Summary
Background
Data are scarce regarding the development of hepatic decompensation in patients with non-alcoholic fatty liver disease (NAFLD) with and without type 2 diabetes. We aimed to assess the risk of hepatic decompensation in people with NAFLD with and without type 2 diabetes.
Methods
We did a meta-analysis of individual participant-level data from six cohorts in the USA, Japan, and Turkey. Included participants had magnetic resonance elastography between Feb 27, 2007, and June 4, 2021. Eligible studies included those with liver fibrosis characterisation by magnetic resonance elastography, longitudinal assessment for hepatic decompensation and death, and included adult patients (aged ≥18 years) with NAFLD, for whom data were available regarding the presence of type 2 diabetes at baseline. The primary outcome was hepatic decompensation, defined as ascites, hepatic encephalopathy, or variceal bleeding. The secondary outcome was the development of hepatocellular carcinoma. We used competing risk regression using the Fine and Gray subdistribution hazard ratio (sHR) to compare the likelihood of hepatic decompensation in participants with and without type 2 diabetes. Death without hepatic decompensation was a competing event.
Findings
Data for 2016 participants (736 with type 2 diabetes; 1280 without type 2 diabetes) from six cohorts were included in this analysis. 1074 (53%) of 2016 participants were female with a mean age of 57·8 years (SD 14·2) years and BMI of 31·3 kg/m2 (SD 7·4). Among 1737 participants (602 with type 2 diabetes and 1135 without type 2 diabetes) with available longitudinal data, 105 participants developed hepatic decompensation over a median follow-up time of 2·8 years (IQR 1·4–5·5). Participants with type 2 diabetes had a significantly higher risk of hepatic decompensation at 1 year (3·37% [95% CI 2·10–5·11] vs 1·07% [0·57–1·86]), 3 years (7·49% [5·36–10·08] vs 2·92% [1·92–4·25]), and 5 years (13·85% [10·43–17·75] vs 3·95% [2·67–5·60]) than participants without type 2 diabetes (p<0·0001). After adjustment for multiple confounders (age, BMI, and race), type 2 diabetes (sHR 2·15 [95% CI 1·39–3·34]; p=0·0006) and glycated haemoglobin (1·31 [95% CI 1·10–1·55]; p=0·0019) were independent predictors of hepatic decompensation. The association between type 2 diabetes and hepatic decompensation remained consistent after adjustment for baseline liver stiffness determined by magnetic resonance elastography. Over a median follow-up of 2·9 years (IQR 1·4–5·7), 22 of 1802 participants analysed (18 of 639 with type 2 diabetes and four of 1163 without type 2 diabetes) developed incident hepatocellular carcinoma. The risk of incident hepatocellular carcinoma was higher in those with type 2 diabetes at 1 year (1·34% [95% CI 0·64–2·54] vs 0·09% [0·01–0·50], 3 years (2·44% [1·36–4·05] vs 0·21% [0·04–0·73]), and 5 years (3·68% [2·18–5·77] vs 0·44% [0·11–1·33]) than in those without type 2 diabetes (p<0·0001). Type 2 diabetes was an independent predictor of hepatocellular carcinoma development (sHR 5·34 [1·67–17·09]; p=0·0048).
Interpretation
Among people with NAFLD, the presence of type 2 diabetes is associated with a significantly higher risk of hepatic decompensation and hepatocellular carcinoma.
Funding
National Institute of Diabetes and Digestive and Kidney Diseases.
Introduction
Non-alcoholic fatty liver disease (NAFLD) affects a third of the global adult population and is a major cause of liver-related morbidity and mortality.1-6 NAFLD includes non-alcoholic fatty liver and non-alcoholic steatohepatitis (NASH), the inflammatory form of NAFLD that can progress to fibrosis and hepatocellular carcinoma.7-14 Nearly 10% of the global population has type 2 diabetes, more than a third of individuals with type 2 diabetes have NASH, and around one in six individuals with type 2 diabetes have advanced fibrosis.15-17
Previous studies have shown that type 2 diabetes is associated with hepatic decompensation among people with cirrhosis, hepatitis C virus, and heavy alcohol consumption.8-25 However, the risk of hepatic decompensation (development of ascites, hepatic encephalopathy, or variceal bleeding) among individuals with NAFLD with and without type 2 diabetes has not been systematically assessed. Therefore, we aimed to examine the association between type 2 diabetes and liver-related outcomes through an individual participant data meta-analysis, accounting for competing risks.
Methods
Search strategy and selection criteria
We previously published an individual participant data meta-analysis26 to examine the association between liver stiffness on magnetic resonance elastography and liver-related outcomes. This individual participant-level data meta-analysis is an extension of the previous study and focuses on the association between type 2 diabetes and liver-related outcomes. A medical librarian conducted a systematic literature search of several databases from inception to April 24, 2023. The databases included MEDLINE on Ovid, Evidence-Based Medicine Reviews, the Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Scopus, Web of Science, and Embase (details of the search strategies are provided in the appendix pp 2-3).26 Additionally, experts in the field were consulted to identify additional published and unpublished primary studies. Two investigators (AMM and TN) independently reviewed the titles and abstracts of all citations identified by the search, and full-text manuscripts for potentially relevant articles were revised. Disagreements were resolved by consensus and a third reviewer (DQH) if needed.
