Abstract
To date, three species of Phaeoacremonium have been associated with phaeohyphomycosis. These are P. parasiticum (formerly Phialophora parasitica), P. inflatipes, and P. rubrigenum. Numerous unknown isolates resembling Phaeoacremonium spp. have in recent years been isolated from human patients as well as from woody plants that appear to be the main environmental source of these fungi. Nine new Phaeoacremonium species, of which six were obtained as etiologic agents of human opportunistic infection, are reported. They can be identified based on their cultural and morphological characters, and the identifications are strongly supported in phylogenetic analyses of partial sequences of the actin, β-tubulin, and calmodulin genes. A multiple-entry electronic key based on morphological, cultural, and β-tubulin sequence data was developed to facilitate routine species identification. Reexamination of all isolates of P. inflatipes associated with human disease showed them to be misidentified and to belong to the new taxa described here.
Phaeoacremonium parasiticum (Ajello, Georg & C.J.K. Wang) W. Gams, Crous & M.J. Wingf., under its original name Phialophora parasitica Ajello, Georg & C.J.K. Wang, was the first species of Phaeoacremonium W. Gams, Crous & M.J. Wingf. reported to cause phaeohyphomycosis in humans (1, 3). Subsequently, P. rubrigenum W. Gams, Crous & M.J. Wingf., P. parasiticum, and P. inflatipes W. Gams, Crous & M.J. Wingf. have been reported from phaeohyphomycosis cases (3, 10, 22). Most reported Phaeoacremonium cases have involved subcutaneous abscesses, cysts, or chronic or acute osteoarthritis in immunocompetent or immunocompromised patients; these cases were often initiated by traumatic inoculation (22). Infections not preceded by injury have also been reported (10). In a few cases involving immunocompromised patients, disseminated infections, fungemia, or endocarditis have been found (22). The occurrence of Phaeoacremonium infections in humans has increased over the past two decades, and although most cases have been ascribed to known species, several unknown species have also been recorded.
Presently there are seven known species of Phaeoacremonium, and species of this genus were recently shown to be connected to teleomorphs in the genus Togninia Berl. (Calosphaeriales) (19). In addition to the three species listed above associated with human infections, P. aleophilum W. Gams, Crous, M.J. Wingf & L. Mugnai has recently been reported from a human subcutaneous infection (10), but the isolate involved is shown to be a member of a species described here as new.
Several morphological identification keys have been developed to distinguish among the known Phaeoacremonium taxa (3, 5, 6), but in practice identifications made using these characters have resulted in incorrect identifications at both the generic and species levels (7, 9). A partial explanation for this lies in the fact that the distinguishing characters noted for several species are relatively minor differences in cultural and microscopic features (7). Recently, we have received numerous isolates that could not be keyed to any known species, suggesting that many undescribed species exist. The existence of undescribed species in Phaeoacremonium was suggested previously by Dupont et al. (7), who showed that several intermediate genotypes could be seen in PCR-restriction fragment length polymorphism analysis of Phaeoacremonium species and that these genotypes could not be accommodated in any of the known species (3).
The aims of this study were to use molecular and phenotypic analyses for isolate identification, to reevaluate the identities of Phaeoacremonium species previously described as infecting humans, and to delineate and describe new species. A further aim was to develop an identification key that can be used in routine laboratory identification for Phaeoacremonium species associated with humans. Morphologically similar Phaeoacremonium species that co-occur in the same or similar environmental reservoirs in woody plants were also included.
MATERIALS AND METHODS
Isolates.
Fifty-two Phaeoacremonium isolates from human cases and from woody hosts were lodged at the Centraalbureau voor Schimmelcultures (CBS) culture collection in Utrecht, The Netherlands (Table 1).
TABLE 1.
Names, accession numbers, and isolation details of Phaeoacremonium sp. strains examined
| Phaeoacremonium species (original identification) | Accession or medical lab report no.a | Source (reference) | Host | GenBank accession no. for:
|
||
|---|---|---|---|---|---|---|
| ACT | β-Tubulin | CAL | ||||
| P. aleophilumb | CBS246.91 | Yugoslavia | Vitis vinifera | AY735497 | AF246811 | |
| P. aleophilum | CBS100397 | Italy | Vitis vinifera | AY735498 | AF246806 | |
| P. aleophilum | CBS110703 | South Africa | Vitis vinifera | |||
| P. aleophilum | CBS111015 | South Africa | Vitis vinifera | |||
| P. alvesiib (P. aleophilum) | CBS 110034, FMR 7682 | Brazil (10) | Subcutaneous infection | AY579234 | AY579301 | AY579284 |
| P. alvesii | CBS 113590, VPRI 22409a | Australia | Dodoneae viscosa | AY579237 | AY579304 | AY579287 |
| P. alvesii (P. inflatipes) | CBS 408.78, CDC 78-042877 | United States | Synovial fluid, keratitis patient | AY579236 | AY579303 | AY579286 |
| P. alvesii (P. inflatipes) | CBS 729.97, 96-034129, CDC B-5747 | United States (22) | Subcutaneous lesion of foot | AY579235 | AY579302 | AY579285 |
| P. amstelodamenseb (P. inflatipes) | CBS 110627 | The Netherlands | Human, elbow joint interior | AY579228 | AY579295 | AY579278 |
| P. australienseb | CBS 113589, VPRI 22016a | Australia | Vitis vinifera | AY579229 | AY579296 | AY579279 |
| P. australiense | CBS 113592, VPRI 22892 | Australia | Vitis vinifera | AY579230 | AY579297 | AY579280 |
| P. griseorubrumb | CBS 111657, UTHSC 02-949 | United States | Human, blood | AY579227 | AY579294 | AY579277 |
| P. griseorubrum (P. rubrigenum) | CBS 566.97 | Japan (16) | Subcutaneous phaeohyphomycosis | AY579226 | AF246801 | AY579276 |
| P. inflatipesb | CBS 391.71 | United States | Quercus virginiana | AY579259 | AF246805 | AY579290 |
| P. inflatipes | CBS 113273, NRRL 32148 | United States | Hypoxylon truncatum | AY579260 | AY579323 | |
| P. inflatipes | CBS 166.75 | Costa Rica | Nectandra sp. | AY579258 | AY579322 | |
| P. krajdeniib (P. inflatipes) | CBS 109479, No. FR 60 | Canada | Human | AY579267 | AY579330 | |
| P. krajdenii | CBS 110118 | South Africa | Vitis vinifera | AY579261 | AY579324 | |
| P. krajdenii | CBS 113588 | South Africa | Vitis vinifera | AY579262 | AY579325 | |
| P. krajdenii (Phialophora repens) | CBS 423.73 | Democratic Republic of Congo (17) | Skin lesion | AY579263 | AY579326 | |
| P. krajdenii | CBS 633.93, No. 657/93 | Norway | Mycetoma on foot | AY579264 | AY579327 | |
| P. krajdenii (Phialophora repens) | CBS 110366, ATCC 58115, BB 903, SM 3531 | Japan (14) | Granuloma on hand | AY579265 | AY579328 | |
| P. krajdenii | CBS 110365, UAMH 5723, BB 725 | United States | Human | AY579266 | AY579329 | |
| P. krajdenii (P. inflatipes) | CBS 110367, CDC B6091, CDC 2001-00-8325 | United States | Mass on foot | AY579268 | AY579331 | |
| P. krajdenii (P. inflatipes) | CBS 110368, CDC B6092, CDC 2001-0 8363 | United States | Foot lesion | AY579269 | AY579332 | |
| P. krajdenii (P. inflatipes) | CBS 110361, CDC B6093, CDC 2001-00-8993 | India | White grain eumycetoma in foot | AY579270 | AY579333 | |
| P. parasiticumb | CBS 860.73, ATCC 26366, IMI 341971, IMI 181115, LCP 88.3537, STE-U 772, UAMH 3629 | United States (1) | Abscess on arm | AY579253 | AF246803 | |
| P. parasiticum | CBS 113585 | South Africa | Vitis vinifera | AY579241 | AY579307 | |
| P. parasiticum | CBS 113586 | South Africa | Vitis vinifera | AY579242 | AY579308 | |
| P. parasiticum | CBS 113591, VPRI 22542b | Australia | Vitis vinifera | AY579243 | AY579309 | |
| P. parasiticum | CBS 113594 | South Africa | Vitis vinifera | AY579244 | AY579310 | |
| P. parasiticum | CBS 109666, FG 00 04652 | United States | Human | AY579245 | AY579311 | |
| P. parasiticum | CBS 109665 | United States | Human | AY579246 | AY579312 | |
| P. parasiticum (P. rubrigenum) | CBS 110033, FMR 7681 | Brazil (10) | Subcutaneous infection | AY579247 | AY579313 | |
| P. parasiticum | CBS 113596, NOMH 568 | Canada | Left lower lobe of lung | AY579248 | AY579314 | |
| P. parasiticum | CBS 984.73, IMI 192879 | Tunisia | Prunus armeniaca | AY579249 | AY579315 | |
| P. parasiticum (P. inflatipes) | CBS 736.94, No. 94 MP 2578 | Finland | Human toenail | AY579250 | AY579316 | |
| P. parasiticum | CBS 184.75 | Iraq | Phoenix dactylifera | AY579251 | AY579317 | |
| P. parasiticum | CBS 101007 | Italy | Actinidia chinensis | AY579252 | AF246804 | |
| P. parasiticum | CBS 514.82, UAMH 5054, No. HD 873 | United States | Human synovial fluid | AY579240 | AY579306 | |
| P. rubrigenumb | CBS 498.94 | United States | Human, pneumonia patient | AY579238 | AF246802 | AY579288 |
| P. rubrigenum | CBS 112046, UTHSC 00-2395 | United States | Human, infected eye | AY579239 | AY579305 | AY579289 |
| P. scolytib | CBS 113597, STE-U 3092 | South Africa | Vitis vinifera | AY579224 | AF246800 | AY579274 |
| P. scolyti | CBS 112585, CCF 3266 | Czech Republic | Larva of Scolytus intricatus | AY579223 | AY579292 | AY579273 |
| P. scolyti | CBS 113593, LCP 97.4002 | France | Vitis vinifera | AY579225 | AY579293 | AY579275 |
| P. subulatumb | CBS 113584, STE-U4655 | South Africa | Vitis vinifera | AY579231 | AY579298 | AY579281 |
| P. subulatum | CBS 113587 | South Africa | Vitis vinifera | AY579232 | AY579299 | AY579282 |
| P. tardicrescensb (P. inflatipes) | CBS 110573, UTHSC 00-14 | United States | Human | AY579233 | AY579300 | AY579283 |
| P. venezuelenseb (Cephalosporium serrae, later P. inflatipes) | CBS 651.85, ATCC 32628, UAMH 4034 | Venezuela (4) | Mycetoma on foot | AY579256 | AY579320 | |
| P. venezuelense | CBS 110119, STE-U 4648 | South Africa | Vitis vinifera | AY579254 | AY579318 | |
| P. venezuelense | CBS 113595, SF 9587 (02) | Canada | Tissue from ankle | AY579255 | AY579319 | |
| P. venezuelense | CBS 113598, STE-U 3697 | Unknown | Unknown | AY579257 | AY579321 | AY579291 |
Culture collections listed: ATCC, American Type Culture Collection, Manassas, Va.; CCF, Culture Collection of Fungi, Department of Botany, Faculty of Science, Charles University, Prague, Czech Republic; CDC, Centers for Disease Control and Prevention, Atlanta, Ga.; FMR, Facultat Medicina dc Reus, Reus, Spain; IMI, CABI Bioscience, Egham, United Kingdom; LCP, Laboratory of Cryptogamy, National Museum of Natural History, Paris, France; NCPF, National Collection of Pathogenic Fungi, Bristol, United Kingdom; NOMH, Ontario Ministry of Health, Toronto, Canada; NRRI, USDA Agricultural Research Service Collection, Peoria, Ill.; STE-U, Department of Plant Pathology, University of Stellenbosch, Stellenbosch, South Africa; UAMH, University of Alberta Microfungus Collection, Devonian Botanic Garden, Edmonton, Canada; UTHSC, University of Texas Health Sciences Center, San Antonio, Tex.; and VPRI, Knoxfield Herbarium, Department of Primary Industries, Knoxfield, Australia.
