Abstract
pls, a gene found in type I staphylococcal cassette chromosome mec (SCCmec) regions of methicillin-resistant Staphylococcus aureus strains, was present in 12 of the 15 human clinical Staphylococcus sciuri isolates studied. Pls was expressed in the S. sciuri isolates, although at a lower level than in S. aureus. Other parts of SCCmec could also be found in the S. sciuri genome.
Methicillin-resistant Staphylococcus aureus (MRSA) is an increasing problem in hospital and community environments worldwide. Resistance towards methicillin is encoded by the mecA gene, carried by a mobile genetic element, staphylococcal cassette chromosome mec (SCCmec). Five types of SCCmec elements, containing slightly differing combinations of mecA, its regulators, and site-specific recombinase (ccr) genes, which enable a site-specific integration into the chromosome, as well as additional type-specific DNA, have so far been characterized (4, 11-13, 20). New MRSA strains are thought to emerge by acquisition of SCCmec by sensitive clones. The origin of mecA, however, has been suggested to be another staphylococcal species, possibly Staphylococcus sciuri or a close evolutionary relative, since a mecA homologue with 88% amino acid sequence similarity (32) is uniformly found in S. sciuri strains (2). S. sciuri is a taxonomically primitive staphylococcal species found on the skins of several animals as well as environmental sources (16, 17). It has occasionally been isolated from humans but has only rarely been associated with infections (6, 9, 21, 28-30).
pls is a part of the type I SCCmec element (11). Pls is a large surface protein with an LPXTG peptidoglycan-anchoring sequence and a so-far-uncharacterized carbohydrate-containing portion (7, 18, 27). Its expression results in reduced adhesiveness to host proteins (27) and decreased cellular invasiveness (14). On the other hand, it mediates bacterial aggregation and binding to glycolipids (10) and to human desquamated nasal epithelial cells (25). It acts as a virulence factor in a mouse model of septic arthritis and sepsis (13a).
The pls gene is common in S. sciuri isolates.
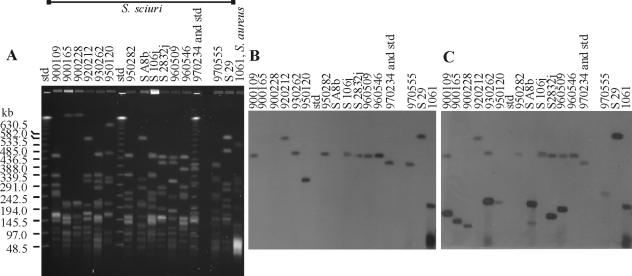
Fifteen S. sciuri clinical human isolates of three subspecies, isolated from various sites (Table 1) (21), were studied for the presence of the pls and mecA genes by Southern hybridization. SmaI-digested DNA separated by pulsed-field gel electrophoresis (PFGE) (26, 27) was hybridized with a digoxigenin-labeled pls probe (Table 1) at 68°C and a mecA probe (Table 1) at 55°C to allow hybridization to both the S. aureus and S. sciuri mec genes. All the strains differed from each other by their PFGE patterns (Fig. 1A). pls was present in 12 out of 15 S. sciuri strains. Three strains, N900165, N900228, and S A8b, all of which are S. sciuri subsp. rodentium, lacked the gene (Fig. 1B). mecA hybridization resulted in a single weak band in 7 of 15 S. sciuri strains (Fig. 1C). All of these strains are oxacillin and methicillin sensitive (Table 1), and primers specific for S. aureus mecA are unable to amplify their DNA or the amplification is weak (21). Eight of 15 of the strains showed either a single strong band (4 of the 8 strains) or a strong and a weak band (4 of the 8 strains). Seven of these eight strains are oxacillin resistant, and their S. aureus mecA genes can be detected by PCR (21). A mecA probe has been shown to hybridize with a large and a smaller fragment in methicillin-resistant S. sciuri strains (2). Based on these data and the better homology of our probe with S. aureus mec than S. sciuri mec, we conclude that the strong bands represent S. aureus mecA and that the weak bands represent its S. sciuri homologue, which is slightly different in sequence. In 10 strains, a mecA-hybridizing fragment, usually the weak one, also hybridized with the pls probe (Fig. 1B and C). The sizes of these fragments were at least 400 kb, and consequently, it is difficult to estimate how near to each other the two genes are in the S. sciuri genome.
TABLE 1.
