Abstract
Background
The main drawback of oral contraceptives (OC) and hormone replacement therapy (HRT) is an increased risk of venous and, to a lesser extent, arterial thrombosis.
Materials and methods
This narrative, case-based review describes the effect of available estrogens and progestogens on the hemostatic system and their potential impact on the risk of thrombosis. Clinical cases are used to illustrate different options for prescribing OC and HRT in the real-word. The aim is to offer discussion topics that could be helpful to guide the choice of different hormonal treatments over a woman’s lifetime and in the presence of risk factors.
Results
We describe physio-pathological changes occurring during the administration of hormonal therapies. Furthermore, we analyze the risk of venous and arterial thrombosis associated with different products, routes of administration and additional risk factors. New hormonal preparations, such as estradiol combined with dienogest, as well as non-oral hormonal therapies, are suggested to decrease thrombotic risk significantly.
Discussion
The availability of many products and different routes of administration allow most women to safely use contraception, as well as HRT. We encourage careful counselling instead of inflexible or fearful behavior, as expanding options and choices will allow women to make the best decisions for their health.
Keywords: contraceptives, estrogens, progestogens, thrombosis, hormone replacement therapy
INTRODUCTION
Thrombotic events are the possible consequence of rare inherited disorders, but are more often complications of several, heterogeneous clinical conditions or diseases1,2. They can even occur after procedures necessary to control bleeding, such as blood transfusions3. It is well known that sex hormones have an impact on red blood cells, modulating erythrocyte calcium influx4, as well as on coagulation pathways5,6. In women, the menstrual cycle, pregnancy and hormone intake are all responsible for dramatic changes in coagulation parameters, thus conditioning many biological activities7.
The aim of this case-based review is to analyze the effect of estrogens and progestogens on the hemostatic system and the risk of thrombosis. Clinical cases are presented in order to illustrate different solutions to deal with some common scenarios that clinicians face daily. The ultimate aim should be to avoid inflexible or fearful behavior and encourage clinicians to provide the careful counseling that is needed for offering the best individual solution.
CLINICAL CASE 1
A 40-year-old woman with no personal or family history of venous thromboembolism (VTE) would like to start oral contraception after two pregnancies. Her body mass index (BMI) is 24, she smokes 10 cigarettes per day and does not show additional risk factors for VTE. Her gynecologist has concerns about the woman’s thrombotic risk because of her age and smoking habit.
CLINICAL CASE 2
A 24-year-old woman is diagnosed with polycystic ovary syndrome. She is obese (BMI=31), does not smoke and does not show additional risk factors. She needs combined oral contraceptives (COC) to control her menstrual cycles, lower androgen levels and reduce acne.
PROTHROMBOTIC EFFECT OF ESTROGENS AND PROGESTOGENS
During pregnancy, estrogens play a pivotal role in driving a significant increase in procoagulant activity. Indeed, we have observed increased levels of factors VII, X, VIII, fibrinogen and von Willebrand factor, especially from the third trimester to delivery. Furthermore, some authors have reported higher levels of prothrombin factor F 1+2 and thrombin-antithrombin complexes in addition to acquired protein C resistance and inhibition of fibrinolysis7.
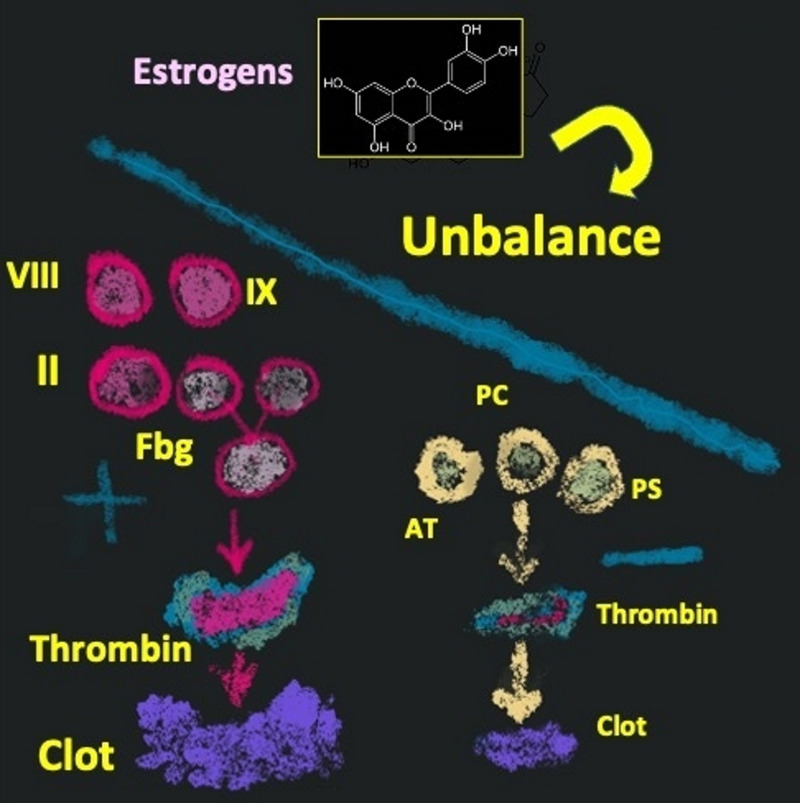
Although the purpose of these changes is to reduce the occurrence of peri-partum hemorrhage, they can foster thromboembolic events. Indeed, the risk of VTE is about eight-fold higher during pregnancy than in non-pregnant women8. Women taking oral contraceptives (OC) that contain estrogens and progestogens show blood coagulation activation similar to that observed during pregnancy. These drugs cause increases in factor II (prothrombin), factor VIII, factor IX, and fibrinogen along with decrease of the natural anticoagulants (antithrombin, protein C and protein S), thus inducing an imbalance between blood coagulation activity and its negative feedback control. It is not surprising that these changes are associated with a higher risk of VTE9 (Figure 1).