Studies were included if they met the following inclusion criteria: (1) characterisation of liver stiffness by magnetic resonance elastography, (2) longitudinal assessment for hepatic decompensation, defined as any of the following: ascites, hepatic encephalopathy, or variceal bleeding, and death, (3) adult patients (aged ≥18 years) with NAFLD, and (4) availability of data for the presence of type 2 diabetes at baseline. NAFLD was defined as hepatic steatosis on imaging or historical liver biopsy without clinically significant alcohol consumption, secondary causes of hepatic steatosis, and other chronic underlying liver disease including viral hepatitis.
Six investigators were approached to obtain individual participant-level data. All six investigators responded and provided these data.
This individual participant data meta-analysis was conducted according to PRISMA-individual participant data guidelines. The study was approved by the institutional review board at each participating site, and analysis used de-identified data.
Data analysis
The following data from each included study were requested, extracted, and systematically checked. No major issues with data quality were identified when checking individual participant data. Demographic data were retrieved from consecutive participants, including age at the time of magnetic resonance elastography, sex, race or ethnicity, and BMI. Data for metabolic comorbidities were recorded, including hypertension, hyperlipidaemia, and type 2 diabetes. Results from the following biochemical tests were requested: albumin, glycated haemoglobin (HbA1c), alanine aminotransferase, aspartate aminotransferase, total bilirubin, alkaline phosphatase, fasting lipid panel, platelet count, international normalised ratio, sodium, and creatinine. The presence of type 2 diabetes at baseline was based on the clinical practice recommendations from the American Diabetes Association and included any of the following criteria: HbA1c greater than or equal to 6·5%; fasting plasma glucose greater than or equal to 126 mg/dL (7·0 mmol/L); plasma glucose greater than or equal to 200 mg/dL (11·1 mmol/L); or medical diagnosis of type 2 diabetes or use of medications to treat type 2 diabetes.27
NAFLD was diagnosed on imaging and clinical criteria consistent with the American Association for the Study of Liver Diseases (AASLD) NAFLD Practice Guidance.6 Liver stiffness data using two dimensional magnetic resonance elastography were obtained.
The risk of bias was assessed by two independent investigators using the QUADAS-2 tool, which consists of four key domains covering patient selection, index test, reference standard, and flow of patients through the study and timing of the index tests and reference standard.28
Outcomes
The primary outcome was hepatic decompensation, defined as any of the following: ascites, hepatic encephalopathy, or variceal bleeding, assessed by the local site investigator. Ascites was defined per AASLD guidance by imaging or physical exam.29 Hepatic encephalopathy was defined as brain dysfunction caused by liver dysfunction or portosystemic shunting, as per practice guidelines.30
The secondary outcome was the development of hepatocellular carcinoma, diagnosed by the Liver Imaging Reporting and Data System for definite hepatocellular carcinoma, Liver Reporting and Data Systems category 5, or histology.
The index date was defined as the date of the baseline magnetic resonance elastography. All participants were followed until death or the last clinical encounter and assessed by chart review. Participants with hepatic decompensation or hepatocellular carcinoma diagnosed within 1 month of the baseline magnetic resonance elastography were excluded from the analyses for incident outcomes.
Statistical analysis
Demographic, laboratory, imaging, and outcome data were presented as mean (SD) or median (IQR) for continuous variables, and as numbers and percentages for categorical variables. Baseline categorical variables were compared using the χ2 test, and continuous data were compared using the Student’s t-test for parametric data and the Wilcoxon rank-sum tests for non-parametric data. Cumulative incidence curves were generated to evaluate the risks of hepatic decompensation and hepatocellular carcinoma, after accounting for competing risks of death without hepatic decompensation, and death without hepatocellular carcinoma, respectively. Participants were censored at the time of death. We did univariable and multivariable logistic regression to determine the odds ratios (ORs) for the presence of hepatic decompensation at baseline. We did competing risk regression using the Fine and Gray subdistribution hazard ratio (sHR)31 with multivariable adjustment to estimate the likelihood of hepatic decompensation after accounting for the competing risk of death without hepatic decompensation, because of the elevated risk of non-liver related mortality in people with NAFLD.32-36 Similarly, the Fine and Gray sHR with multivariable adjustment was used to determine the likelihood of hepatocellular carcinoma after accounting for the competing risk of death without hepatocellular carcinoma. We identified potential confounders based on previous reports in the literature36-40 and selected a minimally sufficient set of confounders using a causal-directed acyclic graph (appendix p 20). The final minimally sufficient set of confounders comprised age, race or ethnicity, and BMI. The proportional hazards assumption was assessed for categorical covariates using graphs of the Kaplan-Meier estimates of the survival function and all seemed to adhere to the proportional hazards assumption. Continuous covariates were assessed by plotting scaled Schoenfeld residuals versus functions of time. All of the smoothed LOESS plots were mostly flat at 0 suggesting that the coefficients did not change over time and that the proportional hazards assumptions held. We explored the functional form of the covariates creating the smooth of a scatter plot of the Martingale residuals from a null model versus each covariate individually. The plots at the higher smoothing parameter values had similar shapes, which seemed to indicate a linear effect. Prespecified subgroup analyses were done for liver stiffness measurements on magnetic resonance elastography of 5 kPa or more and less than 5 kPa, corresponding to the thresholds recommended by the AASLD guidance to identify cirrhosis.6 All statistical analyses were performed using SAS (version 9.4), and p values of less than 0·05 were considered to indicate statistically significant differences.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Six studies were included in this individual participant-level data meta-analysis, of which five41-45 have been published, and one is unpublished (appendix p 21). All included studies were retrospective and were done at tertiary centres, with patients recruited at clinics and through newspaper advertisements. Three studies were from the USA,41,42,45 two from Japan,43,44 and one from Turkey. The risk of bias assessment is shown in the appendix (p 4).