Probes used for dot blot and Southern hybridizations and results of the dot blot hybridization
| Probe | Probe sequencea | Primer(s) used for probe amplificationb | Template DNA in probe amplificationc | SCCmec type(s) in which the primer sequence(s) is foundd | Results of dot blot hybridization at 68°C of S. sciuri strainse:
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxar with S. aureus mecA+f
|
With Oxa S. aureus mecA+
|
Oxas without S. aureus mecA
|
1061, S. aureus | ||||||||||||||||||
| N900109, sc. | N900165, rod. | N930262, sc. | S A8b, rod. | S 2832j, sc. | N960509, car. | S 29, sc. | N900228, rod. | S 106j, rod. | N920212, sc. | N950120, rod. | N960546, sc. | N970234, sc. | N970555, ear. | N950282, sc. | Controlsg | ||||||
| A | Upstream of pls gene | Loci A to H | IA | I | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | +/− |
| B | kdp operon | Loci A to H | II | II | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | +/− |
| C | mecI | Loci A to H | II | II, III | + | − | + | + | + | + | + | − | − | − | − | − | − | − | − | − | +/− |
| D | ORF 5′ to orfX | Loci A to H | IA | I, II, IV | − | − | − | + | + | + | + | − | − | − | − | − | − | − | − | + | +/− |
| E | Between integrated pI258 and Tn554 | Loci A to H | IIIA | III (not IIIB) | − | − | − | − | − | + | − | + | − | − | − | − | − | − | − | − | +/− |
| F | Between Tn554 and orfX | Loci A to H | IIIA | III (not IIIB) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | +/− |
| G | Left junction between IS431 and pUB110 | Loci A to H | II | II, IA | + | + | + | + | + | + | + | + | + | − | − | + | − | + | + | + | +/+ |
| H | Left junction between IS431 and pT181 | Loci A to H | III | III (not IIIA/B) | + | + | + | + | + | + | + | + | + | − | − | + | − | + | + | + | +/+ |
| ccr1 | Part of ccrA1 and -B | β2 | IA | I | − | − | − | − | − | − | − | − | + | − | − | + | − | − | + | + | +/− |
| ccr2 | Part of ccrA2 and -B | β2 | II | II | + | + | + | − | − | − | − | + | + | − | − | + | − | − | + | + | +/− |
| ccr3 | Part of ccrA3 and -B | β2 | IIIA | III | + | + | + | + | + | + | + | + | + | − | − | + | − | − | + | + | +/+ |
| pls | Nonrepetitive A region of pls | 27 | 1061 | I | + | − | + | − | + | + | + | − | + | + | + | + | + | + | + | + | +/− |
| mecA | Part of S. aureus mecA | 477 nt | 1061 | All types | + | − | + | + | + | + | + | − | − | − | − | − | − | − | − | + | +/− |
ORF, open reading frame.
Loci A to H, primers to amplify loci A to H in a multiplex PCR (24); β2, primers β2, specific for the ccrB gene, used together with either primer α2, primer α3, or primer α4, specific for the ccrA1, -2, and -3 genes, respectively (11); 27, primers reported in reference 27; 477 nt, primers 5′ AAGATGGCAAAGATATTCAAC3′ and 5′TTCTTACTGCCTAATTCGAG3′ to amplify an internal 477-nt region of S. aureus mecA.
IA, Iberian; II, UK-EMRSA-16; IIIA, Brazilian; III, Helsinki IV; 1061, clinical MRSA isolate (7, 27).
The strains of these types produce an amplification product in a multiplex PCR (24).
sc., S. sciuri subsp. sciuri; rod., S. sciuri subsp. rodentium; car., S. sciuri subsp. carnaticus.
Strains for which the MIC was >2 μg/ml were considered to be resistant to oxacillin (21).
MRSA DNAs positive and negative for the primer sequences were used as the controls. The symbol to the left side of the slash shows the hybridization signal by the strain used as a positive control, and that to the right side of the slash shows the signal by a negative control.
FIG. 1.
SmaI-digested DNA of S. sciuri strains separated by PFGE (A) was capillary blotted onto a nylon membrane (Roche) and probed with the digoxigenin-labeled MRSA strain 1061 pls gene A region (B) and an internal region of MRSA mecA (C). The lambda ladder PFG (Biolabs) was used as a marker. Molecular size standards (std) were run in three lanes, one of which by mistake was combined with a sample lane (N970234). Marker sizes are shown on the left.
