Abstract
Background
In transfusion-dependent thalassemia patients who started regular transfusions in early childhood, we prospectively and longitudinally evaluated the efficacy on pancreatic iron of a combined deferiprone (DFP) + desferrioxamine (DFO) regimen versus either oral iron chelator as monotherapy over a follow-up of 18 months.
Materials and methods
We selected patients consecutively enrolled in the Extension-Myocardial Iron Overload in Thalassemia network who received a combined regimen of DFO+DFP (No.=28) or DFP (No.=61) or deferasirox (DFX) (No.=159) monotherapy between the two magnetic resonance imaging scans. Pancreatic iron overload was quantified by the T2* technique.
Results
At baseline no patient in the combined treatment group had a normal global pancreas T2* (≥26 ms). At follow-up the percentage of patients who maintained a normal pancreas T2* was comparable between the DFP and DFX groups (57.1 vs 70%; p=0.517).
Among the patients with pancreatic iron overload at baseline, global pancreatic T2* values were significantly lower in the combined DFO+DFP group than in the DFP or DFX groups. Since changes in global pancreas T2* values were negatively correlated with baseline pancreas T2* values, the percent changes in global pancreas T2* values, normalized for the baseline values, were considered. The percent changes in global pancreas T2* values were significantly higher in the combined DFO+DFP group than in either the DFP (p=0.036) or DFX (p=0.030) groups.
Discussion
In transfusion-dependent patients who started regular transfusions in early childhood, combined DFP+DFO was significantly more effective in reducing pancreatic iron than was either DFP or DFX.
Keywords: thalassemia, chelating agents, magnetic resonance imaging, iron overload, pancreas
INTRODUCTION
Thalassemias are genetic disorders deriving from the reduced synthesis of one or more of the globin subunits of normal hemoglobin1. This causes an imbalanced alpha/beta-globin chain ratio, ineffective erythropoiesis, and reduced red blood cell survival, which lead, in patients with transfusion-dependent thalassemia (TDT), to a complete requirement for transfusions since early childhood and to the tendency to develop significant iron overload when iron chelation is not adequate. In the past, much of attention has been devoted firstly to cardiac iron overload as a leading cause of morbidity and mortality2 and thereafter to liver iron overload as an ultimate and extensive cause of complications in patients with TDT3,4. Therefore, a large amount of data is available on the efficacy of iron-chelating agents alone or in combination in the reduction of cardiac and hepatic iron overload and in the prevention of injury and/or failure of these organs5–10.
However, virtually all organs can be involved by iron accumulation and this process could be of relevance as a cause of further morbidity11,12. Among adult patients with TDT, endocrinopathies are the most widespread disorders resulting from iron overload13,14. As for most complications of thalassemia, endocrinopathies progress with aging, but can develop at an early age15. As for the heart and the liver, iron accumulation in endocrine glands can be non-invasively detected by magnetic resonance imaging (MRI)16,17.
In patients with thalassemia, diabetes mellitus (DM) has been historically investigated for its relationship mainly with iron deposition in the pancreatic cells and, to a lesser extent, with the impact of iron on insulin resistance. In a seminal study from 2012, Noetzli showed that, although total body iron burden, age, and body habitus could have an impact on glucose regulation, pancreatic iron was the strongest predictor of beta cell toxicity and glucose dysregulation18. More recently, attention has been focused on the evaluation of a cross-sectional link between pancreatic iron, glucose metabolism and cardiac complications in the largest cohort ever reported of well-treated and well-chelated TDT patients who started regular transfusions in early childhood11. It has been shown that patients with normal glucose metabolism had significantly higher global pancreas T2* values than patients with impaired fasting glucose, impaired glucose tolerance, and DM. Furthermore, a normal global pancreas T2* value has been demonstrated to have a negative predictive value of 100% for disturbances of glucose metabolism and for both cardiac iron and replacement myocardial fibrosis. However, only a single study has evaluated the longitudinal impact of different chelation regimens on modifying either pancreatic iron overload or pancreatic organ function. The three iron chelators in monotherapy were demonstrated to have a comparable efficacy in terms of pancreatic iron removal19.
The aim of this study was to evaluate longitudinally and prospectively the effects of combined desferrioxamine (DFO) and deferiprone (DFP) therapy vs DFP monotherapy and vs deferasirox (DFX) monotherapy on pancreatic iron overload and on glucose metabolism in a large clinical observational setting of TDT patients who have received regular transfusions since early childhood.
MATERIALS AND METHODS
Study population
The Extension-Myocardial Iron Overload in Thalassemia (E-MIOT) project is a network constituted by 11 MRI sites and 66 thalassemia centers, in which MRI examinations are performed using homogeneous, standardized and validated procedures20–22. All centers are linked by a shared database, collecting all clinical, laboratory, and instrumental information.
Among the first 1176 TDT patients who started regular transfusions in early childhood consecutively enrolled in the E-MIOT project from 2015 to 2020, 416 underwent an MRI follow-up study at 18±3 months, according to the protocol. Seventy-six patients in any treatment regimen were excluded because they changed chelation during the follow-up for clinical reasons, without repeating the MRI examination before the modification of the therapy due to logistic reasons. Ninety-two patients were not considered because they received different chelation regimens from those considered in the present study. So, we longitudinally and consecutively evaluated the 248 TDT patients who had maintained combined DFP+DFO therapy or DFP or DFX monotherapy between the two MRI scans. Thus, we identified three groups of patients: 28 treated with combined DFP+DFO, 61 with DFP, and 159 with DFX.
All chelators were prescribed based on the current clinical practice according to clinical, laboratory, and instrumental data. Patients’ interviews and estimates by investigators of each thalassemia center were used to determine compliance to the chelation therapy. Based on the agreement between the self-reported and prescribed chelation regimen, compliance was defined excellent (>80%), good (60–80%), and insufficient (<60%).
All patients had been regularly transfused since early childhood and started chelation therapy from the mid-to-late 1970s onwards, while patients born after the 1970s have received chelation therapy since early childhood. The study complied with the Declaration of Helsinki. All patients gave written informed consent to the protocol. The institutional review board approved this study.
Magnetic resonance imaging
MRI examinations were performed using conventional clinical 1.5 T scanners. Breath-holding in end-expiration and ECG-gating were applied.