Ex-type strains of species.
DNA isolation and amplification.
Genomic DNA was extracted from 50 isolates (15), and the partial β-tubulin and actin (ACT) genes were amplified. The partial calmodulin (CAL) gene was also amplified for a selection of 19 isolates. A fragment of approximately 600 bp of the 5′ end of the β-tubulin gene was amplified using primers T1 (21) and Bt2b (8). Approximately 300 bp of the 5′ end of the ACT gene was amplified using primers ACT-512F and ACT-783R (2). A region of approximately 500 bp of the CAL gene was amplified using primers CAL-228F and CAL-737R (2). Because Groenewald et al. (9) found the internal transcribed spacers (ITS1 and ITS2) and 5.8S rRNA insufficient to distinguish all taxa, this region was excluded in the present study. The reaction mixture contained 5 μl of diluted sample, 1× PCR buffer (Bioline), 2.5 pmol of each primer, 200 μM each of the deoxynucleoside triphosphates (dNTPs), 0.5 U of Taq DNA polymerase (Bioline), and MgCl2 at a concentration optimal for each primer set. Each reaction mixture was made up to a final volume of 25 μl with sterile water. The following optimal MgCl2 concentrations were determined for the primer sets: β-tubulin, 1.5 mM; ACT, 0.5 mM; and CAL, 2.5 mM. The following PCR amplification cycles were run on a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, Calif.): 96°C for 5 min, followed by 36 cycles of denaturation (94°C for 30 s), annealing for 30 s (at 52°C for ACT, 58°C for β-tubulin, and 55°C for CAL), elongation (72°C for 90 s), and a final 7-min extension step at 72°C. PCR products were analyzed by electrophoresis at 85 V for 30 min in a 0.8% (wt/vol) agarose gel in 0.5× TAE buffer (0.4 M Tris, 0.05 M sodium acetate, and 0.01 M EDTA, pH 7.85) and were visualized under UV following ethidium bromide staining. PCR products were purified according to the manufacturer's instructions using a commercial kit (GFX PCR DNA and Gel Band Purification, Amersham Biosciences, Roosendal, The Netherlands). Sequencing reactions were carried out with the PCR primers using a DYEnamic ET Terminator Cycle Sequencing kit (Amersham Biosciences, Roosendal, The Netherlands) according to the manufacturer's recommendations, and the resulting products were analyzed on an ABI Prism 3700 DNA Sequencer (Perkin-Elmer, Norwalk, Foster City, Calif.). A consensus sequence was computed from the forward and reverse sequences with SeqMan from the Lasergene package (DNAstar, Madison, Wis.). Sequences were deposited at GenBank (Table 1), and the alignments and trees were deposited in TreeBase (TreeBASE accession number SN1826).
Phylogenetic analysis.
Sequences were manually aligned in Sequence Alignment Editor version 2.0a11 (23) by inserting gaps. A partition homogeneity test was conducted in PAUP (Phylogenetic Analysis Using Parsimony) v.4.0b10 (27) to test the pairwise congruence between the ACT, β-tubulin, and CAL sequence data sets. Phylogenetic analyses using parsimony were conducted with PAUP. Phialophora richardsiae (Nannf.) Conant (CBS 270.33; GenBank accession number for ACT, AY579271; β-tubulin, AY579334) and Wuestneia molokaiensis Crous & J.D. Rogers (STE-U 3797; GenBank accession number for ACT, AY579272; β-tubulin, AY579335) were used as outgroups in the combined analysis of ACT and β-tubulin, whereas P. inflatipes (CBS 391.71) and P. krajdenii (CBS 109479) were used as outgroups for the alignment containing all three data sets. For the combined analysis of ACT, β-tubulin, and CAL, indels could be scored and were taken as single events. For both analyses, gaps were treated as a fifth character and all characters were unordered and of equal weight. Maximum parsimony analysis was performed using the heuristic search option with 1,000 random taxon additions and tree bisection and reconstruction (TBR) as the branch swapping algorithm. Bootstrap support values for the combined analysis were calculated from 1,000 heuristic search replicates and 100 random taxon additions. Tree length, consistency index (CI), retention index (RI), and the rescaled consistency index (RC) values were also calculated.
Morphology.
Isolates were plated onto malt extract agar (MEA; 2% Oxoid malt extract, 1.5% Difco agar) and potato-dextrose agar (PDA; 3.9% Difco potato-dextrose agar) and placed at 25°C in the dark for 2 to 3 weeks until cultures sporulated. Some plates were placed under near-UV light to enhance sporulation. Microscopic mounts were made from aerial mycelium 2 to 3 cm from the colony margin. Structures that formed on aerial mycelium were mounted in lactic acid on glass slides. Thirty measurements were made using a light microscope (Axioskop 2 plus; Zeiss) of each type of structure. Water agar (WA; 1.2% Oxoid agar technical) and potato-carrot agar (PCA; 50% potato-carrot extract, 0.8% Roko agar) were used to examine the presence and size of hyphal warts. Warts are exudate droplets, perceived as wart-like structures under the light microscope. Morphological terms used follow Hawksworth et al. (13). The 5th and 95th percentiles were determined for all measurements, with the extreme values given in parentheses (where extreme values corresponded with 5th or 95th percentiles, they were omitted). Cardinal temperatures for growth were determined by incubating inoculated MEA plates in the dark at temperatures ranging from 5 to 40°C in 5° intervals, including 37°C to simulate human body temperature. Radial growth was measured after 8 days at 25°C. Surface colony colors, using general color names, were determined from the same plates incubated at 25°C after 8 and 16 days. Yellow diffusible pigment production was observed on PDA after 8 and 16 days at 25°C.
An electronic identification key using BioloMICS.
An electronic identification key was developed based on 23 micromorphological and cultural characters for ex-type strains of 12 Phaeoacremonium species and β-tubulin sequences generated with the primers T1 and Bt2b. Discrete data were scored for the main states as well as for the intermediate states. The minimum, 5th percentile, 95th percentile, and maximum values of size data were used. The micromorphological characters (described fully in the results) include: conidiophore structure and size, occurrence of three phialide types, Type II phialide shape and size, Type III phialide shape, occurrence of phialide percurrent proliferation, extent of wart formation, maximum diameter of warts, mycelial texture, and conidial shape and size. Cultural characters used were colony color on MEA at 25°C after 8 days in the dark, yellow pigment production on PDA, optimal and maximum growth temperature, and radial growth at 25 and 30°C (after 8 days in the dark). A data matrix was compiled on a spreadsheet and imported into BioloMICS (25). Character weights were determined by excluding individual characters and comparing the distance matrices. Coherent coefficients of correlation were determined and used as a measurement to determine if the character positively correlated with β-tubulin sequences. The use of characters weighted according to a subjective perception of their relative usefulness was compared to the use of objective, unweighted characters (meaning that each character was automatically assigned a weight equal to 1). Coherent coefficients of correlation were then calculated. Various algorithms were used to obtain the best fit for each of the data types.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in the course of this study were deposited in the GenBank database, and the accession numbers are listed in Table 1.
RESULTS
Phylogeny.
The combined alignment of ACT and β-tubulin contained 52 strains including the two outgroups and had a total length of 882 characters, of which 267 were constant, 197 were parsimony uninformative, and 418 were parsimony informative. The result of the partition homogeneity test showed that the ACT and β-tubulin data sets were congruent (P = 0.39) and could therefore be combined. From the phylogenetic analysis (Fig. 1) six distinct and well-supported clades were obtained, the first (76% bootstrap support) containing strains related to P. rubrigenum, the second (100% bootstrap support) strains of P. parasiticum, the third (100% bootstrap support) a new species of Phaeoacremonium, the fourth (100% bootstrap support) P. inflatipes, the fifth (100% bootstrap support) another new species of Phaeoacremonium, and the sixth (100% bootstrap support) strains of P. aleophilum. Within clade 1, a large set of isolates appeared to be phylogenetically related to the type strain of P. rubrigenum, CBS 498.94. In most cases, however, this relatedness was not sufficiently close for the isolates to be considered conspecific with P. rubrigenum, especially given that strong morphological divergences were seen among subgroups of isolates within the clade. Strong bootstrap support (above 91%) was found for seven previously unnamed species within this clade. Two of these species are known from only one isolate each (CBS 110627 and CBS 110573). Their distant grouping as well as their distinctive morphological and cultural characteristics supported their species designation. Even though phylogenetic variation was observed within the strains in clades 3 and 4, this variation was not linked to any perceptible morphological differences suggestive of species-level differentiation.
