Summary
Background
Approximately 90% of children with cancer live in low-income and middle-income countries (LMICs), where 5-year survival is lower than 20%. Treatment-related mortality in high-income countries is approximately 3–5%; however, in LMICs, treatment-related mortality has been reported in up to 45% of children with cancer. This study aimed to systematically explore the burden of treatment-related mortality in children with cancer in LMICs and to explore the association between country income level and treatment-related mortality.
Methods
For this systematic review and meta-analysis we identified articles published between Jan 1, 2010, and June 22, 2021, describing treatment-related mortality in paediatric patients (aged 0–21 years) with cancer in LMICs. We searched PubMed, Trip, Web of Science, Embase, and the WHO Global Metric Index databases. The search was limited to full-text articles and excluded case reports (<10 patients) and haematopoietic stem-cell transplantation recipients. Two reviewers independently screened studies for eligibility, extracted data from included publications, and evaluated data quality. Random and mixed-effects models were used to estimate treatment-related mortality burden and trends. The Cochran-Q statistic was used to assess heterogeneity between studies. This study is registered on PROSPERO (CRD42021264849).
Findings
Of 13 269 identified abstracts, 501 studies representing 68 351 paediatric patients with cancer were included. The treatment-related mortality estimate was 6·82% (95% CI 5·99–7·64), accounting for 30·9% of overall mortality (4437 of 14 358 deaths). Treatment-related mortality was inversely related to country income. Treatment-related mortality was 14·19% (95% CI 9·65–18·73) in low-income countries, 9·21% (7·93–10·49) in lower-middle-income countries, and 4·47% (3·42–5·53) in upper-middle-income countries (Cochran-Q 42·39, p<0·0001). In upper-middle-income countries, the incidence of treatment-related mortality decreased over time (slope −0·002, p=0·0028); however, outcomes remained unchanged in low-income (p=0·21) and lower-middle-income countries (p=0·16).
Interpretation
Approximately one in 15 children receiving cancer treatment in LMICs die from treatment-related complications. Although treatment-related mortality has decreased in upper-middle-income countries over time, it remains unchanged in LMICs. There is an urgent need for targeted supportive care interventions to reduce global disparities in childhood cancer survival.
Funding
American Lebanese Syrian Associated Charities and National Cancer Institute.
Introduction
Advances in childhood cancer care have led to improved patient outcomes and overall survival. In high-income countries (HICs), improved treatment regimens and supportive care have enabled 5-year survival to surpass 80%.1–3 In low-income and middle-income countries (LMICs), however, cancer outcomes lag considerably. Although LMICs represent approximately 90% of the 400 000 incident childhood cancer cases annually, the 5-year survival rate is substantially lower, with estimates ranging from less than 10% in low-income countries to 55% in upper-middle-income countries.3–6 Thus, the burden of childhood cancer is disproportionately shifted to countries with scarce resources and reduced capacity to provide quality cancer care.
To address the disproportionate burden of childhood cancer deaths in LMICs, high-intensity treatment regimens are adapted on the basis of the availability of resources and supportive care. Although these regimens offer an increased probability of cure and potentially survival, such protocols may result in increased mortality due to treatment toxicity. Some studies have documented a paradoxical decrease in event-free survival in paediatric cancer patients in sub-Saharan Africa following the adoption of high-intensity treatment protocols designed in HICs—a decrease in relapse counterbalanced by increased mortality due to toxicity.7,8 Such outcomes are largely attributed to the absence of protocol adaptations to local methods of staging, risk stratification, diagnostics, infrastructure, and supportive care.9,10 Conversely, protocols that reduce treatment intensity and adapt to local resources and barriers to care in LMICs, such as treatment abandonment, have been shown to increase overall survival.11–15
Understanding and quantifying the burden of treatment-related mortality in LMICs is necessary to improve outcomes, however, current evidence is scarce. Historically, treatment-related mortality has been vaguely and interchangeably used with terms such as “early”, “toxic”, and “induction” death. Alexander and colleagues16 proposed the first consensus-based definition for treatment-related mortality in 2015 as “death occurring in the absence of progressive cancer”. Conventional understanding of treatment-related toxicity is largely derived from studies conducted in HICs, where primary causes of treatment-related mortality include infection and sepsis, haemorrhage, and encephalopathy.17–19 Hospital and population-level retrospective reviews in HICs report a cumulative incidence of treatment-related mortality of 3–5% in paediatric cancer patients, representing approximately 30–50% of overall deaths from cancer.17,20,21
Literature on treatment-related mortality in LMICs has largely included studies of specific cancers, treatment regimens, or clinical trials in single-centre or regional studies.22 Therefore, reported treatment-related mortality incidence varies widely. For example, a review in India found that in-hospital treatment-related mortality rates ranged from 3·8% to 24% for acute lymphoblastic leukaemia and from 5·7% to 45% for acute myeloblastic leukaemia, with no population-level data available.23 In El Salvador, where one hospital delivers all paediatric cancer care for the country, the incidence of treatment-related mortality was reported to be 35·4% for acute myeloblastic leukaemia.24 However, in another study conducted across El Salvador, Honduras, and Guatemala, the early death rate for acute myeloblastic leukaemia was substantially lower, at 18·3%.25 Despite this variability, treatment-related mortality in LMICs is likely to be higher than in HICs, because LMICs may implement cancer treatment protocols that were designed in HICs with more scarce access to infrastructure to provide appropriate supportive care.14,17,24–26
The aim of this study was to examine the incidence and primary causes of treatment-related mortality in children with cancer in LMICs, as well as to explore the relationship between country income level and treatment-related mortality.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis was completed in accordance with the PRISMA guidelines (appendix p 2).27,28 The protocol is registered on PROSPERO (CRD42021264849).
We conducted a systematic, comprehensive search of the following databases: PubMed (Jan 1, 2010, to June 22, 2021), Trip (Jan 1, 2010, to June 18, 2021), Web of Science (Jan 1, 2010, to June 22, 2021), Embase (Jan 1, 2010, to June 22, 2021), and the WHO Global Metric Index (Jan 1, 2010, to June 22, 2021). The search was designed to identify relevant publications based on a combination of terms capturing “[pediatric]” AND “[neoplasm]” AND “[LMICs]” AND “[treatment-related mortality]”. Medical subject headings terms and keywords were used when available. The search for “treatment-related mortality” extended to other key terms such as “induction”, “toxic” or “early” death and an expansive list of causes of death in children with cancer. The search was limited to full-text articles, English-language abstracts, and studies published between Jan 1, 2010, and June 22, 2021. The complete search strategy is provided in the appendix (pp 3–4).
The systematic review included studies describing treatment-related mortality in paediatric patients (aged 0–21 years) who received cancer-directed treatment (such as chemotherapy, surgery, or radiotherapy) in an LMIC.29 LMICs were classified as low-income, lower-middle-income, or upper-middle-income countries in accordance with the World Bank country classifications in 2021, unless a study explicitly stated its classification as a low-income or middle-income country for the duration of the study. Previous studies varied in their definition or quantification of treatment-related mortality despite the consensus-based classification published in 2015.16 As a result, studies were not excluded on the basis of their definition of treatment-related mortality unless this was in direct contradiction to the consensus classification.