2016 participants (1074 [53·3%] female; 942 [46·7%] male) who underwent magnetic resonance elastography between Feb 27, 2007 and June 4, 2021, and had data indicating the presence or absence of type 2 diabetes were included in this study. The mean age of participants was 57·8 years (SD 14·2) and the mean BMI was 31·3 kg/m2 (SD 7·4; table 1). At baseline, 736 participants had type 2 diabetes and 1280 did not. Participants with type 2 diabetes at baseline were older and had higher BMI, HbA1c, aspartate aminotransferase, fibrosis-4 index (FIB-4), NAFLD fibrosis score, and liver stiffness on magnetic resonance elastography than participants without type 2 diabetes at baseline (table 1).
Table 1.
Baseline characteristics of participants
| Empty Cell | Empty Cell | Overall (n=2016) |
Participants without type 2 diabetes (n=1280) |
Participants with type 2 diabetes (n=736) |
p value |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, years | 57·8 (14·2) | 55·0 (14·9) | 62·8 (11·4) | <0·0001 | |
| Sex | |||||
| Female | 1074 (53·3%) | 673 (52·6%) | 401 (54·5%) | 0·41 | |
| Male | 942 (46·7%) | 607 (47·4%) | 335 (45·5%) | .. | |
| BMI, kg/m2 | 31·3 (7·4) | 31·0 (7·4) | 31·9 (7·5) | 0·011 | |
| Race | |||||
| White | 1184 (58·7%) | 786 (61·4%) | 398 (54·1%) | <0·0001 | |
| Hispanic | 161 (8·0%) | 89 (6·9%) | 72 (9·8%) | .. | |
| Asian | 605 (30·0%) | 353 (27·6%) | 252 (34·2%) | .. | |
| Other | 66 (3·3%) | 52 (4·1%) | 14 (1·9%) | .. | |
| Biochemical profile | |||||
| HbA1c, % | 6·2% (5·6 to 7·2) | 5·8% (5·4 to 6·2) | 6·8% (6·1 to 7·8) | <0·0001 | |
| HbA1c, mmol/mol | 44 (38 to 55) | 40 (36 to 44) | 51 (43 to 62) | <0·0001 | |
| Aspartate aminotransferase, U/L | 40·0 (28 to 59) | 38·0 (27 to 57) | 45·0 (30 to 65) | <0·0001 | |
| Alanine aminotransferase, U/L | 45·0 (28 to 75) | 46·0 (28 to 76) | 44·0 (30 to 70) | 0·64 | |
| Alkaline phosphatase, U/L | 109·0 (76 to 228) | 103·0 (73 to 206) | 124·0 (84 to 267) | <0·0001 | |
| Total bilirubin, mg/dL | 10·3 (6·8 to 15·4) | 10·3 (6·8 to 15·4) | 10·3 (6·8 to 16·9) | 0·87 | |
| Albumin, g/L | 43 (39 to 45) | 43 (40 to 46) | 42 (38 to 44) | <0·0001 | |
| Triglycerides, mg/dL | 1·6 (1·2 to 2·3) | 1·6 (1·1 to 2·2) | 1·8 (1·3 to 2·4) | <0·0001 | |
| HDL, mg/dL | 1·2 (0·9–1·5) | 1·2 (1·0 to 1·5) | 1·2 (1·0 to 1·4) | <0·0001 | |
| LDL, mg/dL | 2·7 (2·1 to 3·4) | 2·8 (2·2 to 3·5) | 2·6 (2·0–3·3) | 0·0004 | |
| Platelet count, 109/L | 197 (142 to 253) | 210 (153 to 266) | 172 (123·5 to 227) | <0·0001 | |
| International normalised ratio | 1·02 (0·99 to 1·10) | 1·00 (0·99 to 1·1) | 1·05 (1 to 1·11) | 0·0056 | |
| Clinical scores | |||||
| Fibrosis-4 index | 1·83 (1·11 to 3·23) | 1·54 (0·96 to 2·54) | 2·44 (1·56 to 4·07) | <0·0001 | |
| NAFLD fibrosis score | −0·59 (−1·97 to 0·90) | −1·34 (−2·52 to −0·20]) | 0·78 (−0·35 to 1·98) | <0·0001 | |
| MELD score | 7·0 (6 to 9) | 7·0 (6 to 9) | 7·0 (6 to 9) | 0·19 | |
| Imaging | |||||
| Magnetic resonance elastography, kPa | 4·14 (2·18) | 3·59 (1·82) | 5·10 (2·42) | <0·0001 | |
| Liver stiffness, kPa | |||||
| <5 | 1485 (73·7%) | 1070 (83·6%) | 415 (56·4%) | <0·0001 | |
| ≥5 | 531 (26·3%) | 210 (16·4%) | 321 (43·6%) | .. | |
Data are mean (SD), n (%), or median (IQR). HbA1c=glycated haemoglobin. NAFLD=non-alcoholic fatty liver disease.