FIG. 1.
One of 10 most parsimonious trees obtained from heuristic searches of a combined alignment of the ACT and β-tubulin gene sequences (length, 1,715 steps; CI, 0.692; RI, 0.896; and RC, 0.620). Bootstrap support values (1,000 replicates) above 65% are shown at the nodes.
An additional gene region, CAL, was sequenced to provide greater clarity about the status of the taxa closely related to P. rubrigenum. Pairwise comparisons were made between ACT, β-tubulin, and the CAL alignments using a partition homogeneity test, and all combinations were found to be congruent. The combined alignment had a total length of 1,339 characters, of which 783 were constant, 218 were parsimony uninformative, and 338 were parsimony informative. This alignment contained 19 strains including the two outgroups. Figure 2 shows the result of the combined analysis, supporting the topology already found.
FIG. 2.
The single-most parsimonious tree obtained from heuristic searches of a combined alignment of the partial ACT, β-tubulin, and CAL gene sequences (length, 924 steps; CI, 0.835; RI, 0.884; and RC, 0.738). All bootstrap support values (1,000 replicates) obtained are shown at the nodes.
Morphology.
The morphological distinctions among isolates were congruent with the distinctions made in the phylogenetic analyses. The morphological characters that proved most useful in distinguishing species included a combination of conidiophore morphology, phialide type and morphology, and, to a lesser extent, conidial size and shape. Useful macromorphological characters included colony color on MEA, yellow pigment production in PDA, growth rate at 25°C, and maximum growth temperature. The extent and size of the mycelial warts proved to be a particularly good distinguishing character for several species. P. parasiticum could, for instance, easily be identified based on the frequent occurrence of very prominent (to 3 μm) warts along the outer walls of hyphae (Fig. 3A). By contrast, the hyphae of other taxa had smaller warts that formed in moderate densities (Fig. 3B). The occurrence of warts was influenced by the age of the culture and the medium used. Warts were not common on mycelium at the margin of a colony and on mycelium from colonies younger than 9 days. Warts were also less frequently observed on the lower-nutrient media PCA and WA than on 2% MEA. Additional mycelial ornamentation was due to irregularities in the cell wall surface, resulting in it varying from verruculose (lightly textured) to verrucose (roughly textured). Conidiophore structure is an important feature, distinguishing among species having predominantly long or frequently branched conidiophores, and species with short and unbranched or infrequently branched conidiophores (Fig. 4). Three types of phialides occurring on aerial mycelium were identified in Phaeoacremonium (11), i.e., types I, II, and III (Fig. 5). They differ in size and shape (Fig. 6), and the predominance of one or more of the three types is distinctive for certain species. Type I phialides, for which the specialized term adelophialides may also be used, were the shortest, 3 to 11 μm long (minimum [min] length, 2 μm; maximum [max] length, 17 μm), and had no basal septum. Type II phialides were medium sized (9 to 14 μm long [min length, 5 μm; max length, 16 μm]) and, in shape, were elongate-ampulliform (swollen at the base and gradually tapering towards an extended, narrow neck) or navicular (literally boat-shaped, meaning elongated with an expanded central region tapering towards narrower regions at both the base and the apex). Type III phialides were long (15 to 23 μm long [min length, 10 μm; max length, 34 μm]), subcylindrical, navicular, or subulate (awl-shaped, i.e., basally subcylindrical but tapering abruptly to form a thin neck). Phialides were mostly monophialidic, that is, they produced conidia from just a single apical aperture. However, some Type II and a few Type I phialides proliferated (i.e., added a new fertile extension after some conidial formation had already taken place) to become polyphialidic (see descriptions of P. scolyti and P. krajdenii below), ultimately terminating in a forked apex consisting of two conidiogenous apertures. This apical branching was mostly inequilateral, with the subapical, more recently proliferated side branch becoming longer than the original phialidic neck. Percurrent proliferation, that is, the growing of a new phialide through the tip of an existing phialide, was observed in some species. In this process, each newly proliferated phialide often becomes strongly swollen at the base, just above the point where it has grown out of the narrow apex of the preceding phialide.
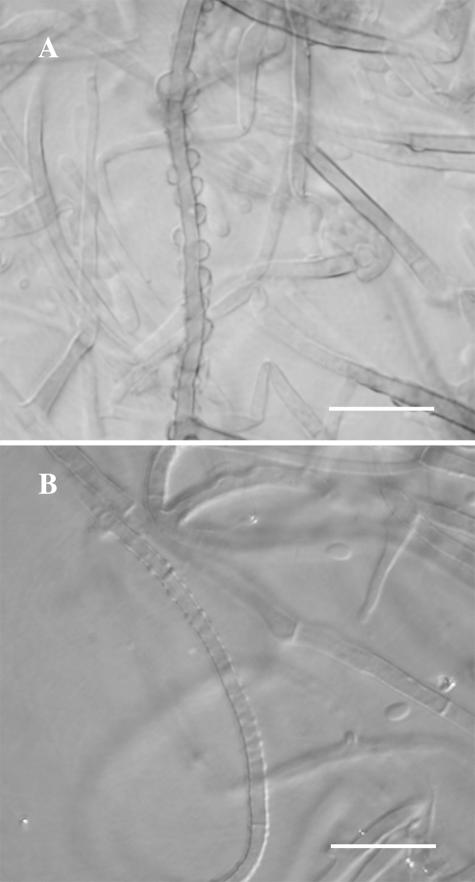
FIG. 3.
(A) Densely occurring, prominent exudate droplets (perceived as warts under the light microscope) on mycelium of P. parasiticum (CBS 860.73) and (B) smaller, less densely occurring exudate droplets on mycelium of P. alvesii (CBS 110034) grown on MEA (scale bar, 10 μm).
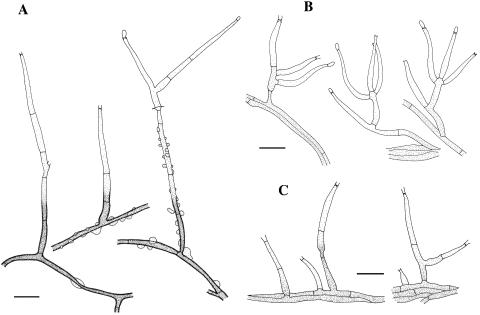
FIG. 4.
Conidiophore morphology pattern of Phaeoacremonium. (A) Long, branched conidiophores of P. parasiticum (CBS 860.73). (B) Branched conidiophores of P. inflatipes (CBS 391.71). (C) Short, usually unbranched conidiophores of P. rubrigenum (CBS 498.94) (scale bar, 10 μm).
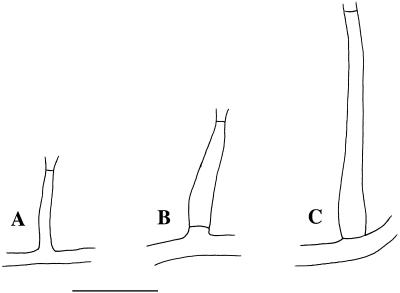
FIG. 5.
Three types of phialides. (A) Type I phialide (adelophialide); (B) Type II phialide; (C) Type III phialide (scale bar, 10 μm).
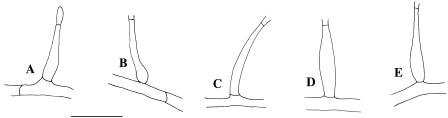
FIG. 6.
Different shapes of Type II and III phialides. (A) Elongate-ampulliform and attenuated at the base; (B) elongate-ampulliform and constricted at the base; (C) subcylindrical; (D) navicular; (E) subulate (scale bar, 10 μm).
The species examined, as presently understood after molecular study, are here formally described and are given detailed morphological descriptions to facilitate phenotypic identification. P. aleophilum, P. rubrigenum, P. inflatipes, and P. parasiticum are redescribed for comparison and to include additional morphological features disclosed by this study. The cardinal temperatures for growth as well as the extent of radial growth after 8 days are given in Table 2.
TABLE 2.
Cardinal temperatures for growth and radial growth distance of Phaeoacremonium species
| Species | Cardinal temp (°C) for growth
|
Radial growth in mm (8 days at 25°C) | ||
|---|---|---|---|---|
| Minimum | Optimum | Maximum | ||
| Phaeoacremonium aleophilum | 10 | 30 | 37/40 | 2.5-11 |
| Phaeoacremonium alvesii | 15 | 30 | 37 | 9.5-11 |
| Phaeoacremonium amstelodamense | 15 | 30 | 40 | 11.5-12.5 |
| Phaeoacremonium australiense | 15 | 30 | 35-37 | 9-10 |
| Phaeoacremonium griseorubrum | 10 | 30 | 40 | 6-7.5 |
| Phaeoacremonium inflatipes | 10 | 25-30 | 35 | 12.5-13 |
| Phaeoacremonium krajdenii | 15 | 30 | 37 | 9-14 |
| Phaeoacremonium parasiticum | 15 | 30 | 40 | 10.5-11.5 |
| Phaeoacremonium rubrigenum | 10 | 30 | 37 | 9.5-10 |
| Phaeoacremonium scolyti | 15 | 25-30 | 37 | 10.5-12 |
| Phaeoacremonium subulatum | 15 | 25-30 | 37 | 8.5-11.5 |
| Phaeoacremonium tardicrescens | 15 | 30 | 40 | 8-9 |
| Phaeoacremonium venezuelense | 15 | 30 | 40 | 9-16 |
Following an increasingly used practice for names of new fungal species that are relatively similar to previously known species, the Latin diagnoses required by international nomenclatural law are truncated, consisting of a distinction from the closest phylogenetic relative among the already described species.
General description of Phaeoacremonium species.
A generic description is provided summarizing general characters common to all Phaeoacremonium species. The species descriptions follow and appear in alphabetical order. For species previously described, a reference to the original publication is provided.