This systematic review excluded conference proceedings, systematic reviews, meta-analyses, editorials, case reports, or case series with fewer than ten patients, letters, and conference abstracts. Studies were only included if the lower age limit of included patients was below 18 years; the upper age limit was 21 years due to common inclusion of patients aged up to 21 years in some paediatric cohorts among searched publications. Haematopoietic stem-cell transplantation recipients (and subsequent reporting of transplant-related complications) were excluded due to the underlying difference in cause and frequency of treatment-related mortality in these patients. Studies were further excluded if they only reported data for a subset of high-risk patients with cancer already experiencing treatment complications, such as solely reporting data for patients with specific infections or critical illness. Studies were not excluded if patients had co-infections, such as HIV, before starting treatment.
Among included studies, only patients meeting all inclusion criteria were included in the analysis. Studies that collected data for both children and adults were included only if data could be extracted for children (aged 0–21 years). Similarly, studies including patients treated with haematopoietic stem-cell transplantation were only included if outcomes data could be extracted for patients who did not receive a transplant. Screening criteria and guidelines are summarised in the appendix (p 5).
Data analysis
Literature search results were extracted to and deduplicated with Covidence software.30,31 Duplicate studies reporting outcomes for an identical or overlapping cohort of patients were manually excluded. In such cases, studies with a smaller sample size, fewer years of observation, or lower quality assessment scores were excluded. All abstracts and full-text articles were screened for inclusion in the study by two reviewers, and disagreements were resolved by a third investigator. Data from included studies were initially extracted in RedCap by one author and subsequently reviewed by a second reviewer.32
Extracted data included study design, years of data observation, study location (country), patient age range, sample size, cancer diagnoses, treatment methods, overall survival, reported statistic for treatment-related mortality, and quantified causes of treatment-related causes of death (see the appendix, pp 6–7, for the case report form). Data were extracted separately in multiple cohorts if a study reported separate outcomes from treatment conducted during different time periods.
All deaths during treatment in the absence of cancer progression or relapse, including induction deaths, were considered as treatment-related mortality. This study did not capture death before treatment initiation or due to external causes. In LMICs, decisions to start treatment are multifactorial and not solely based on the clinical status of the patient (eg, cost, family preference, treatment refusal, and so on), and reporting of this data was inconsistent and difficult to interpret across different studies. Treatment-related mortality was calculated for each study as the number of deaths due to treatment-related mortality divided by the number of treated patients.
The primary outcome of this study was the incidence of treatment-related mortality, and the secondary outcome was causes of death. Causes of death were extracted as listed by study authors, and reviewers applied the criteria described by Alexander and colleagues16 for treatment-related mortality when assessing causes of death. Of studies identifying causes of death, most defined one primary cause. In the few case series with multiple listed causes, reviewers used the available information to identify the primary cause of death.
The methodological quality and bias of included articles was assessed in accordance with STROBE guidelines and based on the Newcastle-Ottawa Quality Assessment Scale and Joanna Briggs Institute critical appraisal checklists for cohort and case-series studies.33,34 Nine items were scored with a maximum point value of 14 based on quality. As part of the bias tool, studies were categorised as including standard-risk, low-risk, or high-risk patients (when described) to account for selection bias. Studies that included early stages of presentation (I or II) were indicated as low risk, and patients in later stages of cancer (III or IV), or those with relapsed or metastatic cancer were indicated as high risk. The overall quality scores were categorised as high (≥12 points), medium (7–11 points), or low (≤6 points) quality on the basis of data trends. The quality assessment tool and associated point values are described in the appendix (pp 8–9).
Descriptive statistics were used to summarise study characteristics, including patient sample size, country income level, and year of study conduct (reported as the midpoint year between start and end dates for patient inclusion). Quality assessment score cutoffs were identified on the basis of treatment-related mortality trends (appendix p 10). Restricted maximum likelihood random-effects and mixed-effects models were used to obtain overall pooled treatment-related mortality estimates (with 95% CIs), as well as treatment-related mortality estimates by cancer subtype and country income group. In the mixed-effects models, “study” was considered as a random effect to adjust for heterogeneity between studies due to differences in patient characteristics and disease distributions. Generalised Cochran heterogeneity statistics (Cochran-Q) was used to assess the heterogeneity of pooled data under the null hypothesis τ2=0 and significance level α1=α2=0·025.35 χ2 and Fisher’s exact tests were used to test associations between categorical data; the non-parametric Wilcoxon rank sum test was used to compare medians in two groups; and medians for three or more groups were compared with Kruskal–Wallis rank sum test. p values less than 0·05 were considered significant. Data were analysed with R (version 4.2.2), and the metafor package (version 3.8–1).36
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Results
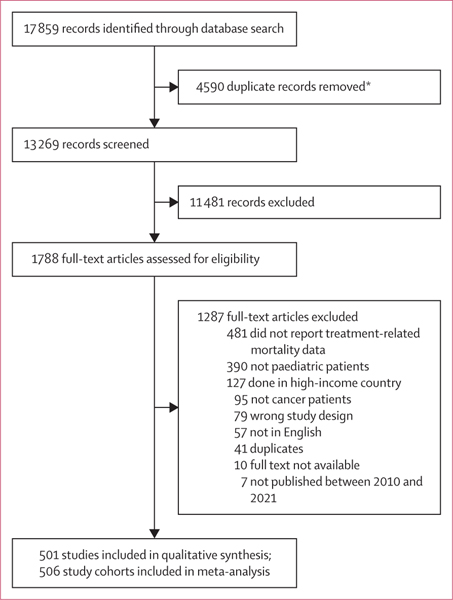
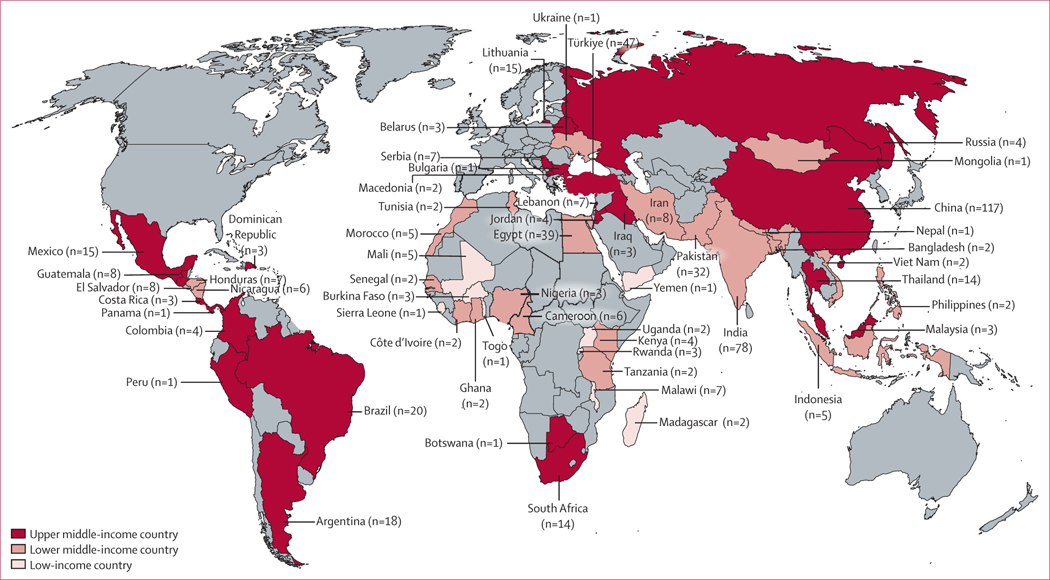
The search identified 13 269 studies after the removal of duplicates. Following abstract screening, 1788 full-text articles were assessed for eligibility. 1287 studies were excluded; the primary reason for exclusion was the absence of quantifiable treatment-related mortality data (n=481). Ultimately, 501 publications met the inclusion criteria (figure 1; appendix pp 11–65). Included studies described data across 506 patient cohorts and comprised 68 351 children with cancer from 66 countries across all WHO regions (figure 2). 286 (57·1%) of 501 studies were conducted in upper-middle-income countries, 207 (41·3%) were conducted in lower-middle-income countries, and 20 (4·0%) were conducted in low-income countries. Studies were done between 1970 and 2021 (median year 2009, mid-year range 1989–2021), and the median time for follow-up was 3·6 years (IQR 2·3–5·0). A complete summary of study characteristics is provided in tables 1 and 2 (appendix p 66).
