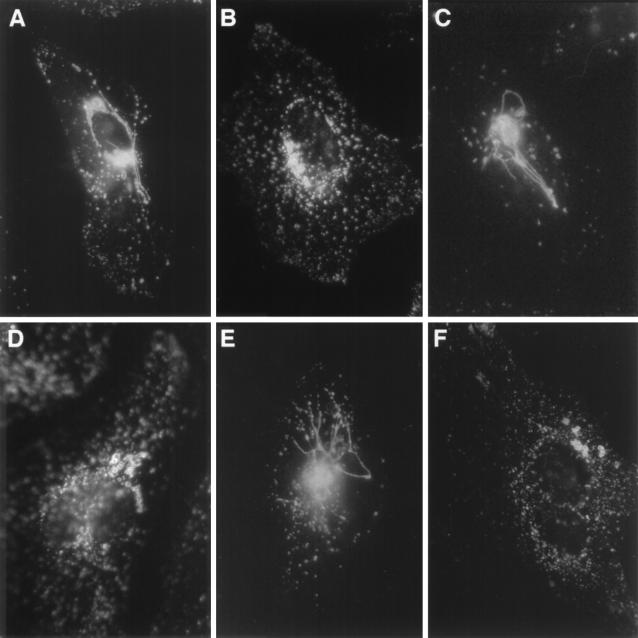
Immunofluorescence micrographs demonstrate the intracellular Sif phenotype associated with porin-deficient mutants, relative to that of wild-type SL1344 (Fig. 2A) and J1-3 (sifA::Tn10 dCm) (Fig. 2F), in HeLa cells 6 h postinvasion (Fig. 2). ompR mutants (ARD3 [Fig. 2B] and CJD359 [Fig. 2D]) were similar to the sifA mutant (J1-3 [Fig. 2F]) in that they were defective for Sif formation. Genetic trans complementation with the cloned ompB locus (pSWLOMP) was able to complement the Sif-negative phenotype of ARD3 (ompR::Mu dJ) in trans (Fig. 2C). The porin-deficient triple mutant, BRD409 (ompC::Tn10, ompF::Mu d1-8, tppB::Mu dJ), was able to induce Sifs at levels comparable to that of wild-type SL1344 (Fig. 1 and 2E).
FIG. 2.
Micrograph illustrating immunofluorescence labeling of Sifs in HeLa cells infected with wild-type SL1344 and porin-deficient mutants at 6 h postinvasion. Shown is a typical Sif (stained with anti-lgp monoclonal antibody) (A) induced by wild-type SL1344. Induction of Sif formation was defective for ompR mutants ARD3 (ompR::Mu dJ) (B) and CJD359 (ompR::Tn10) (D), a situation similar to what was previously shown for J1-3 (sifA::Tn10 dCm) (F) (33). Sif formation was restored to ARD3 (ompR::Mu dJ) (C) complemented in trans with the cloned ompB locus in pSWLOMP. Triple porin-deficient mutant BRD409 (ompC::Tn10, ompF::Mu d1-8, tppB::Mu dJ) induced Sifs similarly to wild-type SL1344 (E).
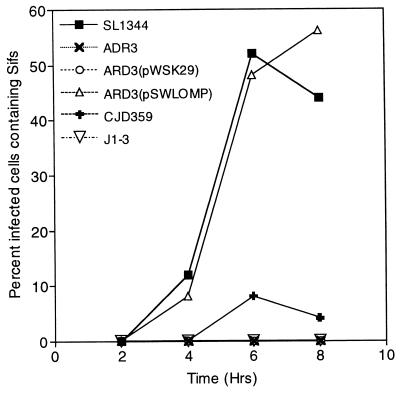
Time course experiments were performed to ascertain whether the mutants under study were defective and not altered kinetically in their abilities to induce Sif formation. In this study, we compared wild-type SL1344, ARD3 (ompR::Mu dJ), CJD359 (ompR::Tn10), and J1-3 (sifA::Tn10 dCm) for their abilities to induce Sifs at 2, 4, 6, and 8 h postinvasion. The results showed that both of the ompR mutants were inhibited or highly reduced for Sif formation over the time course studied, compared with wild-type SL1344 and J1-3 (sifA::Tn10 dCm) (Fig. 3). As shown, at 6 h (Fig. 1), the cloned ompB locus (pSWLOMP), but not the vector alone (pWSK29), was able to complement ARD3 (ompR::Mu dJ) for Sif formation comparable to that of wild-type SL1344 (Fig. 3). These results show that the ompR mutants are defective, and not kinetically altered, for induction of Sif formation.