Figure 1.
Hemostatic changes in women taking the oral contraceptive pill
Fbg: fibrinogen; AT: antithrombin; PC: protein C; PS: protein S.
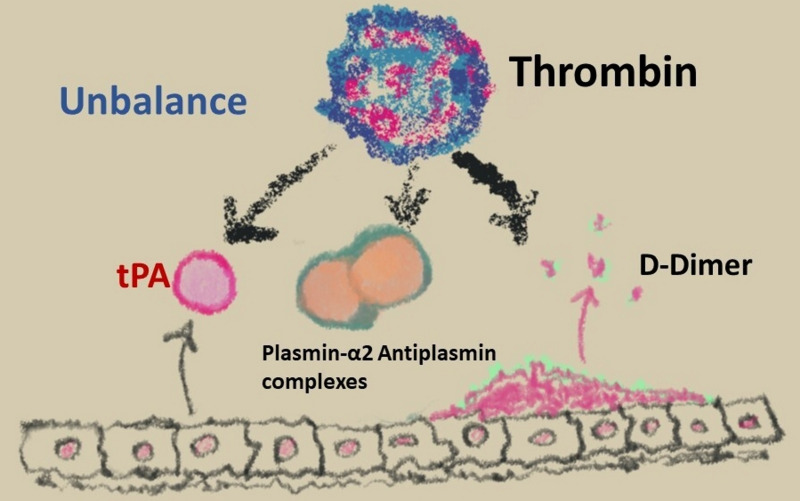
The fibrinolytic system is also affected by OC, as demonstrated by the increase of tissue-plasminogen activator, plasmin-alpha2-antiplasmin complexes and D-dimer in OC users. Parallel to these changes, an increase of anti-fibrinolytic activity is also observed, with a consequent imbalance between blood coagulation and fibrinolysis (Figure 2). These modifications are more pronounced in women taking the third-generation than in those taking second-generation COC9.
Figure 2.
Anti-fibrinolytic activity in women taking the oral contraceptive pill
tPA: tissue plasminogen activator.
COMBINED ORAL CONTRACEPT IVES AND RISK OF VENOUS THROMBOEMBOLISM
The use of COC is associated with a three- to six-fold increased risk of venous thrombosis10 (Table I). However, the absolute risk of COC-associated VTE compared with risk in non-users is quite low (3–15/10,000 woman-years in users vs 1–5/10,000 risk in non-users)11. A recent review focusing on the effectiveness and adverse effects of COC has shown that estrogen-containing products, such as COC, increase the risk of venous thrombosis from 2–10 venous thrombotic events per 10,000 women-years to 7–10 venous thrombotic events per 10,000 women-years12 compared to progestin-only or nonhormonal methods.
Table I.
Summary of information useful for clinical practice
| Preparation | Type of study, Reference No. (year) | Main message |
|---|---|---|
| COC | Review, 10 (2001) | COC users: 3–6 times higher risk of VTE than non-users |
| Historical cohort study, 36 (2012) | COC with 20 μg EE/desogestrel/gestodene vs non-users: 1.5–2 times higher risk of MI | |
| Progestogenonly pill | Systematic review, 31 (2018) | Adjusted RR for VTE, MI and stroke not statistically significant vs non-users |
| LNG-IUD | Large, retrospective study, 43 (2012) | No increased risk of VTE vs non-users |
| Estradiol valerate/dienogest | Prospective, controlled, non-interventional, 42 (2016) | VTE and serious cardiovascular events not significantly different vs EE/LNG users |
| Non-oral preparations | Retrospective study, 43 (2012) | Transdermal patches and vaginal ring vs non-users or users of COC containing LNG: ~7–8 fold higher VTE risk |
| Historical cohort study, 36 (2012) | Vaginal ring vs non-users: 2.5 higher risk of MI | |
| Hormone replacement therapy | Randomized controlled trial, 51 (2002) | VTE, coronary heart disease and stroke 7–8 fold higher in CEE/MPA Estradiol confers a lower VTE risk than CEE w/o progestins or combined preparations |
| Case-control, 54 (2019) | CEE/MPA users show 2-fold higher VTE risk than non-users; estradiol/dydrogesterone users had the lowest risk Transdermal preparations did not increase VTE risk |
COC: combined oral contraceptives; VTE: venous thromboembolism; EE: ethinylestradiol; MI: myocardial infarction; RR: relative risk; LNG: levonorgestrel; CEE: conjugated equine estrogen; MPA: medroxyprogesterone acetate.
High-dose COC with 50 μg or more ethinylestradiol induce a two-fold higher risk of thrombosis than COC containing 20–30 μg of ethinylestradiol13 (Table I). Although the dosage of the estrogens has a crucial role, an important role is also played by the combined progestogens. From the early 1980s until late 1990s a wide range of progestogens became available. At the beginning, norgestimate, desogestrel and gestodene (third-generation products) were marketed as compounds with fewer side effects than norgestrel/levonorgestrel14,15. Afterwards, drospirenone (fourth-generation) entered the market for its potential role in modulating the prothrombotic effect of estrogens16.