114 (5·7%) of 2016 participants (70 participants with type 2 diabetes; 44 participants without type 2 diabetes) had hepatic decompensation at baseline (or within 30 days of the index date); 159 events [ascites, n=91; hepatic encephalopathy, n=43; variceal haemorrhages, n=25). The presence of type 2 diabetes was associated with hepatic decompensation at baseline (OR 2·95 [95% CI 2·00–4·36]; p<0·0001). The association of type 2 diabetes with hepatic decompensation at baseline remained consistent on multivariable analysis (adjusted OR 3·08 [95% CI 1·98–4·78]; p<0·0001) even after adjustment for age, BMI, and race or ethnicity (appendix p 5).
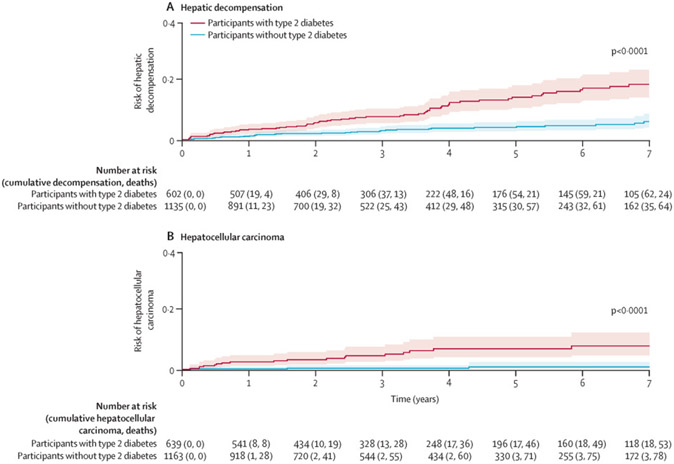
After excluding participants with hepatic decompensation at baseline (n=114), and those without follow-up data (n=165; appendix p 6), 1737 participants (602 with type 2 diabetes and 1135 without type 2 diabetes) were included in the analysis for incident hepatic decompensation. Over a median follow-up of 2·8 years (IQR 1·4–5·5), 205 (11·8%) of 1737 participants developed hepatic decompensation or died without hepatic decompensation; 105 participants (68 with type 2 diabetes, 37 without type 2 diabetes) developed hepatic decompensation (155 events [ascites, n=87; hepatic encephalopathy, n=50; variceal bleeding, n=18), while 100 participants (31 with type 2 diabetes, 69 without type 2 diabetes) died without previous hepatic decompensation. The total number of person-years at risk in participants with type 2 diabetes were 2269 and 3984 among participants without type 2 diabetes. The 1-year, 3-year, and 5-year risks of incident hepatic decompensation were 3·37% (95% CI 2·10–5·11), 7·49% (5·36–10·08), and 13·85% (10·43–17·75) in participants with type 2 diabetes at baseline, and 1·07% (0·57–1·86), 2·92% (1·92–4·25), and 3·95% (2·67–5·60) in participants without type 2 diabetes at baseline (p<0·0001; figure 1A).
Figure 1: Risk of hepatic decompensation (A) and hepatocellular carcinoma (B) in participants with non-alcoholic fatty liver disease, with death as a competing risk, stratified by type 2 diabetes status.
Hepatic decompensation was defined as ascites, hepatic encephalopathy, or variceal bleeding. Cumulative cases of decompensation, deaths, and hepatocellular carcinoma are shown until year 7 of follow-up. Graphs are truncated as the number of participants at risk was low after 7 years.
The presence of type 2 diabetes at baseline was associated with the development of incident hepatic decompensation (sHR 3·29 [95% CI 2·21–4·90]; p<0·0001). Type 2 diabetes remained an independent predictor of incident hepatic decompensation (2·15 [1·39–3·34]; p=0·0006) after adjustment for age, BMI, and race or ethnicity and accounting for competing risks (table 2). HbA1c was an independent predictor for incident hepatic decompensation (1·31 [1·10–1·55]; p=0·0019), after adjustment for age, BMI, and race or ethnicity (appendix p 7).
Table 2.
Predictors of incident hepatic decompensation, accounting for death without hepatic decompensation as a competing risk
| Empty Cell | Univariable sHR (95% CI) |
p value | Multivariable sHR (95% CI) |
p value |
|---|---|---|---|---|
| Age (years) | 1·05 (1·03–1·06) | <0·0001 | 1·05 (1·03–1·07) | <0·0001 |
| BMI (kg/m2) | 1·03 (1·00–1·05) | 0·027 | 1·03 (1·00–1·06) | 0·058 |
| Race (White vs non-White) | 1·64 (1·05–2·56) | 0·029 | 1·82 (1·09–3·04) | 0·022 |
| Presence of type 2 diabetes (vs no type 2 diabetes) | 3·29 (2·21–4·90) | <0·0001 | 2·15 (1·39–3·34) | 0·0006 |
Incident hepatic decompensation was defined as ascites, hepatic encephalopathy, or variceal bleeding. sHR=subdistribution hazard ratio.
After excluding participants with hepatocellular carcinoma at baseline (n=47), and those without follow-up data (n=167), 1802 participants (639 with type 2 diabetes and 1163 without type 2 diabetes) were included in the analysis for incident hepatocellular carcinoma. Over a median follow-up of 2·9 years (IQR 1·4–5·7), 22 participants (18 with type 2 diabetes; four without type 2 diabetes) developed incident hepatocellular carcinoma. The total number of person-years at risk for hepatocellular carcinoma was 2472 person-years in participants with type 2 diabetes and 4139 person-years in participants without type 2 diabetes. The 1-year, 3-year, and 5-year risks of incident hepatocellular carcinoma were 1·34% (95% CI 0·64–2·54), 2·44% (1·36–4·05), and 3·68% (2·18–5·77) in participants with type 2 diabetes at baseline and 0·09% (0·01–0·50), 0·21% (0·04–0·73), and 0·44% (0·11–1·33) in participants without type 2 diabetes at baseline (p<0·0001; figure 1B).