The T2* technique was used for assessment of iron overload. The intra-operator, inter-operator, inter-study, and inter-center reproducibility of this technique had been previously demonstrated20,22,23. Five or more axial slices including the whole pancreas16, a mid-transverse hepatic slice24, and three parallel short-axis views (basal, medium and apical) of the left ventricle25,26 were obtained. T2* images were analyzed by expert operators blinded to the clinical and treatment condition using custom-written, previously validated software (HIPPO MIOT®)27. Three small regions of interest were manually drawn over the head, body, and tail of the pancreas encompassing parenchymal tissue and taking care to avoid large blood vessels or ducts and areas involved in susceptibility artefacts from gastric or colic intraluminal gas28. The global pancreatic T2* value was calculated as the mean of T2* values from the three regions. The lowest threshold of normal T2* pancreatic value was 26 ms16. Hepatic T2* values were calculated in a circular region of interest29 and were converted into liver iron concentration (LIC)30,31. A LIC >3 mg/g dry weight indicated iron overload. The myocardial T2* distribution was mapped into a 16-segment left ventricular model, according to the American Heart Association/American College of Cardiology model32. The global heart T2* value was obtained by averaging all segmental T2* values. A T2* measurement >20 ms was taken as a “conservative” normal value27,33.
Diagnostic criteria
A fasting plasma glucose <100 mg/dL and a 2-h glucose <140 mg/dL were considered to indicated normal glucose tolerance. Impaired fasting glucose was diagnosed in the presence of fasting plasma glucose levels between 100 and 126 mg/dL. Impaired glucose tolerance was defined by a 2-h plasma glucose between 140–200 mg/dL, with a fasting plasma glucose <126 mg/dL. DM was defined by a fasting plasma glucose ≥126 mg/dL or 2-h plasma glucose ≥200 mg/dL during an oral glucose tolerance test or a random plasma glucose ≥200 mg/dL with classic symptoms of hyperglycemia or hyperglycemic crisis34.
Risk classes were defined on the basis of oral glucose tolerance test results from worst to normal: DM → impaired glucose tolerance → impaired fasting glucose → normal glucose tolerance. For patients with alterations of glucose metabolism at baseline, improvement was defined as a transition to a better risk class, stabilization was defined as no change in the risk class, and worsening was defined as a transition to a worse risk class. For patients with normal glucose tolerance, worsening was represented by the transition to a worse risk class.
Statistical analysis
All data were analyzed using the SPSS version 17.0 statistical package (IBM, Armonk, NY, USA).
Continuous variables were described as mean ± standard deviation (SD). Categorical variables were expressed as frequencies and percentages. The normality of distribution of the parameters was assessed using the Kolmogorov-Smirnov test.
For continuous variables the percentage change was calculated as the difference between values at follow-up and baseline MRI scans, divided by the baseline value and multiplied by 100.
For the intra-treatment and the inter-treatment (between two groups) comparisons the changes between final and baseline values were used for each quantitative variable.
The inter-treatment comparison for the continuous variables at baseline and for changes between final and baseline values was made by an independent-samples t-test or by the Mann-Whitney test. Analysis of covariance (ANCOVA) was used to correct for variables significantly different between the two treatment groups at baseline and significantly associated to the dependent variable. The χ2 test was used for the comparison between categorical variables.
The intra-treatment comparison was performed with the Student’s t-test for paired data or the Wilcoxon’s signed-rank test for continuous variables.
Correlation analysis was performed using Pearson’s or Spearman’s test where appropriate.
A two-tailed p<0.05 was considered statistically significant.
RESULTS
Characterization of the whole patient population
The mean administered dosages of the chelators were: (i) DFP in the combined regimen 84.81±14.87 mg/kg body-weight/day with a frequency of 6.86±0.59 days/week and DFO in the combined regimen 37.96±7.79 mg/kg body-weight/day with a frequency of 3.86±1.74 days/week; (ii) DFP as monotherapy 83.93±14.39 mg/kg body-weight/day; (iii) DFX as monotherapy 24.91±7.06 mg/kg body-weight/day if in dispersible tablets (No.=112) and 17.89±4.23 mg/kg body-weight/day if in film-coated tablets (No.=47).
The percentage of patients with excellent/good levels of compliance to the chelation treatment was significantly lower in the combined DFP+DFO group than in either the DFP monotherapy group (82.1 vs 96.7%; p=0.030) or the DFX monotherapy group (82.1 vs 98.7%; p=0.001).
The mean time between the two MRI scans was comparable between the combined treatment and DFP monotherapy groups (18.02±2.44 vs 18.47±2.16 months; p=0.445) and between the combined treatment and DFX monotherapy groups (18.02±2.44 vs 18.98±1.88 months; p=0.065).
The clinically and instrumentally relevant baseline findings in the three treatment groups are summarized in Table I. The combined DFO+DFP group was less frequently splenectomized than the DFP group. Mean serum ferritin levels were significantly higher in the combined DFO+DFP group than in either the DFP or DFX monotherapy groups. Global heart T2* and global pancreas T2* values were significantly lower in the combined DFO+DFP group than in the DFP group or the DFX group.
Table I.