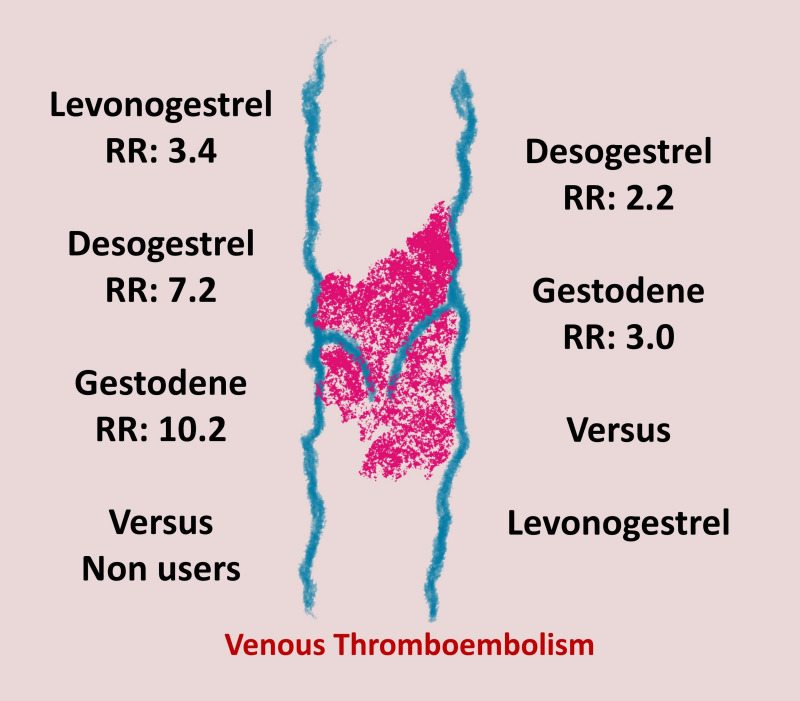
Several case-control studies showed that COC with the third- and fourth-generation progestogens increase the risk of VTE up to three-fold when compared with the risk of those containing second-generation progestogens. Figure 3 depicts the relative risk (RR) of VTE in users of levonorgestrel, desogestrel, and gestodene in comparison with that of non-users and levonorgestrel, respectively17. In 2018, a meta-analysis, including 17 studies and almost 24 million women, confirmed these findings and added information on drospirenone which, in combined preparations, showed an odds ratio (OR) of 1.5418.
Figure 3.
Relative Risk of venous thromboembolism in women taking third vs second generation oral contraceptive pills
RR: relative risk.
RISK FACTORS
Age
A series of observational studies found that age is an independent risk factor for VTE in the setting of women taking COC11, with a hazard ratio (HR) of 1.86 (1.41–2.46) for those in the age range of 10–34 years and 1.35 (1–1.82) in the range of 35–55 years19.
Body mass index
Obesity, defined as a BMI above 30, is per se an important risk factor for VTE20. The association of obesity with OC use and VTE was first investigated in 3,834 women with a first venous thrombotic event and in 4,683 controls21. Obese COC users had an OR for VTE of 23.8 (95% CI: 13.4–42.3) in comparison with non-obese non-users22. A comprehensive analysis of the literature performed in 2017 showed a ten-fold higher VTE risk in obese women11, whereas COC users who were overweight (BMI >25<30) had an OR of 11.6 ( 95% CI: 7.5–18.1)22.
Family history
COC use in women without a family history of thrombotic events is associated with an adjusted OR of 2.6 (95% CI: 1.9–3.6), whereas COC users with family history show an adjusted OR of 5.9 (95% CI: 3.3–11). These findings are derived from a case-control study, which evaluated 1,288 women (18–64 years) with a first VTE event and 1,327 age-matched controls23.
We underscore that clinicians should pay attention to how they collect family history. Indeed, there are potential differences in assessing and understanding family history among patients and medical professionals24. However, a patient’s maintenance of her pedigree provides increased patient awareness, ultimately improving health care and research25.
THROMBOPHILIA
Table II summarizes inherited and acquired thrombophilias according to severity. Mild thrombophilia (factor V Leiden or prothrombin G20210A heterozygosis) significantly increases VTE risk in COC users (RR=5.89; 95% CI: 4.21–8.23)26. Absolute estimates show that VTE risk is far higher in COC users with severe thrombophilia than in those with mild thrombophilia (4.3 to 4.6 vs 0.49 to 2.0 per 100 pill-years, respectively)26. Whether or not it is helpful to investigate thrombophilia before taking COC in order to avoid VTE is still debated. The World Health Organization (WHO) recommends against universal thrombophilia screening before prescribing COC, because of the low prevalence of thrombophilia and high screening costs. However, this approach does not take into account the long-term implications of VTE and/or the lifetime benefits of awareness of inherited thrombophilia24. Screening for the most frequent thrombophilias (factor V Leiden and prothrombin mutation) could be helpful, especially in women older than 35 years, i.e. when the VTE risk per se is significantly higher27.
Table II.
Mild and severe thrombophilic defects
| Mild thrombophilia | Heterozygosity for factor V Leiden, heterozygosity for prothrombin G20210A |
| Severe thrombophilia | Homozygosity for factor V Leiden, homozygosity for prothrombin G20210A, protein C, protein S, antithrombin deficiency |
Antiphospholipid antibodies, combined defects.
Lastly, in the absence of “ad hoc” studies, the use of hormones (oral pill, vaginal ring, transdermal patch) should be discouraged in women with positive antiphospholipid antibodies (with or without definite antiphospholipid syndrome)26. However, some authors suggest that a progestogen-only pill or contraceptives other than COC (intrauterine devices or implants ) may be used in this setting28.