The pls genes of five S. sciuri strains (N960509, N960546, N970234, N970555, and S 29) were characterized in more detail by hybridization and PCR analyses and found to be similar but not identical to S. aureus pls. DNA of each strain, isolated as described previously for S. aureus (27) and digested with StuI and PstI, hybridized with enhanced-chemiluminescence-labeled probes for repeat regions R1, R2, and R3 and the nonrepeat region A of S. aureus strain 1061 Pls (27; results not shown). However, only the R2 region of all five strains and the A region of strain N970555 were amplifiable by PCR with the primers used to prepare these probes (27; data not shown).
Pls is expressed by S. sciuri.
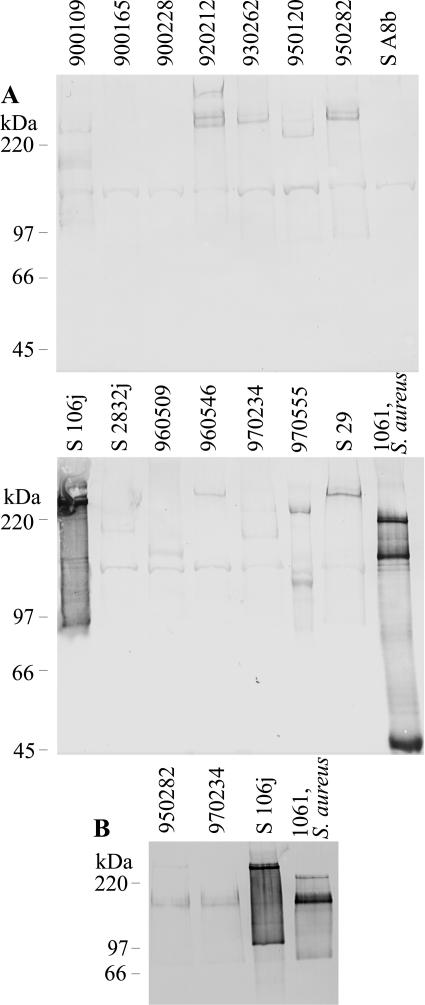
To study the expression of Pls in these 15 S. sciuri strains, surface proteins were solubilized by incubating stationary-phase cells with lysostaphin in the presence of raffinose essentially as described previously for S. aureus (1) and analyzed by Western blotting for Pls. Pls was expressed by all of the 12 pls+ strains (Fig. 2A). However, based on both Coomassie staining (data not shown) and the Western analysis, the level of expression (except in one strain, S 106j) was clearly lower than in S. aureus strain 1061, whose level of Pls is typical of Pls+ S. aureus strains studied (27). In most of the S. sciuri strains, Pls was even larger than the 230 kDa found for S. aureus strain 1061. In some cases more than one band was seen, possibly due to proteolytic cleavage, as was previously suggested for S. aureus 1061 Pls (7). A band of approximately 150 kDa was seen in all strains, including the ones without pls genes, and probably represents a cross-reactive protein.
FIG. 2.
Western analysis of lysostaphin-extracted surface proteins (A) or WGA-purified Pls proteins (B) of S. sciuri and S. aureus strains separated in a sodium dodecyl sulfate-8% polyacrylamide gel and blotted on 0.2-μm-pore-size nitrocellulose membranes (Protran). The membranes were blocked with 2% (wt/vol) bovine serum albumin-phosphate-buffered saline. Pooled mouse monoclonal anti-Pls antibodies (14) followed by alkaline phosphatase conjugated rabbit anti-mouse antibodies (Dako) and alkaline phosphatase substrate were used for detection. (A) The samples originate from 100 μl (40 μl for strain 1061) of a stationary-phase culture. (B) Twenty microliters of the peak fractions that eluted from strains N950282 and N970234 and 3 μl from strains S 106j and 1061 were used.
Pls proteins of three S. sciuri strains and S. aureus strain 1061 were affinity purified from lysostaphin digests by using wheat germ agglutinin (WGA) Sepharose and elution with 100 mM N-acetylglucosamine (7) and then analyzed by Western blotting for Pls (Fig. 2B). Pls of S. sciuri, like that of S. aureus, bound to WGA and thus seems to have a carbohydrate moiety containing N-acetylglucosamine.
Analysis of the “SCCmec region” in S. sciuri.