Descriptive statistics of the treatment groups at baseline. The p-values are for the comparisons between the combined desferrioxamine (DFO) + deferiprone (DFP) and DFP monotherapy groups and between the combined DFO+DFP and deferasirox monotherapy groups
| p-value | DFP (No.=61) | Combined DFO+DFP (No.=28) | DFX (No.=159) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 0.542 | 36.64±11.20 | 34.79±10.62 | 35.58±10.97 | 0.615 |
| Females, No. (%) | 0.149 | 27 (44.3) | 17 (60.7) | 86 (54.1) | 0.516 |
| Age at start of regular transfusions (months) | 0.933 | 12.59±11.08 | 12.04±9.29 | 16.40±17.22 | 0.728 |
| Units transfused in the last 12 months | 0.782 | 39.79±11.62 | 40.62±11.01 | 38.28±10.96 | 0.389 |
| Age at start of chelation (years) | 0.553 | 3.82±3.17 | 3.40±1.45 | 4.07±3.55 | 0.842 |
| Splenectomy, No. (%) | 0.019 | 44 (72.1) | 13 (46.4) | 85 (53.5) | 0.492 |
| Pre-transfusion hemoglobin (g/dL) | 0.169 | 9.51±0.40 | 9.66±0.47 | 9.58±0.46 | 0.478 |
| Mean ferritin (ng/mL) | 0.005 | 815.15±1,140.32 | 1,359.76±1,187.56 | 782.33±637.71 | 0.009 |
| MRI LIC (mg/g dw) | 0.421 | 4.85±5.21 | 8.33±10.27 | 5.76±8.77 | 0.090 |
| MRI LIC >3 mg/g dw, No. (%) | 0.296 | 32 (52.5) | 18 (64.3) | 64 (40.3) | 0.018 |
| Global heart T2* (ms) | 0.002 | 38.47±6.55 | 26.69±15.39 | 38.20±8.76 | <0.0001 |
| Global heart T2* <20 ms, No. (%) | <0.0001 | 2 (3.3) | 10 (35.7) | 12 (7.5) | <0.0001 |
| Global pancreas T2* (ms) | <0.0001 | 11.94±9.57 | 6.23±4.59 | 15.08±11.56 | <0.0001 |
| Global pancreas T2* <26 ms, No. (%) | 0.062 | 54 (88.5) | 28 (100) | 129 (81.1) | 0.012 |
| Altered glucose metabolism, No. (%) | 0.447 | 34 (55.7) | 18 (64.3) | 44 (27.7) | <0.0001 |
DFP: deferiprone; DFO: desferrioxamine; DFX: deferasirox; No.: number; MRI: magnetic resonance imaging; LIC: liver iron concentration; dw: dry weight.
Alterations of glucose metabolism were found in 96 (38.7%) patients: 17 had impaired fasting glucose, 33 impaired glucose tolerance, and 46 DM. The combined DFO+DFP group showed a significant higher frequency of alterations of glucose metabolism than the DFX group.
No patient in the combined group had a normal global pancreas T2* at the baseline MRI. At the follow-up the percentage of patients who maintained a normal global pancreas T2* value was 57.1% in the DFP group (4/7 patients) and 70% in the DFX group (21/30 patients) (p=0.517).
Baseline characteristics in patients with a baseline global pancreas T2* value <26 ms
At baseline 211 patients had a global pancreas T2*<26 ms: 28 in the combined DFO+DFP group, 54 in the DFP group, and 129 in the DFX group.
The mean administered dosages of the chelators were: (i) DFP in patients who received the combined regimen 84.81±14.87 mg/kg body-weight/day with a frequency of 6.86±0.59 days/week and DFO in combined regimen patients 37.96±7.79 mg/kg body-weight/day with a frequency of 3.86±1.74 days/week; (ii) DFP as monotherapy 84.52±14.31 mg/kg body-weight/day; (iii) DFX as monotherapy 24.97±7.07 mg/kg body-weight/day if in dispersible tablets (No.=98) and 18.31±4.43 mg/kg body-weight/day if in film-coated tablets (No.=31). The percentage of patients with excellent/good levels of compliance to the chelation treatment was significantly lower in the combined DFP+DFO group than in either the DFP group (82.1 vs 98.1%; p=0.016) or the DFX group (82.1 vs 99.2%; p=0.001).
The characteristics of these treatment subgroups at baseline are indicated in Table II. Patients in the combined DFO+DFP group were less frequently splenectomized than were patients in the DFP group. Mean serum ferritin levels were significantly higher in the combined DFO+DFP group compared to those in either the DFP or DFX groups. Global heart T2* and global pancreas T2* values were significantly lower in the combined DFO+DFP group compared to either the DFP or DFX groups. The frequency of alterations of glucose metabolism was significantly higher in the combined DFO+DFP group than in the DFX group.
Table II.
Baseline descriptive statistics of the treatment groups composed of patients with global pancreas T2* value <26 ms. The p-values are for the comparisons between the combined desferrioxamine (DFO) + deferiprone (DFP) and DFP monotherapy groups and between the combined DFO+DFP and deferasirox monotherapy groups
| p-value | DFP (No.=54) | Combined DFO+DFP (No.=28) | DFX (No.=129) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 0.384 | 37.17±11.46 | 34.79±10.62 | 37.08±10.33 | 0.224 |
| Females, No. (%) | 0.215 | 25 (46.3) | 17 (60.7) | 67 (51.9) | 0.399 |
| Age at start of regular transfusions (months) | 0.978 | 12.31±9.06 | 12.04±9.29 | 16.69±17.73 | 0.717 |
| Units transfused in the last 12 months | 0.860 | 40.09±10.97 | 40.62±11.01 | 39.38±10.64 | 0.641 |
| Age at start of chelation (years) | 0.514 | 3.75±3.15 | 3.40±1.45 | 4.32±3.69 | 0.847 |
| Splenectomy, No. (%) | 0.013 | 40 (74.1) | 13 (46.4) | 80 (62.0) | 0.128 |
| Pre-transfusion hemoglobin (g/dL) | 0.219 | 9.53±0.39 | 9.66±0.47 | 9.61±0.48 | 0.777 |
| Mean ferritin (ng/mL) | 0.025 | 894.37±1200.53 | 1359.76±1187.56 | 826.20±667.28 | 0.026 |
| MRI LIC (mg/g dw) | 0.762 | 5.24±5.41 | 8.33±10.27 | 6.52±9.51 | 0.258 |
| MRI LIC>3 mg/g dw, No. (%) | 0.547 | 31 (57.4) | 18 (64.3) | 60 (46.5) | 0.099 |
| Global heart T2* (ms) | 0.003 | 38.14±6.75 | 26.69±15.39 | 37.36±9.27 | 0.001 |
| Global heart T2*<20 ms, No. (%) | <0.0001 | 2 (3.7) | 10 (35.7) | 12 (9.3) | <0.0001 |
| Global pancreas T2* (ms) | 0.003 | 9.00±4.89 | 6.23±4.59 | 10.35±6.07 | <0.0001 |
| Altered glucose metabolism, No. (%) | 0.779 | 33 (61.1) | 18 (64.3) | 44 (34.1) | 0.003 |
DFP: deferiprone; DFO: desferrioxamine; DFX: deferasirox; No.: number; MRI: magnetic resonance imaging; LIC: liver iron concentration; dw: dry weight.
Intra-treatment comparisons in patients with baseline global pancreas T2*<26 ms
At the follow-up mean serum ferritin levels and MRI LIC values were, respectively, 973.92±1501.62 ng/mL and 6.03±5.47 mg/g dry weight in the DFP group, 1007.822±895.15 ng/mL and 9.69±20.83 mg/g dry weight in the combined group, and 815.94±710.91 ng/mL and 5.10±7.53 mg/g dry weight in the DFX group.