THE PROGESTOGEN-ONLY PILL
The progestogen-only pill does not have any significant effects on either the levels of blood coagulation factors or fibrinolytic activity29. These findings suggest that progestogens likely reduce the trigger of blood coagulation in vivo, i.e. tissue factor, by interfering with its natural inhibitor, the tissue factor pathway inhibitor30.
A systematic review, including 19 observational studies (7 cohort and 12 case-control studies), found that the risk of VTE, myocardial infarction, hypertension and diabetes were not significantly higher in users than in non-users (Table I)31. In contrast, the RR for injectable progestogen alone revealed an increased VTE risk (RR=2.62, 95% CI: 1.74–3.94).
It is worth noting that recent large nationwide case-control studies demonstrate that higher doses of norethindrone acetate and medroxyprogesterone acetate are significantly associated with two- to three-fold higher VTE risk compared to non-users (including those using levonorgestrel intrauterine devices [LNG-IUD], etonogestrel implants, and oral progesterone)32,33.
COMBINED ORAL CONTRACEPT IVES AND RISK OF ARTERIAL EVENTS
In a case-control study, 248 women (18–49 years old) who had had a myocardial infarction and 925 age-matched controls showed an OR for COC of 2.0 (96% CI: 1.5–2.8). Common inherited thrombophilia did not affect the risk, whereas smoking significantly increased the odds of having a myocardial infarction during COC use (OR=13.6, 95% CI: 7.9–23.4)34.
As for ischemic stroke, another case-control study35, including 203 women with ischemic stroke and 925 controls, showed that COC users had a significantly higher risk, with an OR of 2.3 (95% CI: 1.6–3.3).
Later, a 15-year cohort study, carried out in a sample of 1,626,158 women, confirmed that smoking significantly increases the risk (OR=4.4, 95% CI: 2.7–7.3) of myocardial infarction and stroke in women between 15–49 years with a negative clinical history of cardiovascular disease36 (Table I). The combination of ethinylestradiol 20 μg and different progestogens showed an overall risk of cardiovascular disease of 1.5 (1.3–1.9) for desogestrel, 1.7 (1.4 to 2.1) for gestodene and 0.9 (0.2 to 3.5) for drospirenone36. As for the route of administration, vaginal rings increased the risk of arterial events (RR=2.5, 95% CI: 1.4–4.4), whereas transdermal patches did not (RR=3.2, 95% CI: 0.8–12.6).
NEW HORMONAL PREPARATIONS
In 2009, COC containing estradiol, instead of ethinylestradiol, entered the market. Estradiol had a lesser effect than ethinylestradiol on some metabolic and hepatic parameters and blood pressure37,38. Furthermore, some markers of blood activation, such as F1+2 peptide and D-dimer, were unchanged after estradiol administration if combined with dienogest (not with levonorgestrel)39–41.
All these reassuring biochemical findings prompted planning of the INAS-SCORE, a large international prospective, controlled, non-interventional cohort study, designed to compare the occurrence of VTE and other cardiovascular events in users of estradiol valerate/dienogest and other ethinylestradiol preparations. This study recruited 53,750 women from 2009 to 201642 (Table I). The authors reported a lower incidence rates of VTE and serious cardiovascular events in users of estradiol valerate/dienogest than in users of other estrogens and levonorgestrel preparations (adjusted HR of 0.4 and 0.5 for estradiol valerate/dienogest and levonorgestrel preparations, respectively). Notably, this difference was not statistically significant, as although the upper bounds of the 95% confidence intervals were 0.98 (VTE) and 0.96 (serious cardiovascular events), the 95% confidence intervals included unity.
NON-ORAL HORMONAL THERAPY
Progestin-only, long-acting methods, such as the LNG-IUD and subdermal implants, are effective contraceptive systems, with a rate of less than 1 pregnancy per 100 women per year12. The safety of these methods has been examined in a large retrospective Danish study carried out in about 10 million women43 (Table I). Non-users of hormonal contraception showed VTE rate of 2.1 per 10,000 woman years, whereas progestin-only and non-hormonal methods, such as implants and condoms, were associated with rare serious risks. Users of transdermal patches and vaginal rings showed VTE incidences per 10,000 exposure years of 9.7 and 7.8 events, respectively12.
The etonogestrel subdermal implant is a new long-lasting contraceptive method. It is effective for up to 5 years and is easily placed or removed44. Available data suggest that, as for LNG-IUD and the progestin-only pill, subdermal implants are associated with the lowest risks12,45.
Consistent with these data, the trend in choosing type of contraception has been changing. Indeed, in Northern Europe women older than 35 are mainly prescribed a LNG-IUD or progestin-only pills and younger women primarily use second-generation COC46–48. These changes in prescription habits are expected to prevent 300 first venous thromboses annually among young Danish women46.
Clinical case 1 (continued)
This woman has two risk factors: age above 35 years and cigarette smoking. She should stop smoking before starting COC or should be addressed to a progestin-only pill. Consistent with current guidelines, she should be informed that does not need to undergo thrombophilia screening before prescription. However, should she undergo screening for any other reason and was found to have a state of mild thrombophilia, the VTE risk would be between 0.49 and 2.0 per 100 pill-years26. Smoking significantly increases the odd of arterial events. Transdermal patches, LNG-IUD or an etonogestrel implant are options for her, as all show the lowest thrombotic risk12,49. In conclusion, thrombophilia screening does not add further information to guide the prescription of safer contraception methods.