The presence of areas homologous to different types of S. aureus SCCmec regions in S. sciuri DNA was examined. Dot blot hybridization was used for all 15 strains, and additionally, Southern hybridization was used for strains N920212, N950120, and N960546. Thirteen digoxigenin-labeled probes designed to differentiate among type I to IV SCCmec regions were PCR amplified (Table 1). Four micrograms of total DNA was dot blotted, or SmaI-digested DNA separated by PFGE (5, 23) was capillary blotted, onto positively charged nylon membranes. The dot blot hybridizations were performed at 68°C (Table 1), and the Southern hybridizations were performed at 55°C (results not shown). To make sure that hybridization to S. aureus SCCmec regions was not examined, it was safest to look closely only at the S. sciuri strains not containing the S. aureus mecA gene. All the S. sciuri strains, even if positive for pls, gave negative results with probe A binding to a region upstream of pls in type I SCCmec regions. At 68°C, the strains without S. aureus mecA bound the mecA probe very weakly and not at all probe C, containing the mecI sequence. In a PCR analysis of 28 human S. sciuri isolates by Couto et al., the presence of mecI was always connected to the presence of a copy of S. aureus mecA (3). Similarly, mecA regions sequenced from four S. sciuri strains by Wu et al. revealed mecR1 and mecI adjacent to mecA genes of the S. aureus type but not adjacent to those of the S. sciuri type (31). It seems that mecI is not a part of the native S. sciuri mec region. Probes G, H, and ccr3 bound to negative-control DNA and were thus unable to differentiate between the SCCmec types. There were, however, three to four strains that did not bind to these probes at all, suggesting that IS431 or plasmids pUB101 and pT181 sometimes but not always are a part of the S. sciuri genome. All the other strains except the four S. sciuri strains that did not bind to any of the ccr probes probably have some kind of site-specific recombinase genes. Probes B, D, and F did not bind to the DNA of any strains having only the S. sciuri copy of mecA, suggesting that these regions of SCCmec were not a part of the S. sciuri genome.
The Southern analysis (data not shown) of strain N920212 at a low stringency gave a positive signal with pls, mecA, and the C and A probes, all of which hybridized with the largest fragment. One fragment of strain N950120 DNA hybridized with mecA and probe C, and another fragment hybridized with pls. This strain was one of the few strains having pls and mecA in different SmaI fragments (Fig. 1). One fragment of strain N960546 DNA hybridized with pls, mecA, and C, another one hybridized with the ccr probes and G, another one hybridized with the ccr probes and A, and yet another one hybridized with the H probe. Thus, sequences related to mecI and the upstream region of pls seem to exist in S. sciuri.
The idea that pls, like mecA, might originate from S. sciuri is supported by the much greater frequency of pls in S. sciuri strains than in S. aureus strains. The strains used in this study were human clinical isolates and may not represent the S. sciuri populations in their natural habitats. There is a possibility that pls was a part of an S. aureus SCCmec region in some of the S. aureus mecA+ S. sciuri strains. Half of the strains, however, did not contain an S. aureus mecA gene. We do not know what the function of Pls in S. sciuri is, but the low level of expression suggests that the effects may be different from those in S. aureus.
Some of the SCCmec sequences were present in S. sciuri strains and even localized to the same chromosomal areas. Whether mec genes are a part of an SCCmec element also in species other than S. aureus is not known. SCC elements without mecA have recently been found in S. aureus as well as some other staphylococcal species, and they are thought to act as mobile genetic elements transferring any useful genetic information between species (8, 15, 19, 22). The presence of pls in S. sciuri as well as S. aureus is yet another piece of evidence that there is constant genetic exchange between staphylococcal species.
Acknowledgments
Jérôme Etienne is thanked for kindly providing the S. sciuri strains.
The study was financially supported by the Academy of Finland (projects 173854 and 1206356 to P.K. and K.J.) and The Paulo Foundation (a grant to K.J.)