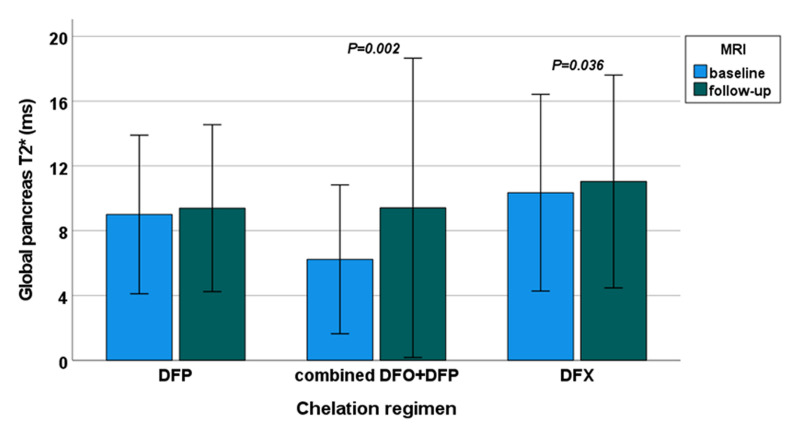
A significant improvement in global pancreas T2* values was detected in the combined DFO+DFP group (from 6.23±4.59 to 9.41±9.24 ms; p=0.002) and in the DFX group (from 10.35±6.07 to 11.04±6.57 ms; p=0.036) but not in the DFP group (from 9.00±4.89 to 9.39±5.15 ms; p=0.192) (Figure 1).
Figure 1.
Intra-treatment comparison between final and basal global pancreas T2* values in patients with a baseline global pancreas T2* value <26 ms
MRI: magnetic resonance imaging; DFP: deferiprone; DFO: desferrioxamine; DFX: deferasirox.
A significant negative association was detected between changes in global pancreas T2* values and baseline global pancreas T2* values (R=−0.192; p=0.005). As a consequence, the percent change in global pancreas T2* values, normalized for the baseline values, were also taken into account.
Table III shows the mean percent changes between final and baseline global pancreas T2* values and their correlates for each treatment group. There was no correlation between the percent changes in global pancreas T2* values and iron chelator dosage. Percentage changes in global pancreas T2* values were not influenced by initial serum ferritin or MRI LIC values in any chelation group. Percentage changes in global pancreas T2* values correlated with percent changes in MRI LIC values in both the combined DFO+DFP and DFX monotherapy groups but not in the DFP monotherapy group and with percent changes in serum ferritin levels in the DFX group. No association was detected between percent changes in pancreatic and cardiac T2* values.
Table III.
Clinical correlates of percent changes in global pancreas T2* values for each treatment group
| DFP (No.=54) | Combined DFO+DFP (No.=28) | DFX (No.=129) | |
|---|---|---|---|
| Descriptive data (mean ± SD) | |||
| Change in serum ferritin (ng/mL) | 77.40±398.86 | −200.36±625.37 | −44.86±425.74 |
| % change in serum ferritin | 12.98±52.921 | 3.01±60.83 | 9.81±69.28 |
| Change in MRI LIC values (mg/g dw) | 0.79±3.75 | 1.37±13.50 | −1.42±6.83 |
| % change in MRI LIC values | 12.58±95.60 | −0.79±45.01 | 0.81±67.07 |
| Change in global pancreas T2* values (ms) | 0.39±3.74 | 3.18±6.36 | 0.69±4.27 |
| % change in global pancreas T2* values | 11.68±37.28 | 60.44±101.41 | 18.48±63.19 |
| Change in global heart T2* values (ms) | 1.37±5.37 | 2.69±5.89 | 0.56±4.98 |
| % change in global heart T2* values | 6.40±19.97 | 23.82±48.77 | 4.35±20.63 |
| Correlation with % changes in global pancreas T2* values (R-squared; p-value) | |||
| DFP dose | R=−0.047; p=0.738 | R=0.162; p=0.420 | |
| DFO dose | R=0.340; p=0.082 | ||
| DFX dose dispersible tablets film-coated tablets | R=0.077; p=0.475 R=0.032; p=0.878 |
||
| Age | R=0.238; p=0.083 | R=0.270; p=0.165 | R=−0.015; p=0.868 |
| Baseline ferritin | R=−0.182; p=0.215 | R=−0.098; p=0.633 | R=0.098; p=0.291 |
| % changes in ferritin | R=−0.145; p=0.358 | R=−0.279; p=0.198 | R=−0.213; p=0.025 |
| Baseline MRI LIC | R=0.049; p=0.726 | R=−0.181; p=0.358 | R=0.205; p=0.080 |
| % changes in MRI LIC | R=−0.141; p=0.310 | R=−0.463; p=0.013 | R=−0.483; p<0.0001 |
| % change in global heart T2* | R=0.014; p=0.992 | R=0.055; p=0.430 | R=0.117; p=0.185 |
| % changes in global pancreas T2* values: differences between groups | |||
| Gender (males vs females) | 13.55±36.08 vs 9.51±39.26 (p=0.609) | 52.20±122.08 vs 65.78±89.23 (p=0.359) | 17.71±75.36 vs 21.12±49.91 (p=0.206) |
| Splenectomy (no vs yes) | 11.86±33.13 vs 11.61±39.02 (p=0.851) | 35.26±69.02 vs 89.51±125.99 (p=0.134) | 24.56±71.59 vs 16.37±57.71 (p=0.564) |
| Altered glucose metabolism at basal MRI (no vs yes) | 0.74±44.14 vs 18.64±30.92 (p=0.077) | 51.75±77.43 vs 65.28±114.41 (p=0.962) | 15.63±48.30 vs 26.93±85.12 (p=0.800) |
DFP: deferiprone; DFO: desferrioxamine; DFX: deferasirox; N: number; MRI: magnetic resonance imaging; LIC: liver iron concentration; dw: dry weight.
Inter-treatment comparisons in patients with baseline global pancreas T2*<26 ms
The percentage of patients who showed a normal global pancreas T2* value at the follow-up MRI was comparable between the combined DFO+DFP group versus either the DFP group (7.1 vs 1.9%; p=0.267) or the group DFX (7.1 vs 3.1%; p=0.291).