Clinical case 2 (continued)
Caution should be taken when suggesting COC to obese women21. We therefore suggest our patient to reduce her BMI. In the meantime, which hormones can we suggest? The WHO states that in similar cases, use of COC “generally outweighs the theoretical or proven risks”. Based on the available literature, we suggest LNG-containing COC. However, a progestin-only pill, LNG-IUD or implant are also possible options for this case50.
CLINICAL CASE 3
Our third case is a 47-year-old woman in perimenopause with abnormal uterine bleeding and iron-deficient anemia. She has undergone hysteroscopy and endometrial biopsy, which has revealed glandular hyperplasia without any sign of dysplasia or atypia. She does not have additional risk factors.
Her gynecologist suggests using progestogen. Should we use other products? Should she be given HRT?
HORMONE REPLACEMENT THERAPY
In 2002, a randomized controlled phase III study was carried out in the USA with the aim of investigating risks and benefits of estrogens plus progestins in healthy postmenopausal women aged 50 to 79 years. The women received conjugated equine estrogens (CEE), 0.625 mg/day plus medroxyprogesterone acetate, 2.5 mg/day in one tablet (No.=8,506) or placebo (No.=8,102)51 (Table I). The study was designed to have a long follow-up (8.5 years).
Results were not in favor of the estrogen-progestin combination, as the absolute excess risks per 10,000 person-years were seven-fold higher for coronary heart disease, eight-fold higher for strokes, eight-fold higher for pulmonary embolism and more than eight-fold higher for invasive breast cancer. Although the small increase of absolute risks and the inclusion in the trial of women within a wide range of age (50 to 79 years), the findings caused concern52 and a consequent reduction in the use of HRT. The NICE guidelines published in 201553 underscored a crucial point: the need to counsel women about the net benefit of HRT, sharing with them the choice of starting and duration of treatment.
In 2019, a large case-control study was planned in the UK to assess possible associations between VTE risk and all types of HRT from 1998 to 2017. The study enrolled about 90,000 women between 40–79 years with a primary diagnosis of VTE and 400,000 controls54. Oral HRT was demonstrated to confer an increased risk of VTE in women exposed in comparison with those not exposed (OR=1.58, 95% CI: 1.52–1.64); this finding included both estrogen-only products (OR=1.40, 95% CI: 1.32–1.48) and combined preparations (OR=1.73, 95% CI: 1.65–1.81). Estradiol showed a lower risk than CEE without progestins (OR=0.85, 95% CI: 0.76–0.95) and combined preparations (OR=0.83, 95% CI: 0.76–0.91). Use of CEE with medroxyprogesterone acetate was associated with a higher risk than no exposure (OR=2.10, 95% CI: 1.92–2.31), whereas estradiol with dydrogesterone did not increase the risk (OR=1.18, 95% CI: 0.98–1.42). It is noteworthy that transdermal preparations (different regimens) were not associated with increased VTE risk (overall adjusted OR=0.93, 95% CI: 0.87–1.01)54 (Table I).
As for the risk of ischemic stroke, literature data are not univocal55. Results from the double-blind randomized controlled trial WHI showed a higher risk of ischemic stroke in women administered HRT (HR=1.44, 95% CI: 1.09–1.90) than in those receiving placebo56. Conversely, another randomized controlled trial of HRT vs placebo, the HERS study, showed no significant difference in the number of ischemic strokes in the two groups (OR=1.18, 95% CI: 0.83–1.67) after a mean follow-up of 4 years57.
Early administration of HRT after menopause has been associated with lower mortality. Indeed, a Cochrane systematic review58 showed that women who started HRT less than 10 years after menopause had lower mortality (RR=0.70, 95% CI: 0.52–0.95) and lower mortality from cardiovascular causes and non-fatal myocardial infarction (RR=0.52, 95% CI: 0.29–0.96), although VTE risk was higher (RR=1.74, 95% CI: 1.11–2.73) compared to that of women taking a placebo or no treatment. In contrast, women who started HRT more than 10 years after the menopause had an increased risk of stroke (RR=1.21, 95% CI: 1.06–1.38) and VTE (RR=1.96, 95% CI: 1.37–2.80). A recent position statement by The North American Menopause Society59 suggests HRT in women younger than 60 years, who are within 10 years from the onset of menopause and do not have contraindications, because of the favorable benefit-risk ratio for HRT.
Using transdermal estradiol in association with micronized progesterone or dydrogesterone may limit both the VTE risk associated with oral estrogens and the risk of breast cancer associated with synthetic progestogens60.
In women with previous thrombotic events, multiple risk factors or severe thrombophilia, we need to be more cautious and consider alternative strategies. For instance, non-hormonal therapies, such as certain antidepressant agents, gabapentinoids, and clonidine may offer some relief from hot flushes but have their own adverse effects. Likewise, cognitive behavioral therapy may have positive effects on vasomotor symptoms61.
Clinical case 3 (continued)
Abnormal uterine bleeding affects 10–30% of middle-aged women and is the reason for about one-third of all outpatient gynecological visits62. Hormonal management is considered the first line of medical therapy for patients with acute abnormal uterine bleeding without known or suspected bleeding disorders63.
Our patient can safely use a progestin-only pill or LNG-IUD. However, after counseling taking into account some aspects of her sexuality and her psycho-relational discomfort, she could also be administered HRT.
CONCLUSIONS
Hormonal therapy is undoubtedly burdened with a thrombotic risk. However, inflexible or fearful behaviors should be avoided. In daily practice, clinicians should find the best solution for each woman, taking into account all the possible choices.