Figure 1: Study selection.
*Deduplicated using Covidence software.
Figure 2: Map of included studies.
World map depicting number of studies included in this systematic review from each country. Countries in grey represent those without included published studies.
Table 1:
Characteristics of included studies
| Included studies (n=501) | |
|---|---|
| Type of study | |
| Single-centre | 431 (86·0%) |
| Multicentre (1 country) | 56 (11·2%) |
| Multicentre (≥2 countries) | 14 (2·8%) |
| Study design | |
| Randomised controlled trial | 9 (1·8%) |
| Prospective cohort or case-series | 98 (19·6%) |
| Retrospective cohort or case-series | 367 (73·3%) |
| Unspecified cohort or case-series | 22 (4·4%) |
| Other | 5 (1·0%) |
| Country income level * | |
| Low income | 20 (4·0%) |
| Lower-middle income | 207 (41·3%) |
| Upper-middle income | 286 (57·1%) |
| WHO region † | |
| Western Pacific region | 124 (24·8%) |
| South-East Asia region | 99 (19·8%) |
| Region of the Americas | 72 (14·4%) |
| Eastern Mediterranean region | 100 (20·0%) |
| European region | 63 (12·6%) |
| African region | 46 (9·2%) |
Data are n (%).
12 studies involved more than one country income level.
One study involved four WHO regions.
Table 2:
Characteristics of included cohorts
| Included cohorts (n=506) | |
|---|---|
| Mid-year* of data observation | 2009 (2006–2012) |
| Years of follow-up | 3·6 (2·3–5·0) |
| Age range (years) Starting age (lower limit) |
1·0 (0·3–2·5) |
| Ending age (upper limit) | 15·9 (13·0–18·0) |
| Median sample size (IQR) | 59 (28–152) |
| Type of cancer-directed treatment | |
| Surgery | 219 (43·3%) |
| Chemotherapy | 469 (92·7%) |
| Radiotherapy | 179 (35·4%) |
| Other† | 22 (4·3%) |
Data are median (IQR) or n (%), unless otherwise stated.
Defined as the midpoint year between initial and final years of study conduct.
Other cancer-directed treatment includes modalities such as immunotherapies and gene therapies.
The median lower age limit of children included in this study was 1·0 years and the upper age limit was 15·9 years. 51 186 (74·9%) of 68 351 patients were diagnosed with haematological malignancies, 6240 (9·1%) were diagnosed with CNS tumours, and 10 785 (15·8%) were diagnosed with non-CNS solid tumours. The most common cancer diagnoses were acute lymphoblastic leukaemia (33 507 [49·0%]), acute myeloblastic leukaemia (6826 [10·0%]), non-Hodgkin lymphoma (6009 [8·8%]), and retinoblastoma (5034 [7·4%]). Studies conducted in low-income countries had a higher proportion of patients with CNS tumours and a lower proportion with haematological malignancies than in lower-middle-income and upper-middle-income countries (appendix p 67).
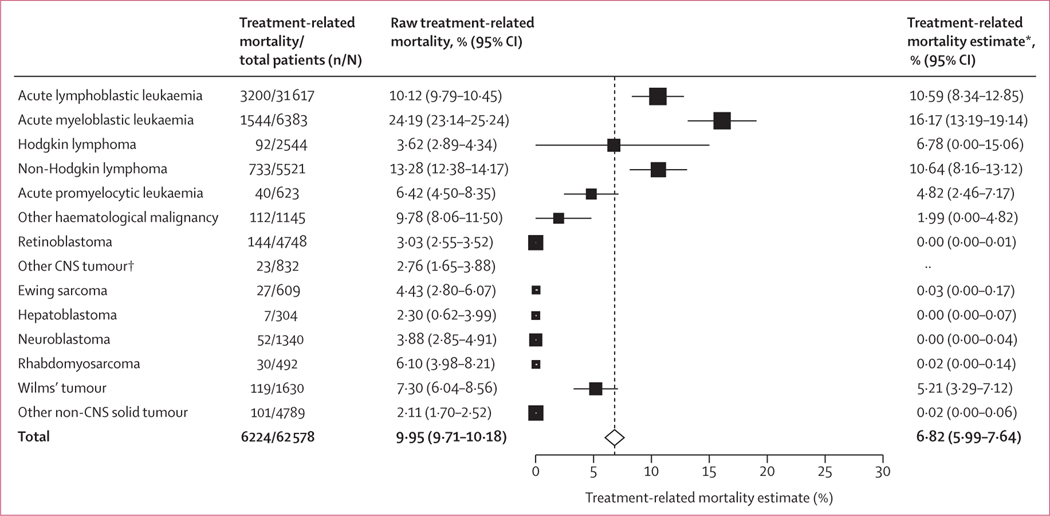
Treatment-related mortality occurred in 6976 (10·2%) of 68 351 patients. Based on the random-effects model, the aggregated treatment-related mortality estimate was 6·82% (95% CI 5·99–7·64). This estimate was significantly higher in patients with haematological malignancies (10·42% [95% CI 9·26–11·58]) than in those with non-CNS solid tumours (2·91% [1·49–4·34]) and CNS tumours (1·96% [0·00–4·05]; p<0·0001). There was significant heterogeneity in treatment-related mortality across all studies (Cochran-Q 5434851·38, p<0·0001) and among cancer subtype: haematological malignancy versus CNS and solid tumours (Cochran-Q 86·07, p<0·0001). The treatment-related mortality estimate was 10·59% (95% CI 8·34–12·85) for acute lymphoblastic leukaemia, 16·17% (13·19–19·14) for acute myeloblastic leukaemia, and 10·64% (8·16–13·12) for non-Hodgkin lymphoma (figure 3; appendix pp 68–69).