Type 2 diabetes at baseline was associated with the development of incident hepatocellular carcinoma (sHR 7·72 [95% CI 2·61–22·87]; p=0·0002). Type 2 diabetes remained an independent predictor of incident hepatocellular carcinoma (5·34 [1·67–17·09]; p=0·0048) after adjustment for age, BMI, and race or ethnicity and accounting for competing risks (table 3). HbA1c was an independent predictor of incident hepatocellular carcinoma (1·32 [1·02–1·71]; p=0·034), after adjusting for confounders (appendix p 8).
Table 3.
Predictors of incident hepatocellular carcinoma, accounting for death without hepatocellular carcinoma as a competing risk
| Empty Cell | Univariable sHR (95% CI) |
p value |
Multivariable sHR (95% CI) |
p value |
|---|---|---|---|---|
| Age (years) | 1·07 (1·03–1·11) | 0·0006 | 1·05 (1·01–1·09) | 0·029 |
| BMI (kg/m2) | 0·96 (0·90–1·03) | 0·27 | 0·99 (0·91–1·08) | 0·77 |
| Race (White vs non-White) | 0·44 (0·19–1·05) | 0·064 | 0·65 (0·23–1·84) | 0·42 |
| Presence of type 2 diabetes (vs no type 2 diabetes) | 7·72 (2·61–22·87) | 0·0002 | 5·34 (1·67–17·09) | 0·0048 |
sHR=subdistribution hazard ratio.
Type 2 diabetes remained an independent predictor for hepatic decompensation (sHR 1·90 [95% CI 1·21–2·96]; p=0·005; figure 2A; appendix p 9) and hepatocellular carcinoma (5·50 [1·63–15·67]; p=0·005; figure 2B; appendix p 10) after adjustment for liver stiffness on magnetic resonance elastography. Among participants with liver stiffness on magnetic resonance elastography of less than 5 kPa at baseline (n=1312), the 1-year, 3-year, and 5-year risk of incident hepatic decompensation was 1·21% (95% CI 0·41–2·90), 2·70% (1·26–5·06), and 4·48% (2·29–7·77) in participants with type 2 diabetes, and 0·23% (0·05–0·80), 0·94% (0·42–1·88), and 1·39% (0·67–2·58) in participants without type 2 diabetes (p=0·0038 for 5-year risk). Among participants with liver stiffness on magnetic resonance elastography of 5 kPa or higher at baseline (n=425), the 1-year, 3-year, and 5-year risk of incident hepatic decompensation was 6·45% (95% CI 3·77–10·12), 14·45% (9·90–19·81), and 27·96% (20·42–35·97) in participants with type 2 diabetes versus 5·62% (2·75–9·96), 13·22% (8·05–19·70), and 16·99% (10·67–24·56) in participants without type 2 diabetes (p=0·13 for 5-year risk). Type 2 diabetes was an independent predictor of hepatic decompensation in participants without cirrhosis (sHR 2·48 [95% CI 1·10–5·61]; p=0·029; appendix p 11), but not those with cirrhosis (appendix p 12). Type 2 diabetes was an independent predictor of hepatocellular carcinoma among participants with cirrhosis (5·25 [95% CI 1·12–24·67]; p=0·036; appendix p 13), but not those without cirrhosis (appendix p 14); however, these analyses were limited by the small number of events in participants without cirrhosis.
Figure 2: sHR for hepatic decompensation (A) and hepatocellular carcinoma (B).
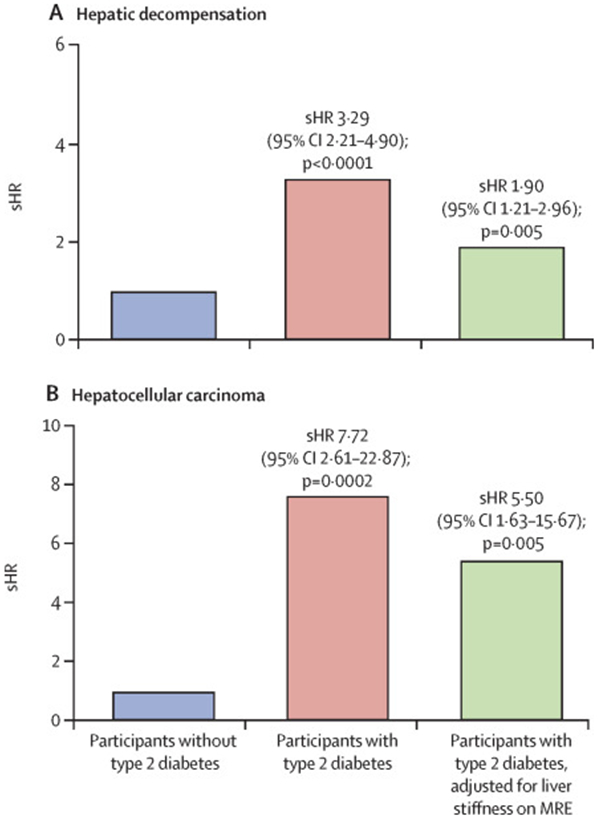
sHR=subdistribution=hazard ratio. MRE=magnetic resonance elastography.