Several additional risk factors need to be taken into account when evaluating net clinical benefit. The identification of an individual risk profile is helpful to define the better strategy for each woman. Therefore, counseling has a pivotal role to discuss the pros and cons of each approach, allowing women to make the best final decision for their health.
Footnotes
AUTHORS’ CONTRIBUTIONS: DB and FM conceived the study and prepared the original draft of the manuscript; DB, EG and FM were responsible for the methodology and wrote, reviewed and edited final version.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Muñoz M, Stensballe J, Ducloy-Bouthors AS, Bonnet MP, De Robertis E, Fornet I, et al. Patient blood management in obstetrics: prevention and treatment of postpartum haemorrhage. A NATA consensus statement. Blood Transfus. 2019;17:112–136. doi: 10.2450/2019.0245-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grandone E, Tiscia GL, Ostuni A, di Mauro L, Mastroianno M, Coffetti N, et al. FCSA (Italian Federation of Anticoagulation Clinics) Anemone study: prevalence of risk factors for superficial vein thrombosis in a large Italian population of blood donors. J Thromb Thrombolysis. 2020;50:689–696. doi: 10.1007/s11239-020-02140-5. [DOI] [PubMed] [Google Scholar]
- 3.Grandone E, Colaizzo D, Mastroianno M, Petruzzelli F, di Mauro L, Carella M, et al. Pulmonary embolism associated with transfusion after severe post-partum haemorrhage: is less more? Blood Transfus. 2020;18:13–19. doi: 10.2450/2019.0060-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang F, Hazegh K, Sinchar D, Guo Y, Page GP, Mast AE, et al. Sex hormone intake in female blood donors: impact on haemolysis during cold storage and regulation of erythrocyte calcium influx by progesterone. Blood Transfus. 2019;17:263–273. doi: 10.2450/2019.0053-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kangasniemi MH, Arffman RK, Joenväärä S, Haverinen A, Luiro K, Tohmola T, et al. Ethinylestradiol in combined hormonal contraceptive has a broader effect on serum proteome compared with estradiol valerate: a randomized controlled trial. Hum Reprod. 2023;38:89–102. doi: 10.1093/humrep/deac250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandone E, Chiocca S, Castelvecchio S, Fini M, Nappi R representatives for Gender Medicine of Scientific Hospitalization and Treatment Institutes-Italian Ministry of Health. Thrombosis and bleeding after COVID-19 vaccination: do differences in sex matter? Blood Transfus. 2023;21:176–184. doi: 10.2450/2022.0060-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004;114:409–414. doi: 10.1016/j.thromres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 8.James AH. Thrombosis in pregnancy and maternal outcomes. Birth Defects Res C Embryo Today. 2015;105:159–166. doi: 10.1002/bdrc.21106. [DOI] [PubMed] [Google Scholar]
- 9.Meijers JC, Middeldorp S, Tekelenburg W, van den Ende AE, Tans G, Prins MH, et al. Increased fibrinolytic activity during use of oral contraceptives is counteracted by an enhanced factor XI-independent down regulation of fibrinolysis: a randomized cross-over study of two low-dose oral contraceptives. Thromb Haemost. 2000;84:9–14. [PubMed] [Google Scholar]
- 10.Vandenbroucke JP, Rosing J, Bloemenkamp KWM, Middeldorp S, Helmerhorst FM, Bouma BN, et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med. 2001;344:1527–1535. doi: 10.1056/NEJM200105173442007. [DOI] [PubMed] [Google Scholar]
- 11.Practice Committee of the American Society for Reproductive Medicine. Combined hormonal contraception and the risk of venous thromboembolism: a guideline. Fertil Steril. 2017;107:43–51. doi: 10.1016/j.fertnstert.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Teal S, Edelman A. Contraception selection, effectiveness, and adverse effects: a review. JAMA. 2021;326:2507–2518. doi: 10.1001/jama.2021.21392. [DOI] [PubMed] [Google Scholar]
- 13.Dragoman MV, Tepper NK, Fu R, Curtis KM, Chou R, Gaffield ME. A systematic review and meta-analysis of venous thrombosis risk among users of combined oral contraception. Int J Gynaecol Obstet. 2018;141:287–294. doi: 10.1002/ijgo.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandi G, Del Savio MC, Facchinetti F. The paradigm of norgestimate: a third-generation testosterone-derivative progestin with a peripheral anti-androgenic activity and the lowest risk of venous thromboembolism. Expert Rev Clin Pharmacol. 2021;14:211–224. doi: 10.1016/j.contraception.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Morimont L, Haguet H, Dogné JM, Gaspard U, Douxfils J. Combined oral contraceptives and venous thromboembolism: review and perspective to mitigate the risk. Front Endocrinol (Lausanne) 2021;12:769187. doi: 10.3389/fendo.2021.769187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lete I, Chabbert-Buffet N, Jamin C, Lello S, Lobo P, Nappi RE, et al. Haemostatic and metabolic impact of estradiol pills and drospirenone-containing ethinylestradiol pills vs. levonorgestrel-containing ethinylestradiol pills: a literature review. Eur J Contracept Reprod Health Care. 2015;20:329–343. doi: 10.3109/13625187.2015.1050091. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Effect of different progestagens in low oestrogen oral contraceptives on venous thromboembolic disease. World Health Organization collaborative study of cardiovascular disease and steroid hormone contraception. Lancet. 1995;346:1582–1588. [PubMed] [Google Scholar]