Figure 3: Estimated treatment-related mortality by cancer diagnosis.
Forest plot of estimated treatment-related mortality by cancer diagnosis by use of a mixed-effects model. Estimated treatment-related mortality is shown with 95% CIs. The size of the square representing the estimate corresponds to the sample size of patients. The vertical dotted line represents the aggregated treatment-related mortality estimate for all patients included in this systematic review and meta-analysis. *Treatment-related mortality estimates based on random-effects model. †Subcategories of diagnoses with a small number of studies, where treatment-related mortality could not be estimated.
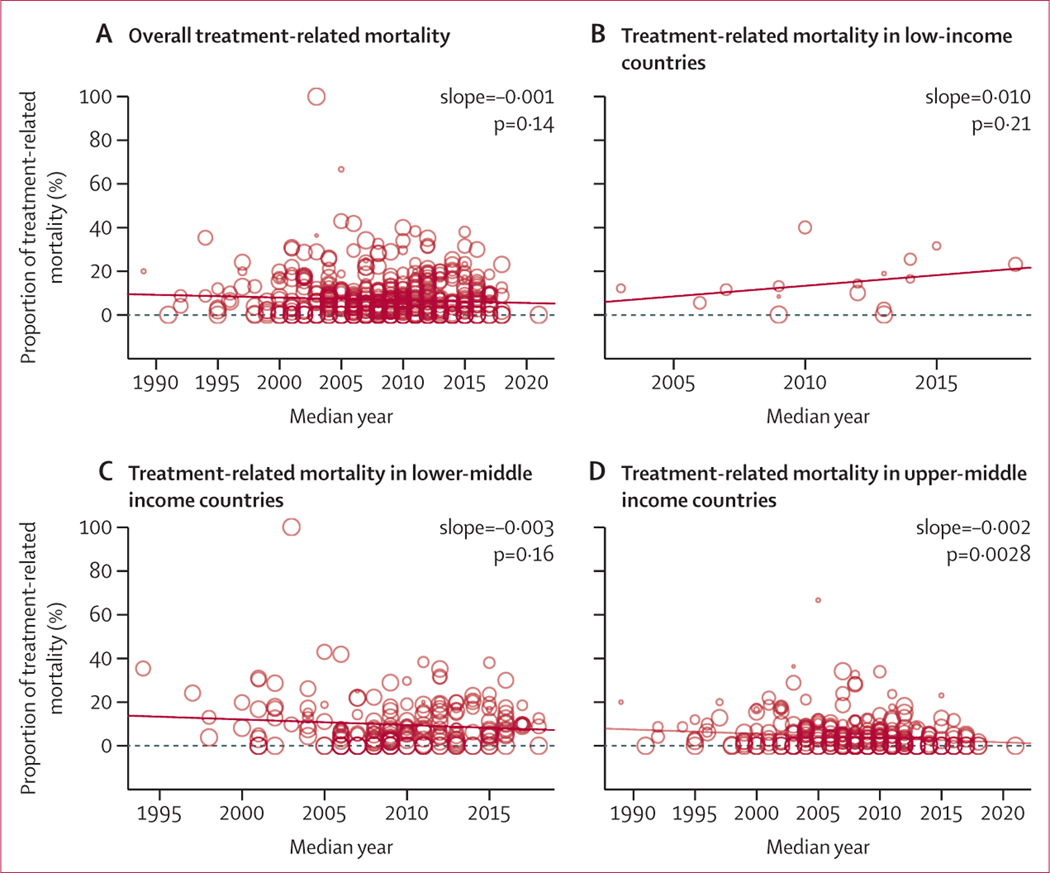
Treatment-related mortality was inversely related to country income group, with low-income countries having a higher treatment-related mortality point estimate (14·19%, 95% CI 9·65–18·73) than lower-middle-income countries (9·21%, 7·93–10·49) and upper-middle-income countries (4·47%, 3·42–5·53; p<0·0001). There was significant heterogeneity between income groups (Cochran-Q 42·39, p<0·0001). Using the mid-year of study data, the overall estimate for treatment-related mortality did not change between 1989 and 2021 (slope −0·001, p=0·14). Although treatment-related mortality remained unchanged in low-income countries (slope over time 0·010, p=0·21) and lower-middle-income countries (slope over time −0·003, p=0·16), treatment-related mortality decreased in upper-middle-income countries (slope over time −0·002, p=0·0028; figure 4).
Figure 4: Trends in treatment related-mortality over time.
The figure depicts treatment-related mortality by study mid-year, calculated as the middle year of study conduct (average between start and stop of study inclusion years). Treatment-related mortality rates are depicted for all study patients (A), patients receiving treatment in low-income countries (B), patients receiving treatment in lower-middle-income countries (C), and patients receiving treatment in upper-middle-income countries (D). The size of the circle represents the size of the study sample. Mixed-effects model was used to explore the relationship between treatment-related mortality estimates and the year of study conduct (as depicted by the trend line shown).
Causes of treatment-related mortality were unspecified for 2604 (37·3%) of 6976 patients, and broadly characterised as “induction deaths” in 803 (11·5%). Among specified causes of death, leading causes included sepsis or infection (2560 [71·7%] of 3569), haemorrhage (379 [10·6%]), and unspecified complications secondary to tumour lysis syndrome (174 [4·9%]; the complete list of causes is shown in table 3). Causes of treatment-related mortality differed significantly between patients with haematological malignancies and solid (CNS and non-CNS) tumours (Fisher’s exact test, p<0·0001). Infection or sepsis accounted for a higher proportion of treatment-related deaths in patients with haematological malignancies (2069 [73·9%] of 2798) than in those with CNS (28 [56·0%] of 50) and other solid tumours (101 [50·8%] of 199; Fisher’s exact test, p<0·0001). Conversely, treatment-related mortality was more likely to be caused by surgical complications in children with CNS (seven [14·0%] of 50) and solid tumours (25 [12·6%] of 199) than in those with haematological malignancies (two [0·1%]; Fisher’s exact test, p<0·0001; table 3).