Colonies on MEA are flat with entire (nonragged) margins, mostly moderately dense, predominantly felty, and sometimes woolly textured. Most colonies are brown in color, with frequently seen shades including pale brown, medium brown, orange-brown, gray-brown, and dark brown. Paler colonies may range from pale yellow to beige. Pink-colored colonies are in shades ranging from pale to dark pink. Mycelium consists of branched, septate hyphae that occur singly or are bundled together in fascicles mostly consisting of 10 or fewer individual hyphae. Hyphae are medium brown, becoming paler brown to hyaline near areas where conidia are formed, and are smooth, verruculose, or verrucose. Hyphae vary in the density and size of the warts present. Conidiophores are branched in the basal region or are unbranched. They arise from aerial or submerged hyphae and in some cases undergo percurrent proliferation. Conidiophores are erect, nearly cylindrical when unbranched, slightly tapered, straight or flexuous, variable in length, 0- to 7-septate, and may have small warts or verruculose ornamentation at their bases. They are mostly pale brown, becoming increasingly pale towards the tip. The apical cell of a conidiophore can produce up to three phialides. Phialides are terminal or lateral and are mostly monophialidic, but sometimes they proliferate to become polyphialidic. They bear few if any warts and may be verruculose or smooth and pale brown to hyaline. Three distinct size classes of phialides (Types I to III) can be observed. Phialides have a terminal, narrowly funnel-shaped collarette. Conidia are aggregated into round, slimy heads at the apices of phialides. They are hyaline to subhyaline, aseptate, and smooth walled. They generally vary from oblong-ellipsoidal to obovate (egg-shaped, with the apical end broader than the basal end), cylindrical, reniform (kidney shaped), or allantoid (sausage-like) in shape, or they are uncommonly fusiform-ellipsoidal (ellipsoidal with two somewhat pointed ends) or globose. They have homogeneous cellular contents when young but can develop two conspicuous vacuoles (after 7 to 14 days), probably containing oil or other metabolic storage products at maturity; in common morphological terminology, they are thus two-guttulate.
Phaeoacremonium aleophilum (3).
Colonies on MEA are honey-brown or beige. Colonies on PDA produce a yellow diffusible pigment in the agar. Hyphae are mostly verruculose, medium to pale brown, and 1 to 2.5 μm wide. Warts on hyphae are up to 1.5 μm wide. Conidiophores are mostly short and usually unbranched, 0- to 3-septate, 17 to 42 μm long (min length, 15 μm; max length, 46 μm) and 1.5 to 2.5 μm wide. The apical cell of conidiophores usually produces one phialide. Type I phialides are cylindrical, occasionally wider at the base, 2 to 9 μm long (min length, 1.5 μm; max length, 11 μm) by 1 to 1.5 μm wide. Type II phialides are either elongate-ampulliform and attenuated at the base or are navicular, tapering towards the apex, 9 to 14 μm long (min length, 6 μm; max length, 15 μm) by 1.5 to 2.5 μm wide. Type III phialides are subcylindrical or elongate-ampulliform and attenuated at the base, 15 to 22 μm long (min length, 14 μm) by 1.5 to 2 μm wide, tapering gradually to a long neck. Collarettes are 1 to 1.5 μm long and 1.5 to 2 μm wide. Conidia are mostly oblong-ellipsoidal or cylindrical, occasionally reniform, 3 to 5 μm long by 1 to 2 μm wide.
Holotype.
Yugoslavia. On roots and stems of Vitis vinifera, 1990, M. Muntaňola-Cvetković (herb. CBS 246.91 dried specimen, cultures ex-type CBS 246.91 STE-U 776, dried ISOTYPE lodged at PREM).
Notes.
P. aleophilum can be distinguished from the species with brown colonies, i.e., P. parasiticum and P. inflatipes, by its short and usually unbranched conidiophores. P. parasiticum also has prominent warts not observed for the other brown-colored species. These species produce long and/or branched conidiophores. P. krajdenii and P. australiense, also brown-colored species, do not produce a yellow pigment on PDA.
Phaeoacremonium alvesii L. Mostert, Summerb. & Crous, sp. nov.
Differt a simili P. rubrigeno conidiophoris brevioribus plerumque simplicibus, raro ramosis, et pigmentis flavis in culturis formatis (PDA) (Fig. 7).
FIG. 7.
(A to D) P. alvesii (CBS 110034), Type I phialide, Type II phialide, Type III phialides, and conidia; (E to H) P. scolyti (CBS 113597), Type I phialide, Type II phialide, Type III phialides, and conidia; (I to L) P. griseorubrum (CBS 111657), Type I phialide, Type II phialide, Type III phialides, and conidia; (M to P) P. amstelodamense (CBS 110627), Type I phialide, Type II phialide, Type III phialides, and conidia; (Q to T) P. australiense (CBS 113589), Type I phialide, Type II phialide, Type III phialides, and conidia (scale bars, 5 μm).
Colonies on MEA are medium pink or beige, becoming pale brown with age. Yellow pigment is produced on PDA but was observed in one strain only after 2 weeks. Hyphae are verruculose, medium to pale brown, and 1 to 2.5 μm wide. Warts on hyphae are up to 0.5 μm wide. Conidiophores are mostly short and usually unbranched, occasionally narrower at the base, 0- to 2-septate, 17 to 43 μm long (min length, 14 μm; max length, 50 μm) by 1.5 to 2 μm wide. The apical cell of conidiophores usually produces one phialide. Type I phialides are cylindrical, occasionally wider at the base, 4 to 12 μm long (min length, 3 μm) by 1 to 1.5 μm wide. Type II phialides are subcylindrical to navicular, rarely swollen at the base, tapering towards the apex, 10 to 14 μm long by 1.5 to 2 μm wide. Type III phialides are predominant, navicular to subcylindrical, between 14 and 22 μm long (min length, 13 μm) by 1.5 to 2.5 μm wide, tapering gradually to a long neck. Collarettes are 2 to 2.5 μm long and 1 to 1.5 μm wide. Conidia are mostly obovoid or oblong-ellipsoidal, occasionally reniform to allantoid, 3 to 4 μm long (max length, 6 μm) by 1 to 1.5 μm wide (max width, 2 μm).
Holotype.
Brazil. Human subcutaneous infection, 2000, S.H. Alves (dried specimen in herb. CBS 7958, culture ex-type CBS 110034, FMR 7682).
Etymology.
In honor of Sydney Hartz Alves (Microbiologia Departamento Análises Clinicas e Toxicologicas; Laboratório de Pesquisas Micológias [LAPEMI], Universidade Federal de Santa Maria, Santa Maria, Brasil), who collected this species.
Notes.
According to the DNA phylogeny, P. alvesii is most closely related to P. rubrigenum, but these species differ in several aspects. P. rubrigenum has medium pink to pale pink colonies, while those of P. alvesii are medium pink to beige. P. alvesii, compared to P. rubrigenum, has relatively simple, infrequently branched conidiophores as well as darker brown mycelium.
Phaeoacremonium amstelodamense L. Mostert, Summerb. & Crous, sp. nov.
Differt a simili P. rubrigeno conidiis minoribus, 2 to 4 μm long (max length, 6 μm) by 1 to 2 μm wide, et coloniis bubalinis (MEA) (Fig. 7).
Colonies on MEA are overall beige, becoming pale brown in the center with age. Hyphae occur mostly singly and are verruculose, pale orange-brown, and 1.5 to 2.5 μm wide. Warts on hyphae are up to 1 μm wide. Conidiophores are mostly short and usually unbranched, constricted at the septa with swollen bases, 0- to 5-septate, 16 to 61 μm long (min length, 15 μm; max length, 90 μm), and 1.5 to 3 μm wide. Percurrent proliferation occurs often. The apical cell of each conidiophore can produce up to two phialides. Type I phialides are mostly cylindrical, 2 to 8 μm long by 1 to 1.5 μm wide. Type II phialides are predominant, mostly elongate-ampulliform, and constricted at the base, tapering towards the apex, 6.5 to 14 μm long (min length, 5 μm) by 1.5 to 3 μm wide. Type III phialides are elongate-ampulliform and attenuated at the base, or subcylindrical, 14 to 19 μm long (min length, 13 μm; max length, 20 μm) by 1.5 to 2.5 μm wide, tapering towards the apex. Collarettes are 1 to 1.5 μm long and 1.5 to 2 μm wide. Conidia are mostly oblong-ellipsoidal or obovoid, occasionally allantoid, 2 to 4 μm long (max length, 6 μm) by 1 to 2 μm wide.
Holotype.
The Netherlands. Amsterdam, human, elbow joint interior, Jun 2002, J. Bruins, (dried herbarium specimen in herb. CBS 7960, culture ex-type CBS 110627).
Etymology.
Named after the place where the only strain of this species was isolated, Amsterdam.
Notes.
This strain produced very little aerial mycelium, so microscopic observations were made from concentrated tufts on the agar. P. amstelodamense can be distinguished by the combination of its beige colonies, its high level of percurrent proliferation of the conidiophores, and its Type II phialides with elongate-ampulliform, strongly constricted bases.
Phaeoacremonium australiense L. Mostert, Summerb. & Crous, sp. nov.
Differt a simili P. rubrigeno coloniis centro brunneis, margine lato aurantiaco-albido cinctis (post 16 dies, MEA) (Fig. 7).
Colonies on MEA are pale brown, becoming medium brown in the center with age. Hyphae are verruculose, pale brown, and 1.5 to 3 μm wide. Warts on hyphae are up to 1 μm in diameter. Conidiophores are mostly short and usually unbranched, often constricted at the septa, 1- to 4-septate, 17 to 50 μm long (min length, 14 μm; max length, 64 μm) and 1.5 to 2.5 μm wide. The apical cell of conidiophores can produce up to three phialides. Type I phialides are cylindrical, occasionally wider at the base, tapering towards the apex, between 3 to 8 μm long by 1 to 2 μm wide. Type II phialides are elongate-ampulliform, sometimes attenuated at the base, or navicular, tapering towards the apex, 8.5 to 14 μm long (min length, 8 μm) by 1.5 to 2.5 μm wide. Type III phialides are subcylindrical to navicular, 13.5 to 20 μm long (min length, 12 μm; max length, 22 μm) by 1.5 to 2.5 μm wide, gradually tapering to a long and narrow neck. All three phialide types occur in equal proportions. Collarettes are slightly flaring, 2 to 2.5 μm long and 1.5 to 2.5 μm wide. Conidia are oblong-ellipsoidal to obovoid, occasionally cylindrical or reniform, 3 to 4 μm long (min length, 2.5 μm; max length, 4.5 μm) by 1 to 2 μm wide.