- 18.Oedingen C, Scholz S, Razum O. Systematic review and meta-analysis of the association of combined oral contraceptives on the risk of venous thromboembolism: the role of the progestogen type and estrogen dose. Thromb Res. 2018;165:68–78. doi: 10.1016/j.thromres.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Committee on Gynecologic Practice. ACOG Committee Opinion Number 540. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239–1242. doi: 10.1097/aog.0b013e318277c93b. [DOI] [PubMed] [Google Scholar]
- 20.Gregson J, Kaptoge S, Bolton T, Pennells L, Willeit P, Burgess S, et al. Emerging risk factors collaboration. Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol. 2019;4:163–173. doi: 10.1001/jamacardio.2018.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pomp ER, le Cessie S, Rosendaal FR, Doggen CJ. Risk of venous thrombosis: obesity and its joint effect with oral contraceptive use and prothrombotic mutations. Br J Haematol. 2007;139:289–296. doi: 10.1111/j.1365-2141.2007.06780.x. [DOI] [PubMed] [Google Scholar]
- 22.Bloemenkamp KV, Rosendaal FR, Helmerhorst FR, Buller HR, Vandenbroucke JP. Enhancement by factor V Leiden of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet. 1995;346:1593–1596. doi: 10.1016/s0140-6736(95)91929-5. [DOI] [PubMed] [Google Scholar]
- 23.Sonnevi K, Bergendal A, Adami J, Lärfars G, Kieler H. Self-reported family history in estimating the risk of hormone, surgery and cast related VTE in women. Thromb Res. 2013;132:164–169. doi: 10.1016/j.thromres.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Walter FM, Emery J. ‘Coming down the line’ -- patients’ understanding of their family history of common chronic disease. Ann Fam Med. 2005;3:405–414. doi: 10.1370/afm.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinton RB., Jr The family history: reemergence of an established tool. Crit Care Nurs Clin North Am. 2008;20:149–158. doi: 10.1016/j.ccell.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Vlijmen EF, Wiewel-Verschueren S, Monster TB, Meijer K. Combined oral contraceptives, thrombophilia and the risk of venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2016;14:1393–1403. doi: 10.1111/jth.13349. [DOI] [PubMed] [Google Scholar]
- 27.Franchini M, Martinelli I, Mannucci PM. Uncertain thrombophilia markers. Thromb Haemost. 2016;115:25–30. doi: 10.1160/TH15-06-0478. [DOI] [PubMed] [Google Scholar]
- 28.Sammaritano LR. Which hormones and contraception for women with APS? Exogenous hormone use in women with APS. Curr Rheumatol Rep. 2021;23:44. doi: 10.1007/s11926-021-01006-w. [DOI] [PubMed] [Google Scholar]
- 29.Schindler AE. Differential effects of progestins on hemostasis. Maturitas. 2003;46(Suppl 1):S31–S37. doi: 10.1016/j.maturitas.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Skouby SO, Sidelmann JJ. Impact of progestogens on hemostasis. Horm Mol Biol Clin Investig. 2018;37(2) doi: 10.1515/hmbci-2018-0041. [DOI] [PubMed] [Google Scholar]
- 31.Glisic M, Shahzad S, Tsoli S, Chadni M, Asllanaj E, Rojas LZ, et al. Association between progestin-only contraceptive use and cardiometabolic outcomes: a systematic review meta-analysis. Eur J Prev Cardiol. 2018;25:1042–1052. doi: 10.1177/2047487318774847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cockrum RH, Soo J, Ham SA, Cohen KS, Snow SG. Association of progestogens and venous thromboembolism among women of reproductive age. Obstet Gynecol. 2022;140:477–487. doi: 10.1097/AOG.0000000000004896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schink T, Princk C, Braitmaier M, Haug U. Use of combined oral contraceptives and risk of venous thromboembolism in young women: a nested case-control analysis using German claims data. BJOG. 2022;129:2107–2116. doi: 10.1111/1471-0528.17268. [DOI] [PubMed] [Google Scholar]
- 34.Tanis BC, van den Bosch MA, Kemmeren JM, Cats VM, Helmerhorst FM, Algra A, et al. Oral contraceptives and the risk of myocardial infarction. N Engl J Med. 2001;345:1787–1793. doi: 10.1056/NEJMoa003216. [DOI] [PubMed] [Google Scholar]
- 35.Kemmeren JM, Tanis BC, van den Bosch MA, Bollen EL, Helmerhorst FM, van der Graaf Y, et al. Risk of Arterial Thrombosis in Relation to Oral Contraceptives (RATIO) study: oral contraceptives and the risk of ischemic stroke. Stroke. 2002;33:1202–1208. doi: 10.1161/01.str.0000015345.61324.3f. [DOI] [PubMed] [Google Scholar]
- 36.Lidegaard Ø, Løkkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257–2266. doi: 10.1056/NEJMoa1111840. [DOI] [PubMed] [Google Scholar]
- 37.Kuhl H. Adverse effects of estrogen treatment: natural versus synthetic estrogens. In: Lippert TH, Mueck AO, Ginsburg J, editors. Sex Steroids and the Cardiovascular System. New York: The Parthenon Publishing Group; 1996. pp. 201–221. [DOI] [Google Scholar]