Table 3:
Identified causes of treatment-related mortality
| All cancers (n=3569) | Haematological malignancy (n=2798) | CNS solid tumour (n=50) | Non-CNS solid tumour (n=199) | |
|---|---|---|---|---|
| Total number of patients* | 68351 | 51186 | 6240 | 10785 |
| Sepsis or infection | 2560 (71·7%) | 2069 (73·9%) | 28 (56·0%) | 101 (50·8%) |
| Haemorrhage | 379 (10·6%) | 306 (10·9%) | 4 (8·0%) | 4 (2·0%) |
| Metabolic causes (tumour lysis syndrome) | 174 (4·9%) | 162 (5·8%) | 0 | 1 (0·5%) |
| Disseminated intravascular coagulation | 13 (0·4%) | 12 (0·4%) | 0 | 0 |
| Surgical complications | 51 (1·4%) | 2 (0·1%) | 7 (14·0%) | 25 (12·6%) |
| Neurological complications (encephalopathy or raised intracranial pressure) | 26 (0·7%) | 21 (0·8%) | 1 (2·0%) | 1 (0·5%) |
| Seizures | 4 (0·1%) | 2 (0·1%) | 2 (4·0%) | 0 |
| Cardiac failure | 43 (1·2%) | 17 (0·6%) | 0 | 15 (7·5%) |
| Respiratory failure | 99 (2·8%) | 80 (2·9%) | 0 | 6 (3·0%) |
| Superior vena cava syndrome | 5 (0·1%) | 5 (0·2%) | 0 | 0 |
| Hyperleukocytosis | 8 (0·2%) | 8 (0·3%) | 0 | 0 |
| Other chemotherapy toxicity | 90 (2·5%) | 32 (1·1%) | 6 (12·0%) | 33 (16·6%) |
| Other organ failure | 98 (2·7%) | 72 (2·6%) | 1 (2·0%) | 6 (3·0%) |
| Other | 19 (0·5%) | 10 (0·4%) | 1 (2·0%) | 7 (3·5%) |
Data are n (%), unless otherwise stated.
Due to the inclusion of studies with multiple diagnoses across several diagnostic categories, the total number of patients with any malignancy is greater than the sum of patients in the given categories.
Of the 506 study cohorts included in this systematic review and meta-analysis, 374 (73·9%) reported overall mortality. 132 (26·1%) of 506 cohorts did not report overall survival outcomes and only described treatment-related mortality. Of all 50 146 patients with identified causes of death, 14 358 (28·6%) died during the study observation period. Treatment-related mortality accounted for 4437 (30·9%) of 14 358 overall deaths. A significantly higher proportion of deaths was attributable to treatment-related mortality in children with haematological malignancies (3355 [37·0%] of 9065 of overall deaths), than in children with CNS tumours (158 [18·2%] of 868) and non-CNS solid tumours (223 [11·9%] of 1881; p<0·0001). The proportion of overall deaths accounted for by treatment-related mortality varied significantly across country income level: 209 (42·1%) of 496 deaths in low-income countries, 1866 (40·5%) of 4613 deaths in lower-middle-income countries, and 2098 (25·2%) of 8313 deaths in upper-middle-income countries (p<0·0001). Causes of treatment-related mortality also varied significantly by country income level (p<0·0001; appendix p 70), and low-income countries reported a significantly higher proportion of treatment-related mortality due to unspecified causes than did middle-income countries (p<0·0001; appendix p 71). The appendix shows causes of death for the most common oncological diagnoses (p 72) and survival outcome data by country income group and WHO region (p 73).
The overall quality of included articles ranged widely. Based on the bias assessment tool, most study cohorts were categorised as high (184 [36·4%] of 506) or medium quality (294 [58·1%]), with a minority (28 [5·5%]) categorised as low quality (appendix p 10). 54 (10·8%) of 501 studies only included high-risk patients, and 18 (3·6%) of 501 included only low-risk patients. Studies with standard-risk patients had significantly higher rates of treatment-related mortality than studies with any other risk categories (7·55% [95% CI 6·64–8·45] vs 3·69% [1·84–5·54]; Cochran-Q 13·44, p=0·0002). The length of patient follow-up was less than 1 year or unspecified in 99 (19·8%) of 501 studies. 81 (16·2%) of 501 studies reported loss of patients to follow-up, and 175 (34·9%) of 501 did not report or account for all causes of death in the sample. Cohorts with no loss of patients to follow-up had a significantly lower incidence of treatment-related mortality than studies with any or unspecified loss to follow-up (p=0·0093). There was no improvement in the quality of studies over time (p=0·36).
The mixed-effects model found that high-quality studies were associated with lower treatment-related mortality estimates than low-quality and medium-quality studies (p=0·0035). Study quality was directly related to country income group; studies conducted in low-income countries were of lower quality than those done in middle-income countries (p=0·0212). When controlling for diagnostic category, country income group, and quality assessment score in the multivariable mixed-effects model, both country income group (low income: p=0·0011, lower middle income: p=0·0084) and oncological diagnosis (haematological malignancy p<0·0001) significantly affected treatment-related mortality, whereas quality score did not (p=0·96; appendix p 74).
Significant heterogeneity in treatment-related mortality estimates was observed among cohorts with or without loss to follow-up (p<0·0001) and among cohorts with low or medium study quality compared to those with high study quality (p<0·0001). There was also significant heterogeneity in treatment-related mortality within countries, probably due to the variability of oncological diagnoses captured in included studies (appendix p 75).
Discussion
This systematic review and meta-analysis analysed mortality data across 506 patient cohorts and 68 351 children with cancer from 66 LMICs. Approximately one in 15 children with cancer receiving treatment in LMICs die from treatment-related complications (based on the treatment-related mortality estimate of 6·82%), which accounts for almost a third (30·9%) of overall deaths and is higher than treatment-related mortality estimated in HICs (3–5%).17 Our study established an inverse, stepwise relationship between country income level and treatment-related mortality. Compared to published data in HICs, our findings demonstrate an approximately two-times increase in treatment-related mortality in lower-middle-income countries (9·21%), and an approximately three-times increase in treatment-related mortality in low-income countries (14·19%).17,20,21 Although this study is the most comprehensive systematic assessment of treatment-related mortality in LMICs to date, these findings are likely to represent an under estimate of the true treatment-related mortality burden due to under-representation of published studies from low-income countries that report comprehensive patient outcomes.5
Our results showed a lack of improvement in the incidence of treatment-related mortality in LMICs since the 1990s, despite global advancements in cancer-directed therapy. However, when modelling outcomes by income level, the incidence of treatment-related mortality was found to decrease over time in upper-middle-income countries, while remaining unchanged in low-income and lower-middle-income countries. Although overall survival has increased in LMICs over time, supportive care interventions have lagged behind treatment advances, resulting in unchanged treatment-related mortality outcomes. These results show widening disparities in treatment-related mortality in children with cancer between high-resource and low-resource settings, representing an increasing threat to equity in childhood cancer outcomes globally.