FIG. 3.
Kinetics of Sif formation in HeLa cells. SL1344 ompR mutants were defective for Sif formation in HeLa cells over a time course of 1 to 8 h postinvasion, compared with wild-type SL1344 and J1-3 (sifA::Tn10 dCm). In these experiments, J1-3 (sifA::Tn10 dCm), ARD3 (ompR::Mu dJ), and ARD3 (pWSK29; cloning vector) did not induce any Sifs, while CJD359 (ompR::Tn10) induced the formation of Sifs at a low frequency. ARD3 complemented in trans with the cloned ompB locus (pSWLOMP) exhibited Sif formation kinetics similar to those of wild-type SL1344. This graph shows results from one of three experiments where 100 infected cells for each S. typhimurium strain were evaluated for Sif formation at 1, 2, 4, 6, and 8 h postinvasion. Results are given as percent infected cells containing Sifs.
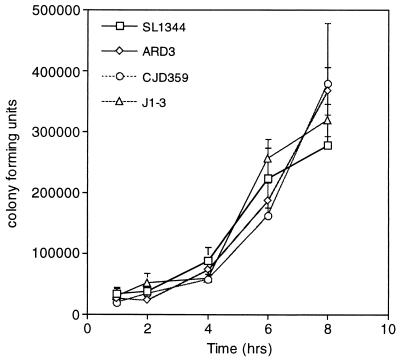
Invasion and replication by the ompR mutants (ARD3 and CJD359) were assessed to determine whether these functions, in addition to Sif formation, were affected relative to their status in wild-type SL1344 and the sifA mutant (J1-3). HeLa cell invasion and replication were assessed by means of the gentamicin protection assay as previously described (25). The results from these experiments show that invasion was not significantly affected in the ompR mutants (Fig. 4). Replication appeared to be slightly enhanced for the ompR mutants, but this was not statistically significant (Fig. 4). These experiments demonstrated that ompR mutants (and envZ mutants [data not shown]) were not adversely affected for invasion and replication.
FIG. 4.
Invasion and replication in HeLa cells are not affected in ompR mutants. This time course replication experiment shows that the ompR mutants (ARD3 and CJD359) are able to replicate as well as, if not better than, wild-type SL1344. Values are mean numbers of CFU recovered from three wells of HeLa cells (5 × 104 cells each) infected with S. typhimurium at 1, 2, 4, 6, and 8 h postinvasion. This graph depicts results from one of three representative experiments.
The present study shows that mutations in the S. typhimurium ompB locus encoding ompR and envZ render the resulting mutants defective for inducing the formation of lgp-containing tubules (Sifs) in HeLa cells. In contrast, mutations in the porin genes known to be regulated by the ompB locus in Salmonella (ompC, ompF, and tppB) had no effect on Sif formation. Disruption of ompD, a Salmonella outer membrane porin gene whose expression is OmpR independent, also had no effect on Sif formation. The role of OmpR in Salmonella pathogenesis, in relation to Sif formation, is interesting because strain SL1344 ompR mutants, such as CJD359, have previously been shown to be avirulent in mice (3, 5). This points to a correlation between earlier in vivo studies in mice and the present in vitro study in HeLa cells: virulence in mice and Sif formation both require a functional ompR gene. Whether Sif formation has any role in pathogenesis is still unclear. Previous 50% lethal dose experiments comparing wild-type SL1344 and the sifA mutant (J1-3) showed that pathogenesis of J1-3 was attenuated, although not to the extent observed for the ompR mutant (33).
In addition to these in vivo data, it has been determined that S. typhimurium ompR mutants do not induce apoptosis in the mouse macrophage cell line J774A.1 (23), while wild-type S. typhimurium does (4, 23, 27). In a study by Lingren et al., the ompR mutants were able to replicate to levels similar to those of wild-type S. typhimurium in J774A.1 cells, and therefore the noncytotoxic phenotype associated with the ompR mutants was not due to nonreplication (23). These results suggested that intracellular fusion of Salmonella-containing vacuoles was inhibited in cells infected with ompR mutants and that this accounted for their lack of cytotoxicity (23). We also observed an apparent lack of fusion of ompR or sifA mutant-containing vacuoles, which remained as individual vacuoles surrounded by an lgp-containing host membrane (Fig. 2B, D, and F). In contrast, vacuoles containing S. typhimurium strains able to induce Sifs generally contained multiple bacteria, presumably as a result of vacuole fusion (Fig. 2A, C, and E). In addition, since Sifs connect Salmonella-containing vacuoles throughout the cell (32a), lack of Sif formation may also be viewed as inhibition of fusion. The main question is, then, what selective advantage is conferred by the ability of Salmonella-containing vacuoles to fuse in macrophages or by Sif formation in epithelial cells? It is interesting to speculate that fusion provides for a dilution of host defense molecules or for the pooling of nutrients, but at the moment there is no clear answer to this question, especially since the absence of fusion does not seem to inhibit intracellular replication.