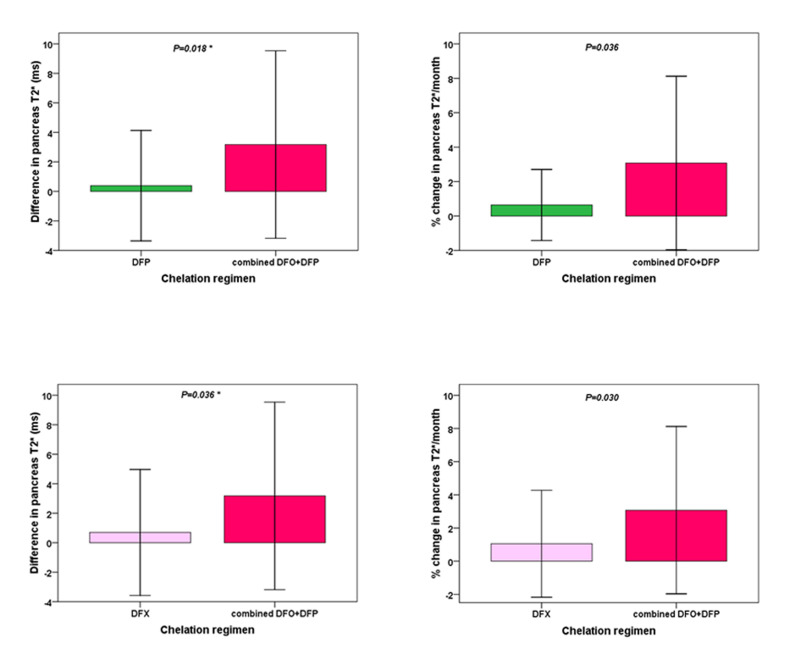
The increase in global pancreas T2* values was higher in the combined DFO+DFP group than in the DFP group and the difference became statistically significant after the correction for baseline global pancreas T2* values (p=0.018). The percent changes in global pancreas T2* values were significantly higher in the combined DFO+DFP group than in the DFP group (p=0.036) (Figure 2).
Figure 2.
Comparison of mean changes in global pancreas T2* values between the combined treatment and deferiprone monotherapy groups (upper panel) and between the combined treatment and deferasirox monotherapy groups (lower panel) in patients with baseline global pancreas T2* value <26 ms
The *symbol indicates that the p-value was adjusted for baseline global pancreas T2* values.
DFP: deferiprone; DFO: desferrioxamine; DFX: deferasirox.
The increase in global pancreas T2* values was higher in the combined DFO+DFP group than in the DFX group and the difference became statistically significant after the correction for baseline global pancreas T2* values (p=0.036). The percent changes in global pancreas T2* values were significantly higher in the combined DFO+DFP group than in the DFX group (p=0.030) (Figure 2).
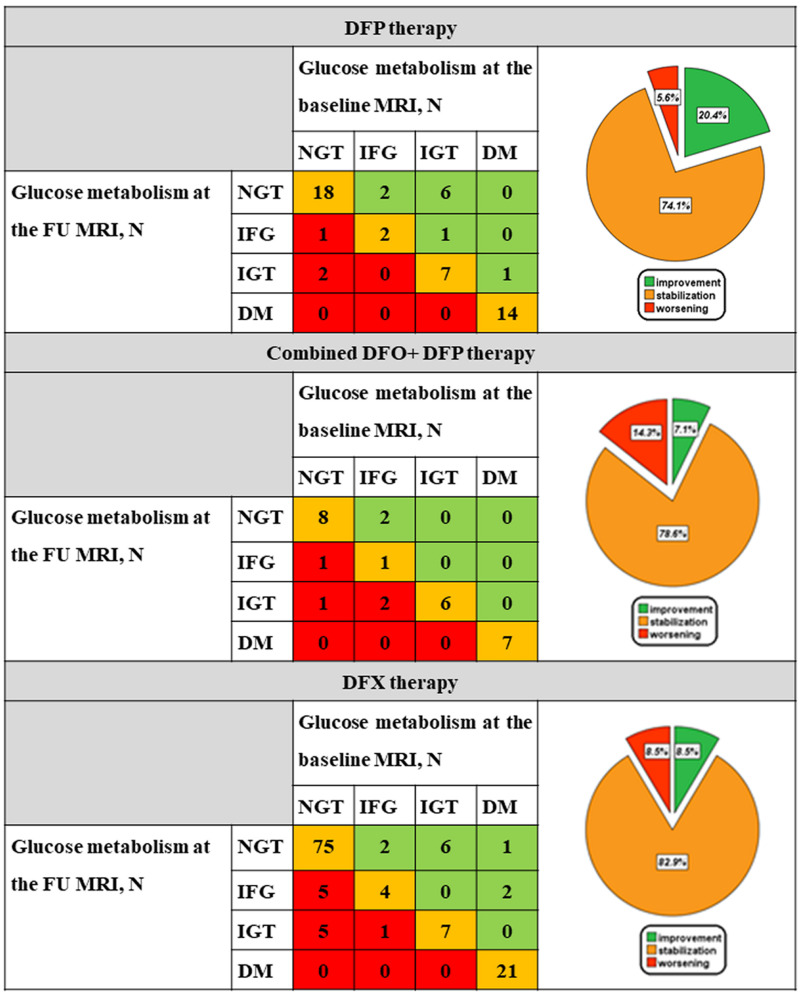
Figure 3 shows glucose metabolism at baseline and at the follow-up MRI scans and the switch from one risk class to another one for each treatment group. In each chelator group about three-quarters of patients remained in the same risk class. The frequency of patients who started with alterations of glucose metabolism and transitioned to a better risk class at the follow-up was 20.4% in the DFP group, 7.1% in the combined DFO+DFP group, and 8.5% in the DFX group. The risk class changes were not different between the combined DFO+DFP and the DFP groups (p=0.076) or between the combined DFO+DFP and the DFX groups (p=0.719).
Figure 3.
Change in glucose metabolism between the baseline and follow-up magnetic resonance imaging scans in the three treatment groups
DFP: deferiprone; MRI: magnetic resonance imaging; N: number; NGT: normal glucose tolerance; IFG: impaired fasting glucose; IGT: impaired glucose tolerance; DM: diabetes mellitus; FU: follow-up; DFO: desferrioxamine; DFX: deferasirox.