Although the incidence of treatment-related mortality reported in this study is higher than in HICs, the patterns of mortality in LMICs reflect those in high-income settings, suggesting that childhood cancer care presents similar challenges globally. As in HICs, infection or sepsis, and haemorrhage were identified as the leading causes of death in this study.17–19,37–39 Additionally, our findings showed that patients with haematological malignancies had significantly higher treatment-related mortality than patients with CNS and other solid tumours.17 Notably, several documented adaptations in treatment regimens that reduced treatment intensity or established additional supportive care guidelines have been shown to reduce treatment-related mortality in LMICs while increasing event-free survival. Future work must assess not only populational-level mortality data, but also disease-specific mortality outcomes based on specific protocol adaptations and patient characteristics.
The WHO Global Initiative for Childhood Cancer outlined the need to implement cost-effective, contextually appropriate supportive interventions such as provision of adequate nutrition, infection prevention and control, and symptom management to curb the impact of treatment toxicity.40 The association between malnutrition and decreased paediatric cancer survival has been well documented, and is likely to be significantly more prevalent in low-income countries.40 Inadequate transportation and insufficient family resources might further contribute to later staging at initial presentation, and subsequently worse outcomes. Moreover, hospital resources, diagnostics, and staffing affect treatment outcomes, and many low-income countries do not have the dedicated paediatric personnel and specific clinician training in paediatric oncology.26,41 Additionally, other essential resources might be more scarce in these settings, because hospitals need to have adequate access to blood bank services and transfusion medicine to prevent delayed delivery of critical blood products.42,43 Future research must further explore the association between hospital-level and patient-level factors and treatment outcomes. Ultimately, implementing interventions to reduce treatment-related mortality would also improve the overall quality of supportive care within resource-limited hospitals, thus strengthening patient care more broadly.
Efforts based on implementation science methods are necessary to adapt sustainable supportive care interventions and promote their use to reduce treatment-related mortality in LMICs. For example, paediatric early warning systems (PEWS) are cost-effective quality-improvement tools to decrease rates of clinical deterioration events and promote early paediatric intensive care unit transfer for paediatric patients with cancer who develop organ dysfunction or sepsis.42–47 PEWS implementation in resource-diverse settings has been shown to improve multilevel outcomes on patient, provider, interdisciplinary team, and institutional levels without requiring additional human or material resources.46–48 Additionally, early recognition of complications and initiation of appropriate treatment, including antimicrobial therapy in sepsis, could improve outcomes of infection and reduce mortality.49,50 Protocols to mitigate the effect of infection, however, must be tailored to epidemiological differences across centres.51 To be effective, programmes must have the ability to diagnose and treat antimicrobial-resistant pathogens that have a higher incidence in LMICs.52
In this systematic review, high-quality studies were more likely to be published in high-resource settings and to report lower treatment-related mortality than in low-resource settings. This association could be explained by the lower incidence of treatment-related mortality in upper-middle-income than in low-income countries. In LMICs, cancer is often detected at late stages, with higher rates of treatment abandonment and loss to follow-up contributing to cancer-related mortality.10,53–55 All of these factors could contribute to a greater proportion of deaths due to disease progression and less accurate capture of mortality data in low-resource settings compared with high-resource settings, further complicating our estimate of treatment-related mortality.
This study also revealed a wide variability in the definition of treatment-related mortality and the quality of reported mortality data.56 Approximately a third of full-text articles screened were excluded because of incomplete reporting of reported causes for mortality. Among included studies, a large subset did not account for all deaths or identify all causes of mortality. Included studies were also likely to face challenges experienced in high-income settings, including under-reporting of early deaths (ie, within 1 month of diagnosis), resulting in underestimation of the true burden of treatment-related mortality.57,58
Ultimately, differences in the quality of studies and the way they reported causes of death might generate less reliable estimates of treatment-related mortality in LMICs and serve as a call to action to standardise the reporting of causes of mortality among paediatric patients with cancer. Future studies should define all terms such as “early” or “induction” death and apply validated, consensus-based criteria of treatment-related mortality.16 The quality of reported data will be further enhanced by identifying and quantifying specific causes of mortality or toxicity within broader categories of complications (ie, infection, haemorrhage, tumour lysis, and so on), which is a necessary step to develop targeted supportive care interventions aimed at the most prevalent complications. Data should also be reported for disease-related mortality and external causes of death preceding treatment initiation. Implementation of hospital and population-based cancer registries in LMICs is crucial to improve and standardise the quality of collected and published treatment outcomes data. Existing tools include the SJCARES Registry tool, a free, cloud-based, paediatric hospital-based cancer registration and reporting system. Other platforms can be strategically used to improve delivery of treatment and supportive care in LMICs, such as Adapted Resource and Implementation Application (ARIA), a collaboration between St Jude and the International Society of Paediatric Oncology—an online clinical resource providing comprehensive, resource-stratified, evidence-based guidelines for treatment of paediatric malignancies across global settings.
This study has several limitations. The search was restricted to English-language abstracts, which might have limited inclusion of all studies published from LMICs. This restriction was necessary given the complexity of extracted data, the need to ensure alignment of causes of treatment-related mortality with the established definition, and to fully assess the quality of included studies. Our study also included substantially fewer studies published in low-income countries, skewing aggregate results away from countries with fewer resources and a higher incidence of treatment-related mortality. Although treatment-related mortality outcomes were reported separately by country income group, certain WHO regions, such as Africa, were under-represented. Selection bias might have also played a role as this analysis was based on published studies, as opposed to registry-based data. Additionally, this study did not collect data about hospital characteristics, such as funding structure (public vs private) or staffing (paediatric vs adult providers), that might affect patient outcomes. Similarly, in this comprehensive analysis of treatment-related mortality, the impact of individual protocols and other treatment factors on outcomes for specific oncological diagnoses could not be assessed, nor could the impact of individual patient characteristics (eg, age and stage at presentation) be evaluated. These important factors should be evaluated in future studies. Last, because studies were frequently excluded because of unclear reporting of treatment-related mortality, a disproportionate number of included studies reported 100% overall survival, where a treatment-related mortality of zero could be assumed. Although we attempted to account for some of this heterogeneity among studies by using random-effects and mixed-effects models, our systematic review probably underestimates the true burden of treatment-related mortality in LMICs.
In conclusion, understanding the global burden of childhood cancer is the first step towards improving outcomes and reducing global disparities. Treatment-related mortality accounts for at least a third of childhood cancer deaths globally, and disparities in treatment-related mortality are widening over time between lower-income and higher-income settings. Furthermore, poor reporting quality and scarce population-based data probably result in an underestimation of the problem. The absence of improvement in patient outcomes in low-resource settings over the past few decades highlights the need for better use of adaptive therapies, improved supportive care, and higher quality data. These findings serve as a call to action to improve the reporting of treatment-related mortality in paediatric oncology studies in LMICs and to develop and adapt effective supportive care interventions to reduce global disparities and improve childhood cancer survival worldwide.
Supplementary Material
Research in context.