At the moment, we can only speculate that S. typhimurium uses the ompB locus to sense its intracellular surroundings after invasion and then react with appropriate gene expression leading to Sif formation. One of the ompR mutants (CJD359) and both envZ mutants (SDM1118 and SDM15265) were able to induce Sifs, albeit at low frequencies (8 to 24%) compared with those of wild-type SL1344 (62%). The other ompR mutant (ARD3) and the ompB deletion mutant (MS123) were completely defective for inducing Sifs (0%). ompR mutants were generally more reduced for induction of Sifs than envZ mutants. These results indicate that the ompB locus plays a regulatory role in the formation of Sifs rather than a direct structural role. Utilization of a two-component regulatory system to regulate intracellular virulence gene expression by S. typhimurium is already well documented, with the phoPQ system being important for macrophage survival and mouse virulence (24). We have previously found that Sif formation is not affected in HeLa cells infected with a phoPQ mutant (unpublished results). The ompR-envZ system has also previously been shown to be important for S. flexneri virulence gene expression and for regulation of expression of the Vi antigen of S. typhi without influencing invasion (2, 28). In conclusion, we have identified a two-component regulatory system (ompR-envZ) which affects Sif formation (independent of ompC, ompF, or tppB), and our findings correlate with this system’s involvement in virulence in mice.
Acknowledgments
We thank A. Richter-Dalfours and C. Pfeifer for critical reading of the manuscript. We thank C. Dorman, G. Dougan, K. Sanderson, C. H. Higgins, A. Richter-Dalfours, and S. Lingren for kindly providing strains and plasmids for this study. We also thank Ken Singh (University of Alabama, Montgomery) for providing anti-OmpC antibodies and Heidi Sawatsky of Hoffman-La Roche Limited (Missassauga, Ontario, Canada) for the kind gift of alafosfalin.
This study was supported by the Medical Research Council of Canada. B.B.F. is an MRC scientist and a Howard Hughes Fellow.
REFERENCES
- 1.Benson N R, Goldman B S. Rapid mapping in Salmonella typhimurium with Mud-P22 prophages. J Bacteriol. 1992;174:1673–1681. doi: 10.1128/jb.174.5.1673-1681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardini M L, Fontaine A, Sansonetti P J. The two-component regulatory system OmpR-EnvZ controls the virulence of Shigella flexneri. J Bacteriol. 1990;172:6274–6281. doi: 10.1128/jb.172.11.6274-6281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatfield S, Dorman C J, Hayward C, Dougan G. Role of ompR-dependent genes in Salmonella typhimurium virulence: mutants deficient in both OmpC and OmpF are attenuated in vivo. Infect Immun. 1991;59:449–452. doi: 10.1128/iai.59.1.449-452.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L M, Kaniga K, Galán J E. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 5.Dorman C J, Chatfield S, Higgins C F, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989;57:2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finlay B B, Ruschkowski S R, Dedhar S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 7.Galán J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 8.Galán J E, Curtiss R., III Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galán J E, Curtiss R., III Distribution of the invA, -B, -C, and -D genes of Salmonella typhimurium among other Salmonella serovars: invA mutants of Salmonella typhi are deficient for entry into mammalian cells. Infect Immun. 1991;59:2901–2908. doi: 10.1128/iai.59.9.2901-2908.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia del-Portillo F, Zwick M B, Leung K Y, Finlay B B. Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc Natl Acad Sci USA. 1993;90:10544–10548. doi: 10.1073/pnas.90.22.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia del-Portillo F, Finlay B B. Salmonella invasion of nonphagocytic cells induces formation of macropinosomes in the host cell. Infect Immun. 1994;62:4641–4645. doi: 10.1128/iai.62.10.4641-4645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia del-Portillo F, Pucciarelli M G, Jeffries W A, Finlay B B. Salmonella typhimurium induces selective aggregation and internalization of host cell surface proteins during invasion of epithelial cells. J Cell Sci. 1994;107:2005–2020. doi: 10.1242/jcs.107.7.2005. [DOI] [PubMed] [Google Scholar]