We assessed the impact of HbA1c as a predictor for hepatic decompensation, stratified by type 2 diabetes status, and a similar trend was observed in participants with type 2 diabetes (1·21 [95% CI 0·99–1·49]; p=0·066; appendix p 15), but not in those without type 2 diabetes (1·40 [0·85–2·30]; p=0·19; appendix p 16). We included sex in the multivariable models and determined consistent results with the main analyses (appendix pp 17-19).
Discussion
In this meta-analysis of individual participant-level data from six international centres, we determined that type 2 diabetes was strongly associated with hepatic decompensation in NAFLD after accounting for appropriate competing risks. The risk of hepatic decompensation was significantly higher in participants with type 2 diabetes than participants without type 2 diabetes. Type 2 diabetes remained an independent predictor of hepatic decompensation after adjustment for multiple confounders and baseline liver stiffness. A higher HbA1c was independently associated with hepatic decompensation after adjustment for confounders. Additionally, type 2 diabetes was associated with a significantly higher risk of hepatocellular carcinoma development and was an independent predictor of hepatocellular carcinoma development after adjustment for confounding factors and baseline liver stiffness.
A prospectively recruited cohort of patients aged 50 years and older with type 2 diabetes showed that the prevalence of advanced liver fibrosis was 14%, although the study was cross-sectional and did not evaluate long-term outcomes.17 A study of 447 patients with NAFLD and paired biopsies determined a faster fibrosis progression rate in patients with type 2 diabetes than those without type 2 diabetes, which is likely to contribute to the higher risk of decompensation and hepatocellular carcinoma in type 2 diabetes.46 The findings of a previous study of 132 patients with NAFLD showed that type 2 diabetes was independently associated with mortality related to liver disease.47 The current study builds on these data by providing high-level evidence that patients with type 2 diabetes are at higher risk for liver-related events. An individual participant data meta-analysis on the risk of liver-related outcomes in participants with NAFLD with and without type 2 diabetes has not been reported, and the current study fills this knowledge gap.
To our knowledge, this is the first pooled analysis of individual participant-level data to assess the risk of hepatic decompensation in patients with type 2 diabetes versus patients without type 2 diabetes. Strengths of our study include the use of individual participant-level data, a large sample size, and ethnically diverse participants from six international centres. However, the study had limitations. The data were retrospectively collected and hence might be subject to bias associated with retrospective studies. There could be unmeasured confounders, such as a family history of cirrhosis, which were not accounted for in the multivariable analyses. Moreover, the data were collected at tertiary centres, which might have introduced a degree of selection bias for participants who were at higher risk of decompensation. We were unable to provide longitudinal data for HbA1c to determine if a reduction in HbA1c was associated with a reduced risk of decompensation. We did not have data on the effect of type 2 diabetes treatment on hepatic decompensation. The follow-up time was modest, and future studies with longer follow-ups might be helpful. The search was also restricted to studies in English, which could have introduced selection bias.
These data have important implications for clinical practice. Type 2 diabetes, and inadequate glycaemic control, were associated with a higher risk of hepatic decompensation and hepatocellular carcinoma in participants with NAFLD. These data highlight the importance of ensuring comparable proportions of participants with type 2 diabetes in the treatment and control groups of clinical trials in NAFLD. These findings indicate the need for a concerted global effort to reduce the morbidity of NAFLD associated with type 2 diabetes.
People with type 2 diabetes have a significantly higher risk of hepatic decompensation and hepatocellular carcinoma than people without type 2 diabetes. Suboptimal glycaemic control is associated with a higher risk of hepatic decompensation and hepatocellular carcinoma. The higher risk of hepatic decompensation and hepatocellular carcinoma in people with type 2 diabetes should be considered when designing clinical trials in NAFLD. These data serve as a call to action to prevent type 2 diabetes and reduce the growing burden of NAFLD and NAFLD-related hepatocellular carcinoma.
Data sharing
Individual participant data will not be made available to maintain participant confidentiality.
Supplementary Material
Research in context.
Evidence before this study
Data regarding the long-term risk of hepatic decompensation in people with non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes are scarce. We searched PubMed for systematic reviews published from database inception until April 24, 2023, using the search terms “nonalcoholic fatty liver disease” and “diabetes mellitus”, but did not identify any individual participant-level data meta-analysis assessing the risk of hepatic decompensation and hepatocellular carcinoma in people with and without type 2 diabetes.
Added value of this study
This meta-analysis of individual participant-level data from participants with NAFLD from six international sites provides high-level evidence that people with type 2 diabetes are at substantially higher risk of hepatic decompensation and hepatocellular carcinoma, compared to people without type 2 diabetes. The association between type 2 diabetes, hepatic decompensation, and hepatocellular carcinoma remained consistent even after adjusting for baseline liver stiffness. This study determined that suboptimal glycaemic control is associated with a higher risk of hepatic decompensation and hepatocellular carcinoma.
Implications of all the available evidence
These data highlight the importance of ensuring comparable proportions of participants with type 2 diabetes in the treatment and control arms of clinical trials in NAFLD. Urgent measures are required to prevent type 2 diabetes to slow the growing burden of NAFLD and NAFLD-related hepatocellular carcinoma.