Evidence before this study
Evidence quantifying the burden of childhood cancer treatment-related mortality in low-income and middle-income countries (LMICs) is scarce. Conventional understanding of treatment toxicity is largely derived from studies conducted in high-income countries. We searched PubMed, Trip, Web of Science, Embase, and the WHO Global Index databases for publications describing paediatric cancer, LMICs, and general terms for treatment-related mortality (ie, “early”, “induction” and “toxic” death). To date, no systematic review has been conducted to estimate the incidence of treatment-related mortality among paediatric cancer patients in LMICs.
Added value of this study
To our knowledge, this is the first systematic review and meta-analysis to describe treatment-related mortality in children with cancer in LMICs. Approximately one in 15 children receiving cancer treatment in LMICs dies from treatment-related complications, with infection or sepsis being the leading causes of death. Treatment-related mortality accounts for a third of overall cancer mortality in these settings. Although treatment-related mortality has decreased over time in upper-middle-income countries, outcomes have remained unchanged in low-income and lower-middle-income countries.
Implications of all the available evidence
Our study demonstrates that disparities in treatment-related mortality between countries of varying income levels are increasing with time. Our findings identify an urgent need for the development of targeted supportive care interventions to improve childhood cancer survival in resource-limited settings and to reduce global disparities in childhood cancer outcomes.
Acknowledgments
We thank Julie Edrington (St Jude Children’s Research Hospital) for assisting with developing and conducting the literature search. This work is supported in part by the American Lebanese Syrian Associated Charities and the US National Cancer Institute (grant number 5P30CA021765).
Footnotes
Declaration of interests
We declare no competing interests.
Data sharing
Data (including data dictionaries) that underlie the results reported in this Article, as well as the study protocol, statistical analysis plan, and analytical code will be made available beginning 6 months and ending 5 years following publication to researchers who provide a methodologically sound proposal. Proposals should be directed to the corresponding author via email; data requestors will need to sign a data access agreement.
References
- 1.Butler E, Ludwig K, Pacenta HL, Klesse LJ, Watt TC, Laetsch TW. Recent progress in the treatment of cancer in children. CA Cancer J Clin 2021; 71: 315–32. [DOI] [PubMed] [Google Scholar]
- 2.Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5—a population-based study. Lancet Oncol 2014; 15: 35–47. [DOI] [PubMed] [Google Scholar]
- 3.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev 2015; 24: 653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Atun R. Estimating the total incidence of global childhood cancer: a simulation-based analysis. Lancet Oncol 2019; 20: 483–93. [DOI] [PubMed] [Google Scholar]
- 5.Bhakta N, Force LM, Allemani C, et al. Childhood cancer burden: a review of global estimates. Lancet Oncol 2019; 20: e42–53. [DOI] [PubMed] [Google Scholar]
- 6.Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Girardi F, Atun R. Global childhood cancer survival estimates and priority-setting: a simulation-based analysis. Lancet Oncol 2019; 20: 972–83. [DOI] [PubMed] [Google Scholar]
- 7.Hesseling PB, Broadhead R, Molyneux E, et al. Malawi pilot study of Burkitt lymphoma treatment. Med Pediatr Oncol 2003; 41: 532–40. [DOI] [PubMed] [Google Scholar]
- 8.Hesseling PB, Njume E, Kouya F, et al. The Cameroon 2008 Burkitt lymphoma protocol: improved event-free survival with treatment adapted to disease stage and the response to induction therapy. Pediatr Hematol Oncol 2012; 29: 119–29. [DOI] [PubMed] [Google Scholar]
- 9.Howard SC, Ortiz R, Baez LF, et al. Protocol-based treatment for children with cancer in low income countries in Latin America: a report on the recent meetings of the Monza International School of Pediatric Hematology/Oncology (MISPHO)—part II. Pediatr Blood Cancer 2007; 48: 486–90. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Galindo C, Friedrich P, Alcasabas P, et al. Toward the cure of all children with cancer through collaborative efforts: pediatric oncology as a global challenge. J Clin Oncol 2015; 33: 3065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard SC, Pedrosa M, Lins M, et al. Establishment of a pediatric oncology program and outcomes of childhood acute lymphoblastic leukemia in a resource-poor area. JAMA 2004; 291: 2471–75. [DOI] [PubMed] [Google Scholar]
- 12.Baez F, Ocampo E, Conter V, et al. Treatment of childhood Hodgkin’s disease with COPP or COPP-ABV (hybrid) without radiotherapy in Nicaragua. Ann Oncol 1997; 8: 247–50. [DOI] [PubMed] [Google Scholar]
- 13.Sackmann-Muriel F, Zubizarreta P, Gallo G, et al. Hodgkin disease in children: results of a prospective randomized trial in a single institution in Argentina. Med Pediatr Oncol 1997; 29: 544–52. [DOI] [PubMed] [Google Scholar]
- 14.Howard SC, Davidson A, Luna-Fineman S, et al. A framework to develop adapted treatment regimens to manage pediatric cancer in low- and middle-income countries: the Pediatric Oncology in Developing Countries (PODC) Committee of the International Pediatric Oncology Society (SIOP). Pediatr Blood Cancer 2017; 64 (suppl 5): e26879. [DOI] [PubMed] [Google Scholar]
- 15.Surapolchai P, Anurathapan U, Sermcheep A, et al. Long-term outcomes of modified St Jude Children’s Research Hospital total therapy XIIIB and XV protocols for Thai children with acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk 2019; 19: 497–505. [DOI] [PubMed] [Google Scholar]
- 16.Alexander S, Pole JD, Gibson P, et al. Classification of treatment-related mortality in children with cancer: a systematic assessment. Lancet Oncol 2015; 16: e604–10. [DOI] [PubMed] [Google Scholar]
- 17.Gibson P, Pole JD, Lazor T, et al. Treatment-related mortality in newly diagnosed pediatric cancer: a population-based analysis. Cancer Med 2018; 7: 707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creutzig U, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol 2004; 22: 4384–93. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor D, Bate J, Wade R, et al. Infection-related mortality in children with acute lymphoblastic leukemia: an analysis of infectious deaths on UKALL2003. Blood 2014; 124: 1056–61. [DOI] [PubMed] [Google Scholar]
- 20.Loeffen EAH, Knops RRG, Boerhof J, et al. Treatment-related mortality in children with cancer: Prevalence and risk factors. Eur J Cancer 2019; 121: 113–22. [DOI] [PubMed] [Google Scholar]
- 21.Pole JD, Gibson P, Ethier MC, et al. Evaluation of treatment-related mortality among paediatric cancer deaths: a population based analysis. Br J Cancer 2017; 116: 540–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bansal D, Davidson A, Supriyadi E, Njuguna F, Ribeiro RC, Kaspers GJL. SIOP PODC adapted risk stratification and treatment guidelines: Recommendations for acute myeloid leukemia in resource-limited settings. Pediatr Blood Cancer 2019; 2019: e28087. [DOI] [PubMed] [Google Scholar]
- 23.Arora RS, Arora B. Acute leukemia in children: a review of the current Indian data. South Asian J Cancer 2016; 5: 155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S, Bonilla M, Fuentes SL, et al. Incidence and predictors of treatment-related mortality in paediatric acute leukaemia in El Salvador. Br J Cancer 2009; 100: 1026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Bonilla M, Valverde P, et al. Treatment-related mortality in children with acute myeloid leukaemia in Central America: incidence, timing and predictors. Eur J Cancer 2012; 48: 1363–69. [DOI] [PubMed] [Google Scholar]
- 26.Muttalib F, González-Dambrauskas S, Lee JH, et al. Pediatric emergency and critical care resources and infrastructure in resource-limited settings: a multicountry survey. Crit Care Med 2021; 49: 671–81. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–12. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Bank. World development indicators. The world by income and region. 2021. https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html (accessed June 27, 2023).