DISCUSSION
Accumulating evidence suggests that the use of tailored iron chelation therapy is the only way to treat or prevent the organ damage caused by iron accumulation. The widespread use of MRI and the higher number of the organs simultaneously assessed are rendering this scenario more complex. Furthermore, as the course of iron loading and clearance following iron chelation therapy between organs seems to reflect specific dynamics and functional associations35, the search for pancreatic and cardiac iron and their correlation both with hepatic iron and altered glucose metabolism is becoming appropriate and intriguing. Preliminary retrospective MRI data showed a delay in mobilization of pancreas iron overload in TDT patients under standard chelation therapy compared to that for the liver and heart36. Indeed, it is well known that intensive chelation therapy, by completely removing iron, may not only prevent new morbidities, but also reverse many cardiac and hepatic complications37. However, excluding case reports38 and a single longitudinal study from the E-MIOT Network19, studies that specifically addressed these changes at a pancreatic level remain limited. In this context, our longitudinal and prospective study evaluated for the first time the effect on pancreatic iron and dysfunction not only of both oral chelators, for which compliance was likely comparable, but also of combined DFO+DFP treatment.
We confirmed that pancreatic iron overload and impaired glucose metabolism are widespread among adult TDT patients who started regular transfusions in their early childhood39,11. Furthermore, patients heavily iron overloaded were those more frequently under combined treatment, whose schedule appears a bit more intensified in terms of dose of DFP and days of DFO administered than in previous Italian reports8,40.
At the intra-treatment analysis, in patients with baseline pancreatic iron overload, both DFX monotherapy and combined treatment, but not DFP monotherapy, were able to lower the global pancreatic iron. Due to the strict inverse linkage between changes in global pancreas T2* values and baseline global pancreas T2*, the analysis was performed also considering mean percent changes between final and baseline values and revealed that for the DFX and the DFO+DFP treatments pancreatic iron clearance occurs simultaneously with liver iron clearance and in parallel with a drop in ferritin level. A recent study found no significant changes in the prevalence of endocrine disease (diabetes, hypothyroidism, hypogonadism, hypoparathyroidism) in patients receiving DFX for a median duration of 6.5 years41, but no data were available on the effect of the drug on iron accumulation. On the other hand, weak data on the effect of DFP, a drug well known for its cardiac chelating efficiency, on both the pancreas and liver could suggest that liver iron clearance is a mandatory condition to remove iron from the pancreas and indicate that, despite being joined by parallel uptake mechanisms, the two organs are not freed of iron similarly by DFP in this clinical setting; however, before this conclusion can be reached, higher dosages of DFP and different iron overload baseline conditions should be investigated.
The observed difference in organ iron burden at baseline led us to further adjust our data for this variable to compare the specific effect of each drug more adequately. At the inter-treatment analysis the comparisons between both the combined DFO+DFP and DFP groups and that between combined the DFO+DFP and DFX groups showed the superiority of the combined regimen in reducing pancreatic iron, also in term of percent changes. Overall these data led to establish a hierarchy in chelating efficiency between chelators.
Nevertheless, although the chelating efficiency of combined treatment on pancreatic iron was so evident in the comparison against either DFP or DFX monotherapy, our longitudinal and prospective data on biochemical and clinical alterations of glucose metabolism were not clinically meaningful. Historical data from Farmaki and coworkers showed that very intensive chelation therapy with combined treatment, by lowering both serum ferritin and LIC to normal values, could significantly ameliorate glucose tolerance and revert some case of DM42. These data, despite lack of information about the effect on pancreatic iron overload, first suggested the contribution of the liver to this effect, as an organ whose iron clearance may not only favorably affect insulin sensitivity, but could also be necessary to reach pancreatic iron clearance. Our findings are not in contrast with this seminal observation, but were likely made in very different baseline and treatment conditions. In fact, our patients were older and less exposed to combined treatment than the Greek study population. Furthermore, it seems that altered glucose function is not an immediate outcome following iron deposition, but develops with latency, as a result of progressive damage to the functional reserve of beta-cell function, whose injury may be not reversible43–45. Therefore, both the advanced mean age of our population and our follow-up iron overload values, very far from normal, suggest that an improvement of glucose metabolism was not an achievable endpoint under our explored “real life” conditions.
A limitation of this study is the unavailability of data about iron intake. However, there were no difference among the three groups in terms of age at the start of regular transfusions or frequency of transfusions.
CONCLUSIONS
The most relevant conclusions of this study are that pancreatic cells are not completely refractory to iron chelation therapy and that combined treatment, more than DFX or DFP monotherapy, is able to remove iron from the pancreas. Further studies are needed to re-examine the findings of Farmaki et al., intensifying iron chelation therapy and/or the duration of exposure until the normalization of pancreatic iron also in adult patients, bearing in mind that reversal or attenuation of glucose dysregulation could not be guaranteed, but toxicity and loss of compliance, due to over chelation, would lie in wait.
ACKNOWLEDGMENTS
We would like to thank all the colleagues involved in the E-MIOT project (https://emiot.ftgm.it/). We also thank all patients and the Italian “L. Giambrone” Thalassemia Foundation for their cooperation.
Footnotes
AUTHORSHIP CONTRIBUTIONS: PR designed the study and drafted the initial manuscript. AM designed the study, analyzed the data, and drafted the initial manuscript. LP was responsible for data collection. MRG, LC, MA, MCP, AS, RR, VC, RR, SR, GP, and AV collected the data. VP developed the software for image analysis and assisted with the methods. EQ and FC contributed to the design and supervision of the study. AP designed and supervised the study and is the guarantor of this work. All Authors assisted with interpretation, commented on drafts of the manuscript, and approved the final version.
DISCLOSURE OF CONFLICTS OF INTEREST: AP has received speakers’ honoraria from Chiesi Farmaceutici S.p.A. The remaining Authors have nothing to disclose.