REFERENCES
- 1.Cheung, A. L., and V. A. Fischetti. 1988. Variation in the expression of cell wall proteins of Staphylococcus aureus grown on solid and liquid media. Infect. Immun. 56:1061-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couto, I., H. de Lencastre, E. Severina, W. Kloos, J. A. Webster, R. J. Hubner, I. Santos Sanchez, and A. Tomasz. 1996. Ubiquitous presence of a mecA homologue in natural isolates of Staphylococcus sciuri. Microb. Drug Resist. 2:377-391. [DOI] [PubMed] [Google Scholar]
- 3.Couto, I., I. Santos Sanchez, R. Sá-Leão, and H. de Lencastre. 2000. Molecular characterization of Staphylococcus sciuri strains isolated from humans. J. Clin. Microbiol. 38:1136-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daum, R. S., T. Ito, K. Hiramatsu, F. Hussain, K. Mongkolrattanothai, M. Jamklang, and S. Boyle-Vavra. 2002. A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J. Infect. Dis. 186:1344-1347. [DOI] [PubMed] [Google Scholar]
- 5.Goering, R. V., and M. A. Winters. 1992. Rapid method for epidemiological evaluation of Gram-positive cocci by field inversion gel electrophoresis. J. Clin. Microbiol. 30:577-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedin, G., and M. Widerström. 1998. Endocarditis due to Staphylococcus sciuri. Eur. J. Clin. Microbiol. Infect. Dis. 17:673-675. [DOI] [PubMed] [Google Scholar]
- 7.Hildén, P., K. Savolainen, J. Tyynelä, M. Vuento, and P. Kuusela. 1996. Purification and characterisation of a plasmin-sensitive surface protein of Staphylococcus aureus. Eur. J. Biochem. 236:904-910. [DOI] [PubMed] [Google Scholar]
- 8.Holden, M. T. G., E. J. Feil, J. A. Lindsay, S. J. Peacock, N. P. J. Day, M. C. Enright, T. J. Foster, C. E. Moore, L. Hurst, R. Atkin, A. Barron, N. Bason, S. D. Bentley, C. Chillingworth, T. Chillingworth, C. Churcher, L. Clark, C. Corton, A. Cronin, J. Doggett, L. Dowd, T. Feltwell, Z. Hance, B. Harris, H. Hauser, S. Holroyd, K. Jagels, K. D. James, N. Lennard, A. Line, R. Mayes, S. Moule, K. Mungall, D. Ormond, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, S. Sharp, M. Simmonds, K. Stevens, S. Whitehead, B. G. Barrell, B. G. Spratt, and J. Parkhill. 2004. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc. Natl. Acad. Sci. USA 101:9786-9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horii, T., Y. Suzuki, T. Kimura, T. Kanno, and M. Maekawa. 2001. Intravenous catheter-related septic shock caused by Staphylococcus sciuri and Escherichia vulneris. Scand. J. Infect. Dis. 33:930-932. [DOI] [PubMed] [Google Scholar]
- 10.Huesca, M., R. Peralta, D. N. Sauder, A. E. Simor, and M. J. McGavin. 2002. Adhesion and virulence properties of epidemic Canadian methicillin-resistant Staphylococcus aureus strain 1: identification of novel adhesion functions associated with plasmin-sensitive surface protein. J. Infect. Dis. 185:1285-1296. [DOI] [PubMed] [Google Scholar]
- 11.Ito, T., Y. Katayama, K. Asada, N. Mori, K. Tsutsumimoto, C. Tiensasitorn, and K. Hiramatsu. 2001. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1323-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito, T., Y. Katayama, and K. Hiramatsu. 1999. Cloning and nucleotide sequence determination of the entire mec DNA of pre-methicillin-resistant Staphylococcus aureus N315. Antimicrob. Agents Chemother. 43:1449-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito, T., X. X. Ma, F. Takeuchi, K. Okuma, H. Yuzawa, and K. Hiramatsu. 2004. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob. Agents Chemother. 48:2637-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Josefsson, E., K. Juuti, M. Bokarewa, and P. Kuusela. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 14.Juuti, K. M., B. Sinha, C. Werbick, G. Peters, and P. I. Kuusela. 2004. Reduced adherence and host cell invasion by methicillin-resistant Staphylococcus aureus expressing the surface protein Pls. J. Infect. Dis. 189:1574-1584. [DOI] [PubMed] [Google Scholar]
- 15.Katayama, Y., F. Takeuchi, T. Ito, X. X. Ma, Y. Ui-Mizutani, I. Kobayashi, and K. Hiramatsu. 2003. Identification in methicillin-susceptible Staphylococcus hominis of an active primordial mobile genetic element for the staphylococcal cassette chromosome mec of methicillin-resistant Staphylococcus aureus. J. Bacteriol. 185:2711-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kloos, W. E., D. N. Ballard, J. A. Webster, R. J. Hubner, A. Tomasz, I. Couto, G. L. Sloan, H. P. Dehart, F. Fiedler, K. Schubert, H. de Lencastre, I. Santos Sanchez, H. E. Heath, P. A. Leblanc, and Å. Ljungh. 1997. Ribotype delineation and description of Staphylococcus sciuri subspecies and their potential as reservoirs of methicillin resistance and staphylolytic enzyme genes. Int. J. Syst. Bacteriol. 47:313-323. [DOI] [PubMed] [Google Scholar]