- 13.Garcia del-Portillo F, Finlay B B. Targeting of Salmonella typhimurium to vesicles containing lysosomal membrane glycoproteins bypasses compartments with mannose 6-phosphate receptors. J Cell Biol. 1995;129:81–97. doi: 10.1083/jcb.129.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson M M, Price M, Higgins C F. Genetic characterization and molecular cloning of the tripeptide permease (tpp) genes of Salmonella typhimurium. J Bacteriol. 1984;160:122–130. doi: 10.1128/jb.160.1.122-130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson M M, Ellis E M, Graeme-Cook K A, Higgins C F. OmpR and EnvZ are pleiotrophic regulatory proteins: positive regulation of the tripeptide permease (tppB) of Salmonella typhimurium. Mol Gen Genet. 1987;207:120–129. doi: 10.1007/BF00331499. [DOI] [PubMed] [Google Scholar]
- 16.Groisman E A, Ochman H. Cognate gene clusters govern invasion of epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J. 1993;12:3779–3787. doi: 10.1002/j.1460-2075.1993.tb06056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosieth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature (London) 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 18.Hueck C J, Hantman M J, Bajaj V, Johnson C, Lee C A, Miller S I. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol Microbiol. 1995;18:479–490. doi: 10.1111/j.1365-2958.1995.mmi_18030479.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones B D, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones B D, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Leung K Y, Finlay B B. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci USA. 1991;88:11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lingren S W, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP and phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills S D, Finlay B B. Comparison of Salmonella typhi and Salmonella typhimurium invasion, intracellular growth and localization in cultured human epithelial cell lines. Microb Pathog. 1994;17:409–423. doi: 10.1006/mpat.1994.1086. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno T, Mizushima S. Signal transduction and gene regulation through the phosphorylation of two regulatory components: the molecular basis for the osmotic regulation of the porin genes. Mol Microbiol. 1990;4:1077–1082. doi: 10.1111/j.1365-2958.1990.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 27.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickard D, Li J, Roberts M, Maskell D, Hone D, Levine M, Dougan G, Chatfield S. Characterization of defined ompR mutants of Salmonella typhi: ompR is involved in the regulation of Vi polysaccharide expression. Infect Immun. 1994;62:3984–3993. doi: 10.1128/iai.62.9.3984-3993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratt L A, Hsing W, Gibson K E, Silhavy T J. From acids to osmZ: multiple factors influence synthesis of OmpF and OmpC porins in Escherichia coli. Mol Microbiol. 1996;20:911–917. doi: 10.1111/j.1365-2958.1996.tb02532.x. [DOI] [PubMed] [Google Scholar]
- 30.Schmieger H, Backhaus H. Altered cotransduction frequencies exhibited by HT-mutants of Salmonella-phage P22. Mol Gen Genet. 1976;143:307–309. doi: 10.1007/BF00269408. [DOI] [PubMed] [Google Scholar]
- 31.Schnaitman C A, McDonald G A. Regulation of outer membrane protein synthesis in Escherichia coli K-12: deletion of ompC affects expression of the OmpF protein. J Bacteriol. 1984;159:555–563. doi: 10.1128/jb.159.2.555-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S P, Singh S R, Williams Y U, Jones L, Abdullah T. Antigenic determinants of the OmpC porin from Salmonella typhimurium. Infect Immun. 1995;63:4600–4605. doi: 10.1128/iai.63.12.4600-4605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Stein, M. A., et al. Unpublished data.
- 33.Stein M A, Leung K Y, Zwyck M, Garcia del-Portillo F, Finlay B B. Identification of a Salmonella virulence gene required for formation of filamentous structures containing lysosomal membrane glycoproteins within epithelial cells. Mol Microbiol. 1996;20:151–164. doi: 10.1111/j.1365-2958.1996.tb02497.x. [DOI] [PubMed] [Google Scholar]
- 34.Sternberg N L, Maurer R. Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella typhimurium. Methods Enzymol. 1991;204:18–43. doi: 10.1016/0076-6879(91)04004-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]