Holotype.
Australia. Moyhu, Victoria, Vitis vinifera L., T. Knaggs (dried specimen in herb. CBS 7955, culture ex-type CBS 113589).
Etymology.
Both strains representing this species were isolated from grapevines in Australia.
Notes.
Colonies have a distinct brown center, with a broad orange-white outer margin that develops after 16 days. The brown-colored species, P. inflatipes and P. parasiticum, form frequently branched or long conidiophores, respectively, in comparison with the mostly short and unbranched conidiophores of P. australiense. P. australiense has verruculose mycelium in comparison with the verrucose mycelium of two other brown-colored species, P. krajdenii and P. tardicrescens. P. australiense can be distinguished from another brown-colored species, P. aleophilum, by the absence of yellow pigment formation of P. australiense in PDA.
Phaeoacremonium griseorubrum L. Mostert, Summerb. & Crous, sp. nov.
Differt a simili P. rubrigeno coloniis rubescentibus, demum obscure roseis, mycelio denso, ad 6 to 7.5 mm diameter (post 8 dies, 25°C, MEA) (Fig. 7).
Colonies on MEA are dark pink, becoming darker with age. Hyphae are verruculose, occasionally verrucose, pale yellow-brown to hyaline, and 1 to 3 μm wide. Warts on hyphae are up to 1.5 μm wide. Conidiophores are mostly short and usually unbranched, often constricted at the base, 0- to 4-septate, 23 to 70 μm long (min length, 21 μm; max length, 85 μm) and 2 to 3 μm wide. The apical cell of each conidiophore can produce up to three phialides. Type I phialides are cylindrical to navicular, occasionally widened at the base, 2 to 12 μm long by 1 to 2.5 μm wide. Type II phialides are elongate-ampulliform or navicular, tapering towards the apex, 9 to 15 μm long (min length, 6 μm) by 2 to 2.5 μm wide. Type III phialides are subcylindrical or navicular, 16 to 24 μm long (min length, 15 μm; max length, 25 μm) by 2 to 2.5 μm wide, gradually tapering towards the apex. Type II and III phialides are predominant. Collarettes are 1.5 to 2 μm long and 1 to 1.5 μm wide. Conidia are mostly obovoid, occasionally oblong-ellipsoidal or globose, 3 to 3.5 μm long (min length, 2 μm; max length, 5.5 μm) by 1.5 to 2 μm wide (min width, 1 μm).
Holotype.
United States. Maryland, Baltimore, human blood, 2002, D. Sutton (dried specimen in herb. CBS 7954, culture ex-type CBS 111657).
Etymology.
Named after the distinct dark pink colony color this species has on MEA.
Notes.
P. griseorubrum could be distinguished from the other species producing pink colonies on MEA (P. rubrigenum, P. scolyti, and P. alvesii) by its dark pink colonies that are denser than those of the other species, as well as by its slow growth, with colonies reaching a radius of only 6 to 7.5 mm in 8 days at 25°C.
Phaeoacremonium inflatipes (3).
Colonies on MEA are brown to gray-brown. Hyphae are verruculose, pale brown to hyaline, and 1.5 to 3 μm wide. Warts on hyphae are up to 0.5 μm wide. Conidiophores are mostly branched in the basal region, pale brown to hyaline, frequently with a slightly swollen base, 0- to 5-septate, 18 to 40 μm long (min length, 14 μm; max length, 43 μm) and 1.5 to 2 μm wide. Percurrent proliferation occurs. The apical cell of each conidiophore can produce up to three phialides. Type I phialides are cylindrical, tapering towards the apex, 3 to 13 μm long (min length, 2 μm; max length, 16 μm) by 1 to 2 μm wide. Type II phialides are elongate-ampulliform and attenuated at the base, or navicular, tapering towards the apex, 10 to 15 μm long (min length, 7.5 μm) by 1.5 to 2 μm wide. Type III phialides are most common and are subcylindrical to navicular, 12 to 25 μm long (min length, 10 μm; max length, 28 μm) by 1.5 to 2.5 μm wide, tapering very gradually towards the apex. Collarettes are 1.5 to 3 μm long and 1 to 1.5 μm wide. Conidia are mostly oblong-ellipsoidal or obovoid, occasionally reniform or allantoid, 3 to 4.5 μm long (max length, 10 μm) by 1 to 2 μm wide.
Holotype.
United States. Texas, on stems of Quercus virginiana Mill., 1966, R.S. Halliwell (CBS 391.71, IMI 192880, CMW 2027, STE-U 770, dried isotype lodged at PREM).
Notes.
P. inflatipes can be identified based on its branched conidiophores (Fig. 4B), combined with its brown colony color. P. parasiticum differs in having brown colonies with medium brown centers and a colony radius of 12.5 to 13 mm on MEA after 8 days, whereas colonies of P. inflatipes are evenly colored, have brown to gray-brown colonies, and grow more slowly, with a colony radius of 10.5 to 11.5 mm after 8 days. P. inflatipes also has hyphae that are relatively weakly pigmented, shorter, and more frequently branched conidiophores and smaller hyphal warts (up to 0.5 μm) than those observed in P. parasiticum (up to 3 μm). P. inflatipes was somewhat heterogeneous. One isolate, CBS 166.75 from woody plant material, grouped distantly from the ex-type strain, CBS 391.71, in the phylogenetic tree (Fig. 1), and also differed by having a growth optimum of 25°C in contrast to the 30°C optimum observed for the ex-type strain. No conclusive cultural or morphological differences were found, however, to support segregating this isolate as a distinct species. The three strains are therefore referred to as representative of P. inflatipes.
Phaeoacremonium krajdenii L. Mostert, Summerb. & Crous, sp. nov.
Differt a simili P. parasitico hyphis modice verruculosis, verrucis ad 1 μm altis et coloniis plus minusve obscure brunneis (MEA) (Fig. 8).
FIG. 8.
(A to D) P. subulatum (CBS 113584), Type I phialide, Type II phialide, Type III phialides, and conidia; (E to H) P. tardicrescens (CBS 110573), Type I phialide, Type II phialide, Type III phialides, and conidia; (I to L) P. krajdenii (CBS 109479), Type I phialide, Type II phialide, Type III phialides, and conidia; (M to P) P. venezuelense (CBS 651.85), Type I phialide, Type II phialide, Type III phialides, and conidia (scale bars, 5 μm).
Colonies on MEA are brown to dark brown. Hyphae are verrucose and dark to medium brown and 2 to 3 μm wide. Warts are common on hyphae and are up to 1 μm wide. Conidiophores are short and usually unbranched, occasionally constricted at the basal septum; bases of older, percurrently proliferating cells are often inflated, 1- to 5-septate, 20 to 45 μm long (min length, 16 μm; max length, 76 μm) and 1.5 to 3 μm wide. The apical cell of each conidiophore can produce up to two phialides. Phialides are often polyphialidic. Type I phialides are cylindrical, occasionally wider at the base, tapering towards the apex, 4 to 13 μm long (min length, 2 μm; max length, 17 μm) by 1 to 2 μm wide. Type II phialides are predominant, elongate-ampulliform and attenuated at the base, or are subcylindrical, 8.5 to 14 μm long (min length, 8 μm) by 1.5 to 2.5 μm wide. Type III phialides are navicular to subcylindrical or sometimes elongate-ampulliform and attenuated at the base, measure 14 to 21 μm long (max length, 25 μm) by 1 to 2.5 μm wide and gradually taper towards the apex. Collarettes are 1 to 3 μm long and 1 to 2 μm wide. Conidia are subhyaline to hyaline, oblong-ellipsoidal or allantoid, 3 to 5 μm long (max length, 8 μm) by 1 to 1.5 μm wide (max width, 2 μm).
Holotype.
Canada. Ontario, Toronto, human, 2001, S. Krajden (dried specimen in herb. CBS 7959, culture ex-type CBS 109479).
Etymology.
In honor of Sigmund Krajden (St. Joseph's Health Center, Toronto, Ontario M6R 1B5, Canada), a physician who has made many distinguished individual and collaborative contributions to the study of medically important fungi.
Notes.
P. krajdenii can be distinguished by having verrucose hyphae with medium-sized warts (up to 1 μm). This species also produces polyphialides frequently and collarettes that are slightly flaring. With eight strains available from clinical cases, P. krajdenii seems to be approximately equal in prevalence with the better-known P. parasiticum, of which seven clinical strains were available to us for the purpose of this study. P. krajdenii can be distinguished from P. parasiticum in colony color, conidiophore structure, and occurrence of polyphialides and warts. Colonies of P. krajdenii are a darker brown than those of P. parasiticum. P. krajdenii has short, mostly unbranched conidiophores in comparison with the very long conidiophores of P. parasiticum. P. parasiticum can also be distinguished by the absence of polyphialides and by the prominent warts formed on its mycelium.
Phaeoacremonium parasiticum (3).
Colonies on MEA are brown with a medium-brown center and become darker with age. Hyphae occur singly or in bundles of up to 20. Hyphae are verrucose, dark to medium brown, and 1.5 to 3.5 μm wide. Warts up to 3 μm wide are very common on hyphae. Conidiophores are mostly long and branched, medium brown, becoming paler towards the tip, 1- to 7-septate, 27 to 80 μm long (min length, 24 μm; max length, 130 μm) and 1.5 to 2.5 μm wide. Unbranched conidiophores are sometimes slightly swollen at the base. Percurrent proliferation occurs frequently. Apical cells of conidiophores mostly produce one phialide. Type I phialides are cylindrical, occasionally wider at the base, tapering towards the apex, 4 to 17 μm long (min length, 2 μm) by 1 to 2 μm wide. Type II phialides are subcylindrical, tapering towards the apex, 14 to 15 μm long by 1.5 to 2 μm wide. Type III phialides are predominant, mostly cylindrical to subulate, 19 to 29 μm long (max length, 37 μm) by 1.5 to 2 μm wide, tapering very gradually and terminating in a narrow neck. Type I and II phialides are rare. Collarettes are 0.5 to 2 μm long and 1 to 2 μm wide. Conidia measure from 3 to 4 μm long (max length, 6 μm) by 1.5 to 2 μm wide (min width, 1 μm) and are mostly oblong-ellipsoidal or obovoid, sometimes allantoid to broadly oblong. Conidia remain aggregated in masses when mounted in lactic acid because of copious, tenacious mucus produced.