FUNDING: The E-MIOT project receives “no-profit support” from industrial sponsorships (Chiesi Farmaceutici S.p.A. and Bayer). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Weatherall DJ. The thalassaemias. BMJ. 1997;314:1675–1678. doi: 10.1136/bmj.314.7095.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borgna-Pignatti C, Cappellini MD, De Stefano P, Del Vecchio GC, Forni GL, Gamberini MR, et al. Survival and complications in thalassemia. Ann N Y Acad Sci. 2005;1054:40–47. doi: 10.1196/annals.1345.006. [DOI] [PubMed] [Google Scholar]
- 3.Borgna-Pignatti C, Garani MC, Forni GL, Cappellini MD, Cassinerio E, Fidone C, et al. Hepatocellular carcinoma in thalassaemia: an update of the Italian Registry. Br J Haematol. 2014;167:121–126. doi: 10.1111/bjh.13009. [DOI] [PubMed] [Google Scholar]
- 4.Dessi C, Leoni G, Moi P, Danjou F, Follesa I, Foschini ML, et al. Thalassemia major between liver and heart: where we are now. Blood Cells Mol Dis. 2015;55:82–88. doi: 10.1016/j.bcmd.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–1884. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- 6.Pennell DJ, Berdoukas V, Karagiorga M, Ladis V, Piga A, Aessopos A, et al. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738–3744. doi: 10.1182/blood-2005-07-2948. [DOI] [PubMed] [Google Scholar]
- 7.Pennell DJ, Porter JB, Piga A, Lai Y, El-Beshlawy A, Belhoul KM, et al. A 1-year randomized controlled trial of deferasirox vs deferoxamine for myocardial iron removal in beta-thalassemia major (CORDELIA) Blood. 2014;123:1447–1454. doi: 10.1182/blood-2013-04-497842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepe A, Meloni A, Rossi G, Cuccia L, D’Ascola GD, Santodirocco M, et al. Cardiac and hepatic iron and ejection fraction in thalassemia major: multicentre prospective comparison of combined deferiprone and deferoxamine therapy against deferiprone or deferoxamine monotherapy. J Cardiovasc Magn Reson. 2013;15:1. doi: 10.1186/1532-429X-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pepe A, Meloni A, Pistoia L, Cuccia L, Gamberini MR, Lisi R, et al. MRI multicentre prospective survey in thalassaemia major patients treated with deferasirox versus deferiprone and desferrioxamine. Br J Haematol. 2018;183:783–795. doi: 10.1111/bjh.15595. [DOI] [PubMed] [Google Scholar]
- 10.Pepe A, Pistoia L, Gamberini MR, Cuccia L, Lisi R, Cecinati V, et al. National networking in rare diseases and reduction of cardiac burden in thalassemia major. Eur Heart J. 2022;43:2482–2492. doi: 10.1093/eurheartj/ehab851. [DOI] [PubMed] [Google Scholar]
- 11.Pepe A, Pistoia L, Gamberini MR, Cuccia L, Peluso A, Messina G, et al. The close link of pancreatic iron with glucose metabolism and with cardiac complications in thalassemia major: a large, multicenter observational study. Diabetes Care. 2020;43:2830–2839. doi: 10.2337/dc20-0908. [DOI] [PubMed] [Google Scholar]
- 12.Spasiano A, Meloni A, Costantini S, Quaia E, Cademartiri F, Cinque P, et al. Setting for “normal” serum ferritin levels in patients with transfusion-dependent thalassemia: our current strategy. J Clin Med. 2021;10:5985. doi: 10.3390/jcm10245985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- 14.Perera NJ, Lau NS, Mathews S, Waite C, Ho PJ, Caterson ID. Overview of endocrinopathies associated with beta-thalassaemia major. Intern Med J. 2010;40:689–696. doi: 10.1111/j.1445-5994.2010.02254.x. [DOI] [PubMed] [Google Scholar]
- 15.Gamberini MR, De Sanctis V, Gilli G. Hypogonadism, diabetes mellitus, hypothyroidism, hypoparathyroidism: incidence and prevalence related to iron overload and chelation therapy in patients with thalassaemia major followed from 1980 to 2007 in the Ferrara Centre. Pediatr Endocrinol Rev. 2008;6(Suppl 1):158–169. [PubMed] [Google Scholar]
- 16.Restaino G, Meloni A, Positano V, Missere M, Rossi G, Calandriello L, et al. Regional and global pancreatic T*(2) MRI for iron overload assessment in a large cohort of healthy subjects: Normal values and correlation with age and gender. Magn Reson Med. 2011;65:764–769. doi: 10.1002/mrm.22640. [DOI] [PubMed] [Google Scholar]
- 17.Meloni A, Pistoia L, Gamberini MR, Ricchi P, Cecinati V, Sorrentino F, et al. The link of pancreatic iron with glucose metabolism and cardiac iron in thalassemia intermedia: a large, multicenter observational study. J Clin Med. 2021;10:5561. doi: 10.3390/jcm10235561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noetzli LJ, Mittelman SD, Watanabe RM, Coates TD, Wood JC. Pancreatic iron and glucose dysregulation in thalassemia major. Am J Hematol. 2012;87:155–160. doi: 10.1002/ajh.22223. [DOI] [PubMed] [Google Scholar]
- 19.Meloni A, Pistoia L, Ricchi P, Allò M, Rosso R, Cuccia L, et al. Prospective changes of pancreatic iron in patients with thalassemia major and association with chelation therapy. Blood Adv. 2023;7:2237–2240. doi: 10.1182/bloodadvances.2022008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepe A, Positano V, Santarelli F, Sorrentino F, Cracolici E, De Marchi D, et al. Multislice multiecho T2* cardiovascular magnetic resonance for detection of the heterogeneous distribution of myocardial iron overload. J Magn Reson Imaging. 2006;23:662–668. doi: 10.1002/jmri.20566. [DOI] [PubMed] [Google Scholar]
- 21.Ramazzotti A, Pepe A, Positano V, Rossi G, De Marchi D, Brizi MG, et al. Multicenter validation of the magnetic resonance T2* technique for segmental and global quantification of myocardial iron. J Magn Reson Imaging. 2009;30:62–68. doi: 10.1002/jmri.21781. [DOI] [PubMed] [Google Scholar]
- 22.Meloni A, De Marchi D, Pistoia L, Grassedonio E, Peritore G, Preziosi P, et al. Multicenter validation of the magnetic resonance T2* technique for quantification of pancreatic iron. Eur Radiol. 2019;29:2246–2252. doi: 10.1007/s00330-018-5783-6. [DOI] [PubMed] [Google Scholar]