Acknowledgments
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK120515). RL receives funding support from the National Institute of Environmental Health Sciences (5P42ES010337), National Center for Advancing Translational Sciences (5UL1TR001442), National Institute of Diabetes and Digestive and Kidney Diseases (U01DK130190, U01DK061734, R01DK106419, P30DK120515, R01DK121378, R01DK124318), and the National Heart, Lung, and Blood Institute (P01HL147835). DQH receives funding support from the Singaporean Ministry of Health National Medical Research Council (NMRC) under the NMRC Research Training Fellowship (MOH-000595–01).
Footnotes
Declaration of interests
RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89bio, Terns Pharmaceuticals, and Viking Therapeutics; and received research grants (via his institution) from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes, and Terns Pharmaceuticals; is a cofounder of LipoNexus; and has stock options in 89bio and Sagimet Biosciences. DQH has served as a consultant for Gilead Sciences and Eisai. AMA received grants from Novo Nordisk, Pfizer, and Target Pharma; payments were made to her institution. MN received grants from Allergan, Akero, Bristol-Myer Squibb, Gilead, Galectin, Genfit, GlaxoSmithKline, Conatus, Corcept, Enanta, Madrigal, Novartis, Novo Nordisk, Shire, Takeda, Terns, Viking, and Zydus. MN serves as a consultant to Altimmune, Boehringer-Ingelheim, Cytodyn, 89bio, EchoSens, GlaxoSmithKline, Madrigal, Merck, Novo Nordisk, Prespecturm, Roche diagnostic and Siemens, Terns, and Takeda; received speaker fees from Novo Nordisk, EchoSens; served as an advisory board or data safety monitoring board for Altimmune, Boehringer-Ingelheim, Cytodyn, 89bio, EchoSens, GlaxoSmithKline, Madrigal, Merck, Novo Nordisk, Prespecturm, Roche diagnostic and Siemens Altimmune, Boehringer-Ingelheim, Cytodyn, 89bio, EchoSens, GlaxoSmithKline, Madrigal, Merck, Novo Nordisk, Prespecturm, Roche diagnostic and Siemens, Terns and Takeda; and owns stock/stock options in Rivus Pharma, CIMA, and ChronWell. All other authors declare no competing interests.
Contributor Information
Daniel Q Huang, NAFLD Research Center, Division of Gastroenterology and Hepatology, Department of Medicine, University of California San Diego, La Jolla, CA, USA; Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Division of Gastroenterology and Hepatology, Department of Medicine, National University Health System, Singapore.
Nabil Noureddin, NAFLD Research Center, Division of Gastroenterology and Hepatology, Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Veeral Ajmera, NAFLD Research Center, Division of Gastroenterology and Hepatology, Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Maral Amangurbanova, NAFLD Research Center, Division of Gastroenterology and Hepatology, Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Ricki Bettencourt, NAFLD Research Center, Division of Gastroenterology and Hepatology, Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Emily Truong, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Tolga Gidener, Division of Gastroenterology and Hepatolog, Mayo Clinic, Rochester, MN, USA.
Harris Siddiqi, NAFLD Research Center, Division of Gastroenterology and Hepatology, Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Abdul M Majzoub, Evidence-Based Practice Center, Mayo Clinic, Rochester, MN, USA; Division of Gastroenterology and Hepatology, Department of Medicine, University of Missouri, Columbia, MO, USA.
Tarek Nayfeh, Evidence-Based Practice Center, Mayo Clinic, Rochester, MN, USA.
Nobuharu Tamaki, NAFLD Research Center, Division of Gastroenterology and Hepatology, Department of Medicine, University of California San Diego, La Jolla, CA, USA; Department of Gastroenterology and Hepatology, Musashino Red Cross Hospital, Tokyo, Japan.
Namiki Izumi, Department of Gastroenterology and Hepatology, Musashino Red Cross Hospital, Tokyo, Japan.
Masato Yoneda, Department of Gastroenterology and Hepatology, Yokohama City University, Yokohama, Japan.
Atsushi Nakajima, Department of Gastroenterology and Hepatology, Yokohama City University, Yokohama, Japan.
Ramazan Idilman, Department of Gastroenterology, School of Medicine, Ankara University, Ankara, Turkey.
Mesut Gumussoy, Department of Gastroenterology, School of Medicine, Ankara University, Ankara, Turkey.
Digdem Kuru Oz, Department of Radiology, School of Medicine, Ankara University, Ankara, Turkey.
Ayse Erden, Department of Radiology, School of Medicine, Ankara University, Ankara, Turkey.
Alina M Allen, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA.
Mazen Noureddin, Houston Methodist Transplant Center, Houston, TX, USA; Houston Liver Institute, Houston, TX, USA.
Rohit Loomba, NAFLD Research Center, Division of Gastroenterology and Hepatology, Department of Medicine, University of California San Diego, La Jolla, CA, USA.