- 30.McKeown S, Mir ZM. Considerations for conducting systematic reviews: evaluating the performance of different methods for de-duplicating references. Syst Rev 2021; 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016; 104: 240–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison JK, Reid J, Quinn TJ, Shenkin SD. Using quality assessment tools to critically appraise ageing research: a guide for clinicians. Age Ageing 2017; 46: 359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res 2020; 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson D.Confidence intervals for the between-study variance in random effects meta-analysis using generalised Cochran heterogeneity statistics. Res Synth Methods 2013; 4: 220–29. [DOI] [PubMed] [Google Scholar]
- 36.Viechtbauer W.Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. [Google Scholar]
- 37.Gao YJ, Qian XW, Lu FJ, Zhai XW, Wang HS, Li J. Improved outcome for children with non-high risk acute lymphoblastic leukaemia after using an ALL IC-BFM 2002-based protocol in Shanghai, China. Br J Haematol 2013; 160: 363–67. [DOI] [PubMed] [Google Scholar]
- 38.Suarez A, Piña M, Nichols-Vinueza DX, et al. A strategy to improve treatment-related mortality and abandonment of therapy for childhood ALL in a developing country reveals the impact of treatment delays. Pediatr Blood Cancer 2015; 62: 1395–402. [DOI] [PubMed] [Google Scholar]
- 39.Trehan A, Bansal D, Varma N, Vora A. Improving outcome of acute lymphoblastic leukemia with a simplified protocol: report from a tertiary care center in north India. Pediatr Blood Cancer 2017; 64: e26281. [DOI] [PubMed] [Google Scholar]
- 40.WHO. CureAll Framework: WHO global initiative for childhood cancer. Increasing access, advancing quality, saving lives. Oct 28, 2021. https://www.who.int/publications/i/item/9789240025271 (accessed June 27, 2023). [Google Scholar]
- 41.Arias AV, Sakaan FM, Puerto-Torres M, et al. Development and pilot testing of PROACTIVE: A pediatric onco-critical care capacity and quality assessment tool for resource-limited settings. Cancer Med 2022; 12: 6270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walubita M, Sikateyo B, Zulu JM. Challenges for health care providers, parents and patients who face a childhood cancer diagnosis in Zambia. BMC Health Serv Res 2018; 18: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cayrol J, Ilbawi A, Sullivan M, Gray A. The development and education of a workforce in childhood cancer services in low- and middle-income countries: a scoping review protocol. Syst Rev 2022; 11: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agulnik A, Mora Robles LN, Forbes PW, et al. Improved outcomes after successful implementation of a pediatric early warning system (PEWS) in a resource-limited pediatric oncology hospital. Cancer 2017; 123: 2965–74. [DOI] [PubMed] [Google Scholar]
- 45.Agulnik A, Méndez Aceituno A, Mora Robles LN, et al. Validation of a pediatric early warning system for hospitalized pediatric oncology patients in a resource-limited setting. Cancer 2017; 123: 4903–13. [DOI] [PubMed] [Google Scholar]
- 46.Agulnik A, Antillon-Klussmann F, Soberanis Vasquez DJ, et al. Cost-benefit analysis of implementing a pediatric early warning system at a pediatric oncology hospital in a low-middle income country. Cancer 2019; 125: 4052–58. [DOI] [PubMed] [Google Scholar]
- 47.Agulnik A, Muniz-Talavera H, Pham LTD, et al. Effect of paediatric early warning systems (PEWS) implementation on clinical deterioration event mortality among children with cancer in resource-limited hospitals in Latin America: a prospective, multicentre cohort study. Lancet Oncol 2023; published online July 8. 10.1016/S1470-2045(23)00285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirochnick E, Graetz DE, Ferrara G, et al. Multilevel impacts of a pediatric early warning system in resource-limited pediatric oncology hospitals. Front Oncol 2022; 12: 1018224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sterling SA, Miller WR, Pryor J, Puskarich MA, Jones AE. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: a systematic review and meta-analysis. Crit Care Med 2015; 43: 1907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans IVR, Phillips GS, Alpern ER, et al. Association between the New York Sepsis Care Mandate and in-hospital mortality for pediatric sepsis. JAMA 2018; 320: 358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukkada S, Smith CK, Aguilar D, et al. Evaluation of a fever-management algorithm in a pediatric cancer center in a low-resource setting. Pediatr Blood Cancer 2018; 65: e26790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antimicrobial Resistance C.Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedrich P, Lam CG, Itriago E, Perez R, Ribeiro RC, Arora RS. Magnitude of treatment abandonment in childhood cancer. PLoS One 2015; 10: e0135230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta S, Yeh S, Martiniuk A, et al. The magnitude and predictors of abandonment of therapy in paediatric acute leukaemia in middle-income countries: a systematic review and meta-analysis. Eur J Cancer 2013; 49: 2555–64. [DOI] [PubMed] [Google Scholar]
- 55.Chantada GL, Qaddoumi I, Canturk S, et al. Strategies to manage retinoblastoma in developing countries. Pediatr Blood Cancer 2011; 56: 341–48. [DOI] [PubMed] [Google Scholar]
- 56.Ethier MC, Blanco E, Lehrnbecher T, Sung L. Lack of clarity in the definition of treatment-related mortality: pediatric acute leukemia and adult acute promyelocytic leukemia as examples. Blood 2011; 118: 5080–83. [DOI] [PubMed] [Google Scholar]
- 57.Green AL, Furutani E, Ribeiro KB, Rodriguez Galindo C. Death within 1 month of diagnosis in childhood cancer: an analysis of risk factors and scope of the problem. J Clin Oncol 2017; 35: 1320–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furutani E, Rodriguez-Galindo C, Green AL. Early death in pediatric cancer: remaining questions and next steps. Oncotarget 2017; 8: 96478–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data (including data dictionaries) that underlie the results reported in this Article, as well as the study protocol, statistical analysis plan, and analytical code will be made available beginning 6 months and ending 5 years following publication to researchers who provide a methodologically sound proposal. Proposals should be directed to the corresponding author via email; data requestors will need to sign a data access agreement.