Holotype.
United States. California, Stanford University Hospital, isolated from human subcutaneous phaeohyphomycosis, 1971, R.T. Steigbigel (dried specimen at BPI, ex-type cultures CBS 860.73, IMI 181115).
Notes.
P. parasiticum is very distinctive, easily recognized by its predominance of long, branched conidiophores (Fig. 5), long Type II and Type III phialides, dark brown hyphae, and large hyphal warts of up to 3 μm in diameter. In different studies, discrepant optimal growth temperatures have been obtained for P. parasiticum, ranging from 25 (3) to 30°C (6). Results from the present study suggest that the optimum is closer to 30 than to 25°C. Both Crous et al. (3) and Du Pont et al. (7) only used the strains CBS 860.73 and CBS 984.73, which were also included in our study.
Phaeoacremonium rubrigenum (3).
Colonies on MEA are pale to medium pink, becoming purple-pink to brown-pink with age. Hyphae are verruculose, orange to pale brown, and 1.5 to 3 μm wide. They bear warts of up to 1 μm wide. Conidiophores are mostly short and usually unbranched, 0- to 4-septate, 23 to 51 μm long (min length, 20 μm; max length, 70 μm) and 1.5 to 3 μm wide. Percurrent proliferation occurs occasionally, with each newly proliferated segment becoming swollen at the base. The apical cell of each conidiophore can produce up to three phialides. Type I phialides are cylindrical, 4 to 8 μm long (min length, 2 μm; max length, 14 μm) by 1 to 2 μm wide, occasionally wider at the base. Type II phialides are elongate-ampulliform and attenuated at the base or are navicular, and they measure 10 to 15 μm long (min length, 9 μm; max length, 16 μm) by 1.5 to 2.5 μm wide. Type III phialides are predominant and subcylindrical, becoming slightly inflated at or just above the base, 16 to 24 μm long (min length, 15 μm; max length, 28 μm) by 1.5 to 2.5 μm wide and narrowing gradually to a long neck. Collarettes are 1 to 3 μm long and 1 to 2 μm wide. Conidia are oblong-ellipsoidal, obovoid, or occasionally reniform to allantoid and are prominently two-guttulate for one of the two strains examined, 3 to 5 μm long (max length, 7.5 μm) by 1 to 2 μm wide (max width, 2.5 μm).
Holotype.
United States. Bethesda, National Institutes of Health, human patient with pneumonia, 1994, K.J. Kwon-Chung (CBS 498.94 dried specimen and ex-type culture, dried isotype lodged at PREM).
Notes.
P. rubrigenum can be identified by its pink to purplish colony color on MEA.
Phaeoacremonium scolyti L. Mostert, Summerb. & Crous, sp. nov.
Differt a simili P. rubrigeno coloniis pallide roseis translucentibus (MEA) et phialidibus oblonge ampulliformibus (Fig. 7).
Colonies on MEA are medium pink to translucent. Hyphae are mostly verruculose, occasionally verrucose, medium brown to pale brown, and 1 to 2 μm wide. They bear warts of up to 1 μm wide. Conidiophores are mostly short and usually unbranched, subcylindrical to navicular, 0- to 3-septate, 17 to 35 μm long (min length, 15 μm; max length, 39 μm) and 1.5 to 2.5 μm wide. The apical cell of each conidiophore can produce up to three phialides. Phialides are occasionally polyphialidic. Type I phialides are cylindrical, occasionally swollen at the base, 3 to 7 μm long (min length, 2 μm) by 1 to 1.5 μm wide. Type II phialides are predominant, elongate-ampulliform, and constricted at the base, or are navicular, tapering towards the apex, 7 to 14 μm long by 1.5 to 2.5 μm wide. Type III phialides are subcylindrical, subulate to elongate-ampulliform, 14 to 20 μm long (min length, 10 μm) by 1.5 to 2.5 μm wide, tapering gradually to the apex. Collarettes are 1.5 to 2 μm long and 1 to 1.5 μm wide. Conidia are oblong-ellipsoidal or obovoid, occasionally reniform or allantoid, 2.5 to 4 μm long (max length, 6 μm) by 1 to 2 μm wide.
Holotype.
South Africa. Western Cape, Vitis vinifera, 1999, M. Groenewald (dried specimen in herb. CBS 7952, culture ex-type CBS 113597, STE-U 3092).
Etymology.
One of the strains of this species originates from the larvae of a Scolytus intricatus Ratz. bark beetle.
Notes.
P. scolyti can be distinguished by the combination of its distinctive medium pink to translucent colonies on MEA and its elongate-ampulliform Type II phialides, often strongly constricted at the base.
Phaeoacremonium subulatum L. Mostert, Summerb. & Crous, sp. nov.
Differt a simili P. rubrigeno pigmentis flavis in culturis formatis (PDA) et phialidibus subcylindraceis vel subulatis (Fig. 8).
Colonies on MEA are pale yellow to translucent, becoming pale brown with age. Colonies on PDA produce a bright yellow pigment in the agar. Hyphae are verruculose, orange to pale brown, and 1.5 to 2.5 μm wide. They bear warts of up to 0.8 μm wide. Conidiophores are mostly short and usually unbranched, 1- to 7-septate, 18 to 32 μm long (min length, 17 μm; max length, 45 μm) and 1.5 to 2.5 μm wide. The apical cells of conidiophores can produce up to two phialides. Type I phialides are cylindrical, occasionally wider at the base, tapering towards the apex, 3 to 9 μm long by 1 to 1.5 μm wide. Type II phialides are subcylindrical to subulate, occasionally elongate-ampulliform and attenuated at the base, tapering towards the apex, 9 to 13 μm long (min length, 7 μm) by 1.5 to 2 μm wide. Type III phialides are subcylindrical to subulate, 12.5 to 20 μm long (min length, 12 μm; max length, 21 μm) by 1.5 to 2 μm wide, tapering gradually into a long, narrow neck. All three phialide types occur in equal proportions and develop collarettes that are 1 μm long and 1 to 1.5 μm wide. Conidia are oblong-ellipsoidal, cylindrical, occasionally reniform, 3 to 5 μm long (max length, 7 μm) by 1 to 1.5 μm wide (max width, 2 μm).
Holotype.
South Africa. Western Cape, Paarl, Zandrift, Vitis vinifera, 2001, L. Mostert (dried specimen in herb. CBS 7956, culture ex-type CBS 113584).
Etymology.
This species has distinctive, subulately shaped Type II and Type III phialides.
Notes.
P. subulatum can be distinguished by the production of yellow pigmentation on PDA and by its possession of subcylindrical to subulate Type II and Type III phialides.
Phaeoacremonium tardicrescens L. Mostert, Summerb. & Crous, sp. nov.
Differt a simili P. rubrigeno coloniis brunneis, tarde crescentibus (MEA) et hyphis verrucis minutis praeditis (ad 0.5 μm diameter) (Fig. 8).
Colonies on MEA are overall medium brown, becoming olivaceous-brown with age. Hyphae are verrucose to strongly verrucose and medium brown, 1.5 to 2.5 μm wide. They bear warts up to 0.5 μm wide. Conidiophores are mostly short and usually unbranched, 1- to 7-septate, 16 to 52 μm long (min length, 13 μm; max length, 67 μm) and 1 to 2 μm wide. The apical cells of conidiophores mostly produce one phialide. Type I phialides are mostly cylindrical, tapering towards the apex, 2 to 12 μm long by 1 to 1.5 μm wide. Type II phialides are subcylindrical to subulate or occasionally elongate-ampulliform and attenuated at the base, tapering towards the apex, 9 to 14 μm long (min length, 7 μm; max length, 15 μm) by 1 to 2.5 μm wide. Type III phialides are subcylindrical to navicular, 14.5 to 24 μm long (min length, 10 μm; max length, 34 μm) by 1 to 2 μm wide and tapering gradually to a long neck. Type I and Type III phialides are predominant. Collarettes are 1.5 to 2.5 μm long and 1.5 to 2 μm wide. Conidia are oblong-ellipsoidal to allantoid, 4 to 5.5 μm long (min length, 3.5 μm; max length, 7 μm) by 1 and 1.5 μm wide (max width, 2 μm).
Holotype.
United States. Texas, Dallas, human, Levi (dried specimen in herb. CBS 7953, culture ex-type CBS 110573, UTHSC 00-146).
Etymology.
P. tardicrescens was named after the slow growth typical of this species. Of all species studied, it had the smallest colony radius at 30°C.
Notes.
P. tardicrescens had colonies that were distinctly browner on MEA than those of the other species studied. This species also proved to be the slowest growing of the species studied when all were observed at their optimum growth temperature. Microscopically, P. tardicrescens was distinct from several of the other Phaeoacremonium species, particularly the more common pathogen P. parasiticum, by possessing verrucose mycelium with relatively small warts (up to 0.5 μm wide).
Phaeoacremonium venezuelense L. Mostert, Summerb. & Crous, sp. nov.
Differt a simili P. inflatipede coloniis aurantiaco-brunneis, et conidiis fusiformibus-ellipsoideis (Fig. 8).