- 17.Kloos, W. E., K. H. Schleifer, and R. F. Smith. 1976. Characterization of Staphylococcus sciuri sp. nov. and its subspecies. Int. J. Syst. Bacteriol. 26:22-37. [Google Scholar]
- 18.Kuusela, P., P. Hildén, K. Savolainen, M. Vuento, O. Lyytikäinen, and J. Vuopio-Varkila. 1994. Rapid detection of methicillin-resistant Staphylococcus aureus strains not identified by slide agglutination tests. J. Clin. Microbiol. 32:143-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luong, T. T., S. Ouyang, K. Bush, and C. Y. Lee. 2002. Type 1 capsule genes of Staphylococcus aureus are carried in a staphylococcal cassette chromosome genetic element. J. Bacteriol. 184:3623-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma, X. X., T. Ito, C. Tiensasitorn, M. Jamklang, P. Chongtrakool, S. Boyle-Vavra, R. S. Daum, and K. Hiramatsu. 2002. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 46:1147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsou, R., M. Bes, M. Boudouma, Y. Brun, H. Meugnier, J. Freney, F. Vandenesch, and J. Etienne. 1999. Distribution of Staphylococcus sciuri subspecies among human clinical specimens, and profile of antibiotic resistance. Res. Microbiol. 150:531-541. [DOI] [PubMed] [Google Scholar]
- 22.Mongolrattanothai, K., S. Boyle, T. V. Murphy, and R. S. Daum. 2004. Novel non-mecA-containing staphylococcal chromosomal cassette composite island containing pbp4 and tagF genes in a commensal staphylococcal species: a possible reservoir for antibiotic resistance islands in Staphylococcus aureus. Antimicrob. Agents Chemother. 48:1823-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murchan, S., M. E. Kaufmann, A. Deplano, R. de Ryck, M. Struelens, C. E. Zinn, V. Fussing, S. Salmenlinna, J. Vuopio-Varkila, N. El Solh, C. Cuny, W. Witte, P. T. Tassios, N. Legakis, W. van Leeuwen, A. van Belkum, A. Vindel, I. Laconcha, J. Garaizar, S. Haeggman, B. Olsson-Liljequist, U. Ransjo, G. Coombes, and B. Cookson. 2003. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J. Clin. Microbiol. 41:1574-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira, D. C., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roche, F. M., M. Meehan, and T. J. Foster. 2003. The Staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiology 149:2759-2767. [DOI] [PubMed] [Google Scholar]
- 26.Salmenlinna, S., O. Lyytikäinen, P. Kotilainen, R. Scotford, E. Siren, and J. Vuopio-Varkila. 2000. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in Finland. Eur. J. Clin. Microbiol. Infect. Dis. 19:101-107. [DOI] [PubMed] [Google Scholar]
- 27.Savolainen, K., L. Paulin, B. Westerlund-Wikström, T. J. Foster, T. K. Korhonen, and P. Kuusela. 2001. Expression of pls, a gene closely associated with the mecA gene of methicillin-resistant Staphylococcus aureus, prevents bacterial adhesion in vitro. Infect. Immun. 69:3013-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shittu, A., J. Lin, D. Morrison, and D. Kolawole. 2004. Isolation and molecular characterization of multiresistant Staphylococcus sciuri and Staphylococcus haemolyticus associated with skin and soft-tissue infections. J. Med. Microbiol. 53:51-55. [DOI] [PubMed] [Google Scholar]
- 29.Stepanović, S., I. Dakić, S. Djukić, B. Lozuk, and M. Švabić-Vlahović. 2002. Surgical wound infection associated with Staphylococcus sciuri. Scand. J. Infect. Dis. 34:685-686. [DOI] [PubMed] [Google Scholar]
- 30.Tsakris, A., E. Papadimitriou, J. Douboyas, F. Stylianopoulou, and E. Manolis. 2002. Emergence of vancomycin-intermediate Staphylococcus aureus and S. sciuri, Greece. Emerg. Infect. Dis. 8:536-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu, S. W., H. de Lencastre, and A. Tomasz. 1998. Genetic organization of the mecA region in methicillin-susceptible and methicillin-resistant strains of Staphylococcus sciuri. J. Bacteriol. 180:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu, S. W., C. Piscitelli, H. de Lencastre, and A. Tomasz. 1996. Tracking the evolutionary origin of the methicillin resistance gene: cloning and sequencing of a homologue of mecA from a methicillin susceptible strain of Staphylococcus sciuri. Microb. Drug Resist. 2:435-441. [DOI] [PubMed] [Google Scholar]