- 23.Positano V, Meloni A, Santarelli MF, Gerardi C, Bitti PP, Cirotto C, et al. Fast generation of T2* maps in the entire range of clinical interest: application to thalassemia major patients. Comput Biol Med. 2015;56:200–210. doi: 10.1016/j.compbiomed.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Positano V, Salani B, Pepe A, Santarelli MF, De Marchi D, Ramazzotti A, et al. Improved T2* assessment in liver iron overload by magnetic resonance imaging. Magn Reson Imaging. 2009;27:188–197. doi: 10.1016/j.mri.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Meloni A, Positano V, Ruffo GB, Spasiano A, D’Ascola DG, Peluso A, et al. Improvement of heart iron with preserved patterns of iron store by CMR-guided chelation therapy. Eur Heart J Cardiovasc Imaging. 2015;16:325–334. doi: 10.1093/ehjci/jeu191. [DOI] [PubMed] [Google Scholar]
- 26.Meloni A, Positano V, Pepe A, Rossi G, Dell’Amico M, Salvatori C, et al. Preferential patterns of myocardial iron overload by multislice multiecho T*2 CMR in thalassemia major patients. Magn Reson Med. 2010;64:211– 219. doi: 10.1002/mrm.22410. [DOI] [PubMed] [Google Scholar]
- 27.Positano V, Pepe A, Santarelli MF, Scattini B, De Marchi D, Ramazzotti A, et al. Standardized T2* map of normal human heart in vivo to correct T2* segmental artefacts. NMR Biomed. 2007;20:578–590. doi: 10.1002/nbm.1121. [DOI] [PubMed] [Google Scholar]
- 28.Meloni A, De Marchi D, Positano V, Neri MG, Mangione M, Keilberg P, et al. Accurate estimate of pancreatic T2* values: how to deal with fat infiltration. Abdom Imaging. 2015;40:3129–3136. doi: 10.1007/s00261-015-0522-9. [DOI] [PubMed] [Google Scholar]
- 29.Meloni A, Luciani A, Positano V, De Marchi D, Valeri G, Restaino G, et al. Single region of interest versus multislice T2* MRI approach for the quantification of hepatic iron overload. J Magn Reson Imaging. 2011;33:348–355. doi: 10.1002/jmri.22417. [DOI] [PubMed] [Google Scholar]
- 30.Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meloni A, Rienhoff HY, Jr, Jones A, Pepe A, Lombardi M, Wood JC. The use of appropriate calibration curves corrects for systematic differences in liver R2* values measured using different software packages. Br J Haematol. 2013;161:888–891. doi: 10.1111/bjh.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 33.Meloni A, Maggio A, Positano V, Leto F, Angelini A, Putti MC, et al. CMR for myocardial iron overload quantification: calibration curve from the MIOT Network. Eur Radiol. 2020;29:2246–2252. doi: 10.1007/s00330-020-06668-1. [DOI] [PubMed] [Google Scholar]
- 34.De Sanctis V, Soliman AT, Elsedfy H, Yaarubi SA, Skordis N, Khater D, et al. The ICET-A recommendations for the diagnosis and management of disturbances of glucose homeostasis in thalassemia major patients. Mediterr J Hematol Infect Dis. 2016;8:e2016058. doi: 10.4084/MJHID.2016.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noetzli LJ, Carson SM, Nord AS, Coates TD, Wood JC. Longitudinal analysis of heart and liver iron in thalassemia major. Blood. 2008;112:2973–2978. doi: 10.1182/blood-2008-04-148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinto VM, Bacigalupo L, Gianesin B, Balocco M, De Franceschi L, Malago R, et al. Lack of correlation between heart, liver and pancreas MRI-R2*: results from long-term follow-up in a cohort of adult beta-thalassemia major patients. Am J Hematol. 2018;93:E79–E82. doi: 10.1002/ajh.25009. [DOI] [PubMed] [Google Scholar]
- 37.Galanello R, Agus A, Campus S, Danjou F, Giardina PJ, Grady RW. Combined iron chelation therapy. Ann N Y Acad Sci. 2010;1202:79–86. doi: 10.1111/j.1749-6632.2010.05591.x. [DOI] [PubMed] [Google Scholar]
- 38.Pinto VM, Forni GL. Management of iron overload in beta-thalassemia patients: clinical practice update based on case series. Int J Mol Sci. 2020;21:8871. doi: 10.3390/ijms21228771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noetzli LJ, Papudesi J, Coates TD, Wood JC. Pancreatic iron loading predicts cardiac iron loading in thalassemia major. Blood. 2009;114:4021–4026. doi: 10.1182/blood-2009-06-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricchi P, Ammirabile M, Spasiano A, Costantini S, Cinque P, Di Matola T, et al. Combined chelation therapy in thalassemia major with deferiprone and desferrioxamine: a retrospective study. Eur J Haematol. 2010;85:36–42. doi: 10.1111/j.1600-0609.2010.01447.x. [DOI] [PubMed] [Google Scholar]
- 41.Casale M, Citarella S, Filosa A, De Michele E, Palmieri F, Ragozzino A, et al. Endocrine function and bone disease during long-term chelation therapy with deferasirox in patients with beta-thalassemia major. Am J Hematol. 2014;89:1102–1106. doi: 10.1002/ajh.23844. [DOI] [PubMed] [Google Scholar]
- 42.Farmaki K, Angelopoulos N, Anagnostopoulos G, Gotsis E, Rombopoulos G, Tolis G. Effect of enhanced iron chelation therapy on glucose metabolism in patients with beta-thalassaemia major. Br J Haematol. 2006;134:438–444. doi: 10.1111/j.1365-2141.2006.06203.x. [DOI] [PubMed] [Google Scholar]
- 43.Chern JP, Lin KH, Lu MY, Lin DT, Lin KS, Chen JD, et al. Abnormal glucose tolerance in transfusion-dependent beta-thalassemic patients. Diabetes Care. 2001;24:850–854. doi: 10.2337/diacare.24.5.850. [DOI] [PubMed] [Google Scholar]
- 44.Cooksey RC, Jouihan HA, Ajioka RS, Hazel MW, Jones DL, Kushner JP, et al. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology. 2004;145:5305–5312. doi: 10.1210/en.2004-0392. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez-Real JM, McClain D, Manco M. Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care. 2015;38:2169–2176. doi: 10.2337/dc14-3082. [DOI] [PubMed] [Google Scholar]