Colonies on MEA are variable in different strains, ranging from beige to orange-brown. Hyphae are verruculose, orange, orange-brown or pale brown, rarely hyaline, and 1 to 3 μm wide. When colored, hyphae become paler near conidiogenous regions. Warts are up to 1 μm wide. Conidiophores are short and occasionally branched, 1- to 4-septate, 28 to 34 μm long (min length, 20 μm; max length, 52 μm) 1 to 2.5 μm wide. Percurrent proliferation occurs occasionally through an expended phialide, with the newly proliferated segment becoming markedly swollen at the base. Apical cells of conidiophores can produce up to two phialides. Type I phialides are cylindrical, tapering towards the apex, 5 to 14 μm long (min length, 4.5 μm; max length, 16 μm) by 1 to 1.5 μm wide. Type II phialides are mostly subcylindrical to navicular, tapering towards the apex, 12 to 14 μm long by 1.5 to 2 μm wide. Type III phialides are predominant, subcylindrical, navicular to subulate, 15 to 23 μm long (min length, 14 μm; max length, 24 μm) by 1 to 2 μm wide, very gradually tapering towards the apex. Collarettes are 1 to 3 μm long and 1 to 2 μm wide. Conidia are oblong-ellipsoidal or fusiform-ellipsoidal, occasionally reniform-allantoid, 3 to 4.5 μm long (max length, 6 μm) by 1 to 1.5 μm wide (max width, 2 μm).
Holotype.
Venezuela. Human mycetoma, foot (dried specimen in herb. CBS 7957, culture ex-type CBS 651.85, ATCC 32628).
Etymology.
This is the only species represented so far by a strain from Venezuela, and it was named for this country.
Notes.
P. venezuelense was distinct in having beige to orange-brown colonies as well as the production of fusiform-ellipsoidal conidia. The phylogenetic differences observed among isolates of this species were also reflected in phenotypic differences. Strains CBS110119 and CBS113595 had prominent orange-brown mycelium. CBS110119 had more warts than other strains and wooly tufts on the colonies, whereas the other strains were felty to powdery in texture. The phenotypic and genetic differences found in these strains are of low taxonomic value in terms of considering them different species.
For all species described above, Table 3 summarizes major distinctive characteristics, including colony color, conidiophore structure and length, mycelium texture, maximum wart size, predominant phialide type, predominant Type II phialide shape, and production of yellow pigmentation on PDA.
TABLE 3.
Summary of colony color and morphological characters useful for identification of Phaeoacremonium species, listed according to the colony color (ranging from pink to brown to beige)
| Species | Colony color on 2% MEA | Conidiophore structure | Conidiophore length (μm)a | Mycelium texture | Maximum size of warts (μm) | Predominant phialide type | Predominant Type II phialide shape | Yellow pigmentation on PDA |
|---|---|---|---|---|---|---|---|---|
| P. rubrigenum | Medium to purple pink | Mostly short and usually unbranched | 23-51 (20, 70) | Verruculose | 1 | III | Elongate-ampulliform, attenuated at the base | No |
| P. scolyti | Medium pink to translucent | Mostly short and usually unbranched | 17-35 (15, 39) | Mostly verruculose | 1 | II | Elongate-ampulliform, constricted at the base | No |
| P. griseorubrum | Dark pink | Mostly short and usually unbranched | 23-70 (21, 85) | Verruculose | 1.5 | II and III | Elongate-ampulliform or navicular | No |
| P. alvesii | Medium pink or beige | Mostly short and usually unbranched | 17-43 (14, 50) | Verruculose | 0.5 | III | Subcylindrical or navicular | Variable |
| P. inflatipes | Brown to gray-brown | Mostly branched in the basal region | 18-40 (14, 43) | Verruculose | 0.5 | III | Elongate-ampulliform, attenuated at the base | No |
| P. parasiticum | Brown with medium brown center | Mostly long and branched | 27-80 (24, 130) | Verricose | 3 | III | Subcylindrical | No |
| P. krajdenii | Medium brown to dark brown | Short and usually unbranched | 20-45 (16, 76) | Verrucose | 1 | II | Elongate-ampulliform, attenuated at the base | No |
| P. tardicrescens | Medium brown to olivaceous-brown | Mostly short and usually unbranched | 16-52 (13, 67) | Verrucose | 0.5 | I and III | Subcylindrical to subulate | No |
| P. aleophilum | Honey-brown or beige | Mostly short and usually unbranched | 17-42 (15, 46) | Mostly verruculose | 1.5 | II and III | Elongate-ampulliform attenuated at the base | Yes |
| P. australiense | Pale brown to medium brown | Mostly short and usually unbranched | 17-50 (14, 64) | Verruculose | 1 | I, II, and III | Elongate-ampulliform | No |
| P. subulatum | Pale yellow to pale brown | Mostly short and usually unbranched | 18-32 (17, 45) | Verruculose | 0.8 | I, II, and III | Subcylindrical to subulate | Yes |
| P. venezuelense | Beige to orange-brown | Mostly short and occasionally branched | 28-48 (20, 52) | Verruculose | 1 | III | Subcylindrical or navicular | No |
| P. amstelodamense | Beige to pale brown | Mostly short and usually unbranched | 16-61 (15, 90) | Verruculose | 1 | II | Elongate-ampulliform constricted at the base | No |
Conidiophore lengths are given in the form 5th percentile-95th percentile (min, max).
Electronic identification key in BioloMICS software.
In an evaluation of the utility of assigning differing weights to the characteristics used in the analysis, the coefficient of correlation between phenotypic and gold standard phylogenetic analyses was found to be 0.47 when all characters were assigned an equal weight and 0.60 when a priori weightings were used. This indicates that suitably weighted characters correlate positively with β-tubulin sequence data and can thus aid in accurate electronic identification. The morphological and cultural characteristics that were weighted include conidiophore structure, colony color on MEA, and colony radius at 25 and 30°C on MEA.
The Phaeoacremonium BioloMICS identification database has been placed on the CBS (Centraalbureau voor Schimmelcultures) website at http://www.cbs.knaw.nl/phaeoacremonium.htm. A few or multiple characteristics can be entered, and through pairwise comparison the most similar Phaeoacremonium species can be identified. The similarity of each character of the unknown species to those of the known Phaeoacremonium species can also be seen in the output file. This multiple-entry comparison key is similar to the yeast identification database available from CBS (24).
DISCUSSION
Nine new species of Phaeoacremonium were identified and substantiated by combined morphological, cultural, and phylogenetic analyses. Some of these species have already been the subject of published case reports (Table 1). In some cases, the isolates studied were correctly identified as belonging to the genus Phaeoacremonium but were not recognized as undescribed species because of the subtle nature of the morphological differences involved. For example, Padhye et al. (22) ascribed a case of P. alvesiii subcutaneous infection to P. inflatipes. The identification of the case isolate, CBS 729.97, was based largely on the presence of relatively broad phialides with a constricted base. It was also confirmed as P. inflatipes at the time by experts at CBS. The present molecular study, however, clarifies the significance of the fact that this isolate produced mainly single lateral phialides rather than the extensive branching structures typical of P. inflatipes. CBS 729.97 could grow at 37°C, whereas isolates of P. inflatipes have a maximum growth temperature of 35°C. Another isolate of P. alvesii, CBS 110034, was reported from a subcutaneous infection as P. aleophilum (10). It produced yellow rather than red diffusing pigment on several media; thus, its close relatedness to the red-pigmented P. rubrigenum could not be phenotypically recognized. On the other hand, the purely plant-associated P. aleophilum was known to produce similar pigment and to grow at or near body temperature in vitro. P. griseorubrum isolate CBS 566.97, from a Japanese subcutaneous infection, was identified as P. rubrigenum by Matsui et al. (16) because of its dark pink appearance on most media. At the time, however, it was not known that several related species shared the pink coloration of P. rubrigenum to a greater or lesser extent.
Phaeoacremonium isolates have often been incorrectly identified as P. inflatipes. Dupont et al. (7) found with restriction patterns of the internal transcribed sequence (ITS) and β-tubulin genes that several isolates identified as P. inflatipes were P. parasiticum, P. rubrigenum, P. viticola, and P. aleophilum. Our study revealed that isolates obtained from undocumented human infections and deposited in CBS as P. inflatipes are very diverse, with new identifications dispersed among P. alvesii (CBS 408.78), P. amstelodamense (CBS 110627), P. tardicresens (CBS 110573), P. krajdenii (CBS 109479, CBS 110367, CBS 110368, CBS 110361), and P. parasiticum (CBS 736.94).
Prior to 1996, isolates morphologically resembling Phialophora parasitica but possessing shorter and thicker phialides were frequently identified as Phialophora repens (Davidson) Conant. Their identification as P. repens was based on a degree of overlap in phialidic and conidial shapes and measurements, particularly in the curved conidia possessed by P. repens and several undescribed Phaeoacremonium species. The close resemblance between P. repens and Phaeoacremonium spp. was noted by Hawksworth and Gibson (12). P. repens, an apparently rare species from wood, is only reliably known from its ex-type isolate CBS 294.39 and has no pathogenic record in humans or animals. It seems that P. repens grows relatively rapidly, reaching a colony radius of 45 mm in fewer than 12 days at 25°C (26), in contrast with the 4- to 15.5-mm radii recorded for 8-day growth of Phaeoacremonium species. It also tends to produce a proportion of its phialides in penicillate bushes (26), an attribute unknown in Phaeoacremonium spp. Weitzman et al. (28) observed that the ex-type isolate of P. repens could be micromorphologically distinguished from P. parasitica by the absence of warts on its phialides. Fortunately, most medical isolates reported under this name were deposited in public culture collections and two such isolates were available for reexamination. An isolate reported from a scalp nodule in the Democratic Republic of Congo (17), deposited as CBS 423.73, and CBS 110366, from a subcutaneous nodule on the hand of a Japanese man (14), are identified as P. krajdenii, as are two isolates from undocumented clinical cases, CBS 633.93 and CBS 110365.
The only Phaeoacremonium case report known to have been accepted for publication prior to the description of P. parasitica is that of de Albornoz (4). This strain was isolated from a Venezuelan mycetoma case and was identified as Cephalosporium serrae Maffei, an anachronistic synonym of the dark-colored plant pathogen Verticillium nigrescens Pethybr. This isolate was later reidentified by Crous et al. (3) as P. inflatipes and is placed here in P. venezuelense (CBS 651.85). Some strains morphologically consistent with this species were genetically heterogeneous and require further study.