References
- 1.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021; 184: 2537–64. [DOI] [PubMed] [Google Scholar]
- 2.Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 2020; 72: 1605–16. [DOI] [PubMed] [Google Scholar]
- 3.Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2022; 7: 851–61. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023; 77: 1335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chew NWS, Ng CH, Tan DJH, et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab 2023; 35: 414–428. [DOI] [PubMed] [Google Scholar]
- 6.Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023; 77: 1797–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013; 10: 686–90. [DOI] [PubMed] [Google Scholar]
- 8.Anstee QM, Castera L, Loomba R. Impact of non-invasive biomarkers on hepatology practice: past, present and future. J Hepatol 2022; 76: 1362–78. [DOI] [PubMed] [Google Scholar]
- 9.Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab 2022; 34: 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loomba R, Abdelmalek MF, Armstrong MJ, et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol 2023; 8: 511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan DJH, Setiawan VW, Ng CH, et al. Global burden of liver cancer in males and females: changing etiological basis and the growing contribution of NASH. Hepatology 2023; 77: 1150–63. [DOI] [PubMed] [Google Scholar]
- 12.Tan DJH, Ng CH, Lin SY, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol 2022; 23: 521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang DQ, Terrault NA, Tacke F, et al. Global epidemiology of cirrhosis—aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol 2023; published March 28. 10.1038/s41575-023-00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021; 18: 223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019; 71: 793–801. [DOI] [PubMed] [Google Scholar]
- 16.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019; 157: 107843. [DOI] [PubMed] [Google Scholar]
- 17.Ajmera V, Cepin S, Tesfai K, et al. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J Hepatol 2022; 78: 471–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015; 62: 1148–55. [DOI] [PubMed] [Google Scholar]
- 19.Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care 2021; 44: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005; 42: 132–38. [DOI] [PubMed] [Google Scholar]
- 21.Yang JD, Ahmed F, Mara KC, et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology 2020; 71: 907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer 2012; 130: 1639–48. [DOI] [PubMed] [Google Scholar]
- 23.Noureddin N, Noureddin M, Singh A, Alkhouri N. Progression of nonalcoholic fatty liver disease-associated fibrosis in a large cohort of patients with type 2 diabetes. Dig Dis Sci 2022; 67: 1379–88. [DOI] [PubMed] [Google Scholar]
- 24.Elkrief L, Chouinard P, Bendersky N, et al. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology 2014; 60: 823–31. [DOI] [PubMed] [Google Scholar]
- 25.Åberg F, Helenius-Hietala J, Puukka P, Färkkilä M, Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology 2018; 67: 2141–49. [DOI] [PubMed] [Google Scholar]
- 26.Ajmera V, Kim BK, Yang K, et al. Liver stiffness on magnetic resonance elastography and the MEFIB index and liver-related outcomes in nonalcoholic fatty liver disease: a systematic review and meta-analysis of individual participants. Gastroenterology 2022; 163: 1079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes–2018. Diabetes Care 2018; 41 (suppl 1): S13–27. [DOI] [PubMed] [Google Scholar]
- 28.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: background document. http://www.bristol.ac.uk/media-library/sites/quadas/migrated/documents/background-doc.pdf (accessed Feb 11, 2022). [Google Scholar]
- 29.Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2021; 74: 1014–48. [DOI] [PubMed] [Google Scholar]
- 30.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014; 60: 715–35. [DOI] [PubMed] [Google Scholar]
- 31.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 32.Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut 2021; 70: 1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen VL, Oliveri A, Miller MJ, et al. PNPLA3 genotype and diabetes identify patients with nonalcoholic fatty liver disease at high risk of incident cirrhosis. Gastroenterology 2023; 164: 966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jepsen P, Vilstrup H, Andersen PK. The clinical course of cirrhosis: the importance of multistate models and competing risks analysis. Hepatology 2015; 62: 292–302. [DOI] [PubMed] [Google Scholar]
- 35.Pennisi G, Enea M, Romero-Gomez M, et al. Liver-related and extrahepatic events in patients with non-alcoholic fatty liver disease: a retrospective competing risks analysis. Aliment Pharmacol Ther 2022; 55: 604–15. [DOI] [PubMed] [Google Scholar]
- 36.Sanyal AJ, Van Natta ML, Clark J, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med 2021; 385: 1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology 2011; 54: 555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balakrishnan M, Patel P, Dunn-Valadez S, et al. Women have a lower risk of nonalcoholic fatty liver disease but a higher risk of progression vs men: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021; 19: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed HS, Pedersen N, Jayanna MB, Ten Eyck P, Sanchez A, Murali AR. Predictive factors and time to development of hepatic decompensation in patients with non-alcoholic fatty liver disease. J Gen Intern Med 2020; 35: 1523–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rich NE, Oji S, Mufti AR, et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 16: 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gidener T, Ahmed OT, Larson JJ, et al. Liver stiffness by magnetic resonance elastography predicts future cirrhosis, decompensation, and death in NAFLD. Clin Gastroenterol Hepatol 2021; 19: 1915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han MAT, Vipani A, Noureddin N, et al. MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: a multicenter study. Liver Int 2020; 40: 2242–51. [DOI] [PubMed] [Google Scholar]
- 43.Matsui N, Imajo K, Yoneda M, et al. Magnetic resonance elastography increases usefulness and safety of non-invasive screening for esophageal varices. J Gastroenterol Hepatol 2018; 33: 2022–28. [DOI] [PubMed] [Google Scholar]
- 44.Tamaki N, Higuchi M, Kurosaki M, Loomba R, Izumi N. Risk difference of liver-related and cardiovascular events by liver fibrosis status in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2022; 20: 1171–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ajmera V, Nguyen K, Tamaki N, Sharpton S, Bettencourt R, Loomba R. Prognostic utility of magnetic resonance elastography and MEFIB index in predicting liver-related outcomes and mortality in individuals at risk of and with nonalcoholic fatty liver disease. Therap Adv Gastroenterol 2022; 15: 17562848221093869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang DQ, Wilson LA, Behling C, et al. Fibrosis progression rate in biopsy-proven nonalcoholic fatty liver disease among people with diabetes versus people without diabetes: a multicenter study. Gastroenterology 2023; published online April 29. 10.1053/j.gastro.2023.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 2004; 2: 262–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.