- 38.Grandi G, Xholli A, Napolitano A, Piacenti I, Bellafronte M, Cagnacci A. Prospective measurement of blood pressure and heart rate over 24 h in women using combined oral contraceptives with estradiol. Contraception. 2014;90:529–534. doi: 10.1016/j.contraception.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Junge W, Mellinger U, Parke S, Serrani M. Metabolic and haemostatic effects of estradiol valerate/dienogest, a novel oral contraceptive: a randomized, open-label, single-centre study. Clin Drug Investig. 2011;31:573–584. doi: 10.2165/11590220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Klipping C, Duijkers I, Parke S, Mellinger U, Serrani M, Junge W. Hemostatic effects of a novel estradiol-based oral contraceptive: an open-label, randomized, crossover study of estradiol valerate/dienogest versus ethinylestradiol/levonorgestrel. Drugs R D. 2011;11:159–170. doi: 10.2165/11591200-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaussem P, Alhenc-Gelas M, Thomas JL, Bachelot-Loza C, Remones V, Ali FD, et al. Haemostatic effects of a new combined oral contraceptive, nomegestrol acetate/17β-estradiol, (INASCORE with those of levonorgestrel/ethinyl estradiol. A double-blind, randomised study. Thromb Haemost. 2011;105:560–567. doi: 10.1160/TH10-05-0327. [DOI] [PubMed] [Google Scholar]
- 42.Dinger J, Do Minh T, Heinemann K. Impact of estrogen type on cardiovascular safety of combined oral contraceptives. Contraception. 2016;94:328–339. doi: 10.1016/j.contraception.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Lidegaard O, Nielsen LH, Skovlund CW, Løkkegaard E. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001–10. BMJ. 2012;344:e2990. doi: 10.1136/bmj.e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali M, Akin A, Bahamondes L, et al. WHO Study Group on Subdermal Contraceptive Implants for Women. Extended use up to 5 years of the etonogestrel-releasing subdermal contraceptive implant: comparison to levonorgestrel-releasing subdermal implant. Hum Reprod. 2016;31:2491–2498. doi: 10.1093/humrep/dew222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tepper NK, Dragoman MV, Gaffield ME, Curtis KM. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130–139. doi: 10.1016/j.contraception.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kristensen SIP, Lidegaard Ø. Hormonal contraceptive use in Denmark 2010–2019. Dan Med J. 2021;68:A08200599. [PubMed] [Google Scholar]
- 47.Lindh I, Skjeldestad FE, Gemzell-Danielsson K, et al. Contraceptive use in the Nordic countries. Acta Obstet Gynecol Scand. 2017;96:19–28. doi: 10.1111/aogs.13055. [DOI] [PubMed] [Google Scholar]
- 48.Vigoureux S, Le Guen M. Current knowledge on contraceptive knowledge in France: CNGOF Contraception Guidelines. Gynecol Obstet Fertil Senol. 2018;46:777–785. doi: 10.1016/j.gofs.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 49.van Hylckama Vlieg A, Helmerhorst FM, Rosendaal FR. The risk of deep venous thrombosis associated with injectable depot-medroxyprogesterone acetate contraceptives or a levonorgestrel intrauterine device. Arterioscler Thromb Vasc Biol. 2010;30:2297–2300. doi: 10.1161/ATVBAHA.110.211482. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 5th edn. 2015. [Accessed on 13/02/2023]. Available at: https://www.who.int/publications/i/item/9789241549158. [PubMed]
- 51.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 52.Fletcher SW, Colditz GA. Failure of estrogen plus progestin therapy for prevention. JAMA. 2002;288:366–368. doi: 10.1001/jama.288.3.366. [DOI] [PubMed] [Google Scholar]
- 53.2019 surveillance of menopause: diagnosis and management (NICE guideline NG23) [Internet] London: National Institute for Health and Care Excellence (NICE); 2019. Dec 5, [PubMed] [Google Scholar]
- 54.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kremer C, Gdovinova Z, Bejot Y, Heldner MR, Zuurbier S, Walter S, et al. European Stroke Organisation guidelines on stroke in women: management of menopause, pregnancy and postpartum. Eur Stroke J. 2022;7:I–XIX. doi: 10.1177/23969873221078696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 57.Simon JA, Hsia J, Culey JA, Richards C, Harris F, Fong J, et al. Postmenopausal hormone therapy and risk of stroke. The Heart and Estrogen-progestin Replacement Study (HERS) Circulation. 2001;103:638–642. doi: 10.1161/01.cir.103.5.638. [DOI] [PubMed] [Google Scholar]
- 58.Boardman HMP, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, et al. Hormone therapy for preventing cardiovascular disease in postmenopausal women. Cochrane Database Syst Rev. 2015;3:CD002229. doi: 10.1002/14651858.CD002229.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.The North American Menopause Society Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767–794. doi: 10.1097/GME.0000000000002028. [DOI] [PubMed] [Google Scholar]
- 60.Trémollieres FA, Chabbert-Buffet N, Plu-Bureau G, Rousset-Jablonski C, Lecerf JM, Duclos M, et al. Management of postmenopausal women: Collège National des Gynécologues et Obstétriciens Français (CNGOF) and Groupe d’Etude sur la Ménopause et le Vieillissement (GEMVi) Clinical Practice Guidelines. Maturitas. 2022;163:62–81. doi: 10.1016/j.maturitas.2022.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Yuksel N, Evaniuk D, Huang L, Malhotra U, Blake J, Wolfman W, et al. Guideline No. 422a. Menopause: vasomotor symptoms, prescription therapeutic agents, complementary and alternative medicine, nutrition, and lifestyle. J Obstet Gynaecol Can. 2021;43:1188–204. doi: 10.1016/j.jogc.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Shawki O, Wahba A, Magon N. Abnormal uterine bleeding in midlife: the role of levonorgestrel intrauterine system. J Midlife Health. 2013;4:36–39. doi: 10.4103/0976-7800.109634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ACOG committee opinion no. 557. Management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891–896. doi: 10.1097/01.AOG.0000428646.67925.9a. [DOI] [PubMed] [Google Scholar]