An overview of the various cases documented as being caused by the species newly described (P. alvesii, P. amstelodamense, P. griseorubrum, P. krajdenii, and P. tardicrescens) shows an infection pattern consistent with that of P. parasiticum. Mostly posttraumatic subcutaneous invasion and occasional deep infection (including a case isolated from lung and human synovial fluid) have been found associated with isolates of P. parasiticum. No outstanding distinct patterns are recognizable for any of the new species from human sources. All published cases document subcutaneous infections. The majority of the isolates from unpublished cases were from subcutaneous sites, but some were from joints and one was from a blood culture (Table 1). On the other hand, certain closely related species, such as P. australiense and P. subulatum, as well as the redefined P. inflatipes, may be ecologically distinctive in their inability to cause human infections, an attribute often correlated with a low tolerance of elevated temperatures. These apparently nonpathogenic species co-occur with the opportunistic species in a variety of woody plants. These plants are often cultivated, and it must be assumed that they pose at least a small degree of hazard in cases of dermal trauma. The main economically important plant species involved is the cultivated grape, Vitis vinifera, statistically overrepresented in Table 1 because of extensive phytopathological sampling. The date palm (Phoenix dactylifera), the apricot tree (Prunus armeniaca), and the kiwi fruit vine (Actinidia chinensis), as well as the hop bush (Dodoneae viscosa), though it is not a crop plant, may also be supposed to pose a hazard. It must be stated that no case of human Phaeoacremonium infection has been documented to be connected with one of these plants. However, grapevines with esca disease generally host one or more Phaeoacremonium species, often including species known to be opportunists (3). Some degree of caution in handling infected material must therefore be advised.
Four Phaeoacremonium species have thus far only been isolated from human infections: P. rubrigenum, P. griseorubrum, P. amstelodamense, and P. tardicrescens. The ecological habitat of these species is thus completely unknown, but based on comparison with that of the other species, it should be assumed to include woody plants. Of course, most ascomycetous plant-associated fungi will to some extent also be represented by inoculum in soil, even if this inoculum is mainly or entirely in a dormant condition. P. parasiticum has been isolated from soil in Tahiti (7). Therefore, the connection of some subcutaneous Phaeoacremonium infections with soil, e.g., in gardeners, should be expected. Even dust or indoor water sources may occasionally contain such widely environmentally disseminated inoculum, perhaps explaining cases like the above-mentioned case of a Japanese hemodialysis patient (16) who developed a P. griseorubrum infection in a lesion at the back of her foot even though she had been hospitalized for a long period beforehand.
Most of the Phaeoacremonium species are plant pathogens (3, 6, 9). Our study suggests that perhaps the most important single phenetic character both for screening for potentially human pathogenic species in Phaeoacremonium and for initial understanding of the virulence factors involved in opportunism is temperature tolerance. The species included in our study that occurred only in plants (P. australiense, P. inflatipes, and P. subulatum) or, in one case, in both plants and insect larvae (P. scolyti), could not grow at 40°C. P. angustius W. Gams, Crous & M.J. Wingf. and P. mortoniae Crous & W. Gams, two other medically insignificant Phaeoacremonium species that so far have been isolated only from plants, have been found to have maximum growth temperatures between 33 and 35°C (3). Yet another species, P. viticola Dupont, has a still lower maximum growth temperature below 32°C (6). One species so far obtained only from plants, P. aleophilum, grew at 37 and 40°C when tested for comparison with the opportunistic and undescribed species included in the present study. From these growth temperature trends it would appear that most of the species only occurring on plants cannot grow at 40°C, whereas several, but not all, of the human opportunistic species are able to do so. It cannot be ruled out that some species, such as P. australiense, P. subulatum and P. aleophilum, that so far have only been obtained from plants but are able to grow at human body temperature might in future be seen from human infections.
The identification of species of Phaeoacremonium based on their morphological and cultural characters is difficult, as is evident from the numerous incorrect identifications that have been made since the genus was established in 1996 (3). A reliable technique for Phaeoacremonium species identification is needed. The multiple-character electronic identification key we developed is designed to allow users to employ any or all available elements of the combination of morphological, cultural, and β-tubulin sequence data. Numerical analysis of encoded morphological and cultural characters has proven useful in other studies aimed at establishing the identity of fungal species and the patterns of variation found within these species (18, 20).
All known Phaeoacremonium species infecting humans can be identified and distinguished through the use of an electronic key allowing input of morphological, cultural, or β-tubulin sequence data.
Acknowledgments
We thank Hans-Josef Schroers for the informative discussions over Phaeoacremonium morphology. Ursula Bunn, Maria Witkowska, and Frances Jamieson of the Ontario Ministry of Health and Long-term Care are thanked for sending isolates, as is Lynne Sigler of the University of Alberta Microfungus Collection.
REFERENCES
- 1.Ajello, L., L. K. Georg, R. T. Steigbigel, and C. J. K. Wang. 1974. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia 66:490-498. [PubMed] [Google Scholar]
- 2.Carbone, I., and L. M. Kohn. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553-556. [Google Scholar]
- 3.Crous, P. W., W. Gams, M. J. Wingfield, and P. S. Van Wyk. 1996. Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia 88:786-796. [Google Scholar]
- 4.De Albornoz, M. B. 1974. Cephalosporium serrae, agente etiológico de micetomas. Mycopathol. Mycol. Appl. 54:485-498. [DOI] [PubMed] [Google Scholar]
- 5.De Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Hyphomycetes. Genus: Phaeoacremonium, p. 846-852. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 6.Dupont, J., J. Laloui, S. Magnin, P. Larignon, and M.-F. Roquebert. 2000. Phaeoacremonium viticola, a new species associated with Esca disease of grapevine in France. Mycologia 92:499-504. [Google Scholar]
- 7.Dupont, J., S. Magnin, C. Césari, and M. Gatica. 2002. ITS and β-tubulin markers help delineate Phaeoacremonium species, and the occurrence of P. parasiticum in grapevine disease in Argentina. Mycol. Res. 106:1143-1150. [Google Scholar]
- 8.Glass, N. L., and G. C. Donaldson. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 61:1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groenewald, M., J.-C. Kang, P. W. Crous, and W. Gams. 2001. ITS and β-tubulin phylogeny of Phaeoacremonium and Phaeomoniella species. Mycol. Res. 105:651-657. [Google Scholar]
- 10.Guarro, J., S. H. Alves, J. Gené, N. A. Grazziotin, R. Mazzuco, C. Dalmagro, J. Capilla, L. Zaror, and E. Mayayo. 2003. Two cases of subcutaneous infection due to Phaeoacremonium spp. J. Clin. Microbiol. 41:1332-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausner, G., G. G. Eyjoflsdottir, J. Reid, and G. R. Klassen. 1992. Two additional species of the genus Togninia. Can. J. Bot. 70:724-734. [Google Scholar]
- 12.Hawksworth, D. L., and I. A. S. Gibson. 1976. Phialophora parasitica. C.M.I. Descriptions of pathogenic fungi and bacteria, no. 504. Commonwealth Mycological Institute, Kew, Surrey, England.
- 13.Hawksworth, D. L., P. M. Kirk, B. C. Sutton, and D. N. Pegler. 1995. Ainsworth and Bisby's dictionary of fungi, 8th ed. University Press, Cambridge, United Kingdom.
- 14.Hironaga, M., K. Nakano, I. Yokoyama, and J. Kitajima. 1989. Phialophora repens, an emerging agent of subcutaneous phaeohyphomycosis in humans. J. Clin. Microbiol. 27:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, S. B., and J. W. Taylor. 1990. Isolation of DNA from fungal mycelia and single spores, p. 282-287. In M. A. Innis, D. H. Gelfand, J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, New York, N.Y.
- 16.Matsui, T., K. Nishimoto, S. Udagawa, H. Ishihara, and T. Ono. 1999. Subcutaneous phaeohyphomycosis caused by Phaeoacremonium rubrigenum in an immunosuppressed patient. Jpn. J. Med. Mycol. 40:99-102. [DOI] [PubMed] [Google Scholar]
- 17.Meyer, W. M., J. R. Dooley, and K. J. Kwon-Chung. 1975. Mycotic granuloma caused by Phialophora repens. Am. J. Clin. Pathol. 64:549-555. [DOI] [PubMed] [Google Scholar]
- 18.Mordue, J. E. M., R. S. Currah, and P. D. Bridge. 1989. An integrated approach to Rhizoctonia taxonomy: cultural, biochemical and numerical techniques. Mycol. Res. 92:78-90. [Google Scholar]
- 19.Mostert, L., P. W. Crous, J. Z. Groenewald, W. Gams, and R. Summerbell. 2003. Togninia (Calosphaeriales) is confirmed as teleomorph of Phaeoacremonium by means of morphology, sexual compatibility, and DNA phylogeny. Mycologia 95:646-659. [DOI] [PubMed] [Google Scholar]
- 20.Mueller, G. M. 1985. Numerical taxonomic analyses on Laccaria (Agaricales). Mycologia 77:121-129. [Google Scholar]
- 21.O'Donnell, K., and E. Cigelnik. 1997. Two different intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 7:103-116. [DOI] [PubMed] [Google Scholar]
- 22.Padhye, A. A., M. S. Davis, D. Baer, A. Reddick, K. K. Sinha, and J. Ott. 1998. Phaeohyphomycosis caused by Phaeoacremonium inflatipes. J. Clin. Microbiol. 36:2763-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rambaut, A. 2002. Sequence alignment editor, version 2.0. University of Oxford, Oxford, United Kingdom.
- 24.Robert, V., W. Epping, T. Boekhout, M. Smith, G. Poot, and J. Stalpers. 2003, posting date. CBS yeasts database. [Online.] http://www.cbs.knaw.nl/databases/index.htm.
- 25.Robert, V., and S. Szoke. 2003. BioloMICS: biological manager for identification, classification and statistics, version 6.2. BioAware, Hannut, Belgium.
- 26.Schol-Schwarz, M. B. 1970. Revision of the genus Phialophora. Persoonia 6:59-94. [Google Scholar]
- 27.Swofford, D. L. 2000. PAUP* 4.0: phylogenetic analysis using parsimony. Sinauer Associates, Sunderland, Mass.
- 28.Weitzman, I., M. A. Gordon, R. W. Henderson, and E. W. Lapa. 1984. Phialophora parasitica, an emerging pathogen. Sabouraudia J. Med. Vet. Mycol. 22:331-339. [DOI] [PubMed] [Google Scholar]