Abstract
Interaction of many infectious agents with eukaryotic host cells is known to cause activation of the ubiquitous transcription factor nuclear factor κB (NF-κB) (U. Siebenlist, G. Franzoso, and K. Brown, Annu. Rev. Cell Biol. 10:405–455, 1994). Recently, we reported a biphasic pattern of NF-κB activation in cultured human umbilical vein endothelial cells consequent to infection with Rickettsia rickettsii, an obligate intracellular gram-negative bacterium and the etiologic agent of Rocky Mountain spotted fever (L. A. Sporn, S. K. Sahni, N. B. Lerner, V. J. Marder, D. J. Silverman, L. C. Turpin, and A. L. Schwab, Infect. Immun. 65:2786–2791, 1997). In the present study, we describe activation of NF-κB in a cell-free system, accomplished by addition of partially purified R. rickettsii to endothelial cell cytoplasmic extracts. This activation was rapid, reaching maximal levels at 60 min, and was dependent on the number of R. rickettsii organisms added. Antibody supershift assays using monospecific antisera against NF-κB subunits (p50 and p65) confirmed the authenticity of the gel-shifted complexes and identified both p50-p50 homodimers and p50-p65 heterodimers as constituents of the activated NF-κB pool. Activation occurred independently of the presence of endothelial cell membranes and was not inhibited by removal of the endothelial cell proteasome. Lack of involvement of the proteasome was further confirmed in assays using the peptide-aldehyde proteasome inhibitor MG 132. Activation was not ATP dependent since no change in activation resulted from addition of an excess of the unhydrolyzable ATP analog ATPγS, supplementation with exogenous ATP, or hydrolysis of endogenous ATP with ATPase. Furthermore, Western blot analysis before and after in vitro activation failed to demonstrate phosphorylation of serine 32 or degradation of the cytoplasmic pool of IκBα. This lack of IκBα involvement was supported by the finding that R. rickettsii can induce NF-κB activation in cytoplasmic extracts prepared from T24 bladder carcinoma cells and human embryo fibroblasts stably transfected with a superrepressor phosphorylation mutant of IκBα, rendering NF-κB inactivatable by many known signals. Thus, evidence is provided for a potentially novel NF-κB activation pathway wherein R. rickettsii may interact with and activate host cell transcriptional machinery independently of the involvement of the proteasome or known signal transduction pathways.
The rickettsiae are obligate, intracellular, gram-negative bacterial pathogens that cause a variety of diseases in humans. Rickettsia rickettsii, a member of the spotted fever group, is the causative agent of the severe and often fatal rickettsial disease known as Rocky Mountain spotted fever. During disease, R. rickettsii attacks primarily the vascular endothelial cell, and the pathologic sequelae that follow may result from R. rickettsii-induced damage and/or response of this cell type to intracellular infection. Much information regarding the infection has been gained from studies using the cultured human endothelial cell, which is readily infected with R. rickettsii (22). The organism enters the host cell by traversing the cell membrane in a poorly understood process requiring both rickettsial and host cell energy, as well as an intact host cell actin cytoskeleton, and likely involving rickettsial phospholipase A (26). During intracellular infection, R. rickettsii does not remain within membrane-bounded vesicles but resides and replicates in intimate contact with the host cell cytoplasm. Cellular injury, likely caused by generation of reactive oxygen species (14, 24, 25), is a consequence of infection and is manifested by dilatation of the endoplasmic reticulum and mitochondrial swelling (23). Superimposed on this injury process, the host endothelial cell undergoes a number of active cellular responses which include alterations in the pattern of gene expression (18, 27, 28).
Our laboratory recently reported that endothelial cell infection with R. rickettsii results in activation of the transcription factor nuclear factor κB (NF-κB) (29), and such activation likely participates in the infection-induced changes in expression of certain genes (19). NF-κB activation occurs in response to a variety of environmental stimuli and directs expression of several early-response genes (10). Its existence in a preformed cytoplasmic pool complexed with an inhibitory subunit (IκB) or covalently attached to an inhibitory prosequence makes it well suited for rapid, transient activation, which entails degradation of these inhibitory peptides, resulting in exposure of both nuclear translocation sequences and DNA-binding domains (10). Replenishment of the inactive cytoplasmic pool following activation is aided, at least in part, by the rapid increase in expression of IκB which occurs in response to NF-κB activation (10). R. rickettsii-induced NF-κB activation likely requires intracellular uptake of the organisms and follows biphasic kinetics, with the first, transient phase peaking at 3 h and the second, sustained phase appearing at approximately 18 h (29). This activation involves at least two members of this transcription factor family: a heterodimer of the subunits p65 (RelA) and p50 (NF-κB1) which has transactivating activity (1, 10) and a homodimer of the p50 subunit which may inhibit transactivation of κB-dependent genes (1). The p50 subunit is synthesized as a precursor protein (p105) with an inhibitory sequence at its carboxy terminus (10). Thus, the complement of inactive NF-κB species present in endothelial cell cytoplasm likely includes p50-p65 noncovalently complexed with IκB, as well as p65-p105. Cytoplasmic retention of the p50 homodimer may be accomplished either by covalent attachment to its inhibitory prosequence or via noncovalent complexing with other members of the IκB family, such as Bcl-3 (10).
Cell signalling pathways involved in NF-κB activation in response to physiologic agonists such as cytokines, growth factors, and endotoxin are as yet incompletely understood. Diverse signalling pathways likely converge upon a final, common pathway which involves a reactive oxygen intermediate (15), a phosphorylation step (11) which serves as a signal for ubiquitination, and subsequent inhibitory peptide degradation by the proteasome, a multicatalytic proteinase complex which is involved in selective, nonlysosomal degradation of cellular proteins (5, 12). Specifically, removal of IκBα protein from the inactive cytoplasmic pool of NF-κB complex is preceded by phosphorylation of serine residues 32 and 36 on the IκBα molecule. A cytokine-activated protein kinase complex (IκB kinase) which phosphorylates IκBα on the sites that trigger its degradation has recently been identified and purified (2, 9, 31). Cellular signalling pathways involved in R. rickettsii-induced NF-κB activation are not yet understood and may be complex, as there are numerous points at which interaction of the cell with the organism could trigger a relevant signalling pathway.
To gain further understanding of the nature of rickettsia-host cell interaction leading to activation of NF-κB, a cell-free system of NF-κB activation which allowed exposure of host cell cytoplasmic components to purified rickettsial organisms was developed. NF-κB activation was achieved, occurred independently of the presence of cellular membranes and proteasome activity, and involved both the p50-p65 heterodimer and the p50-p50 homodimer. We hypothesize that this novel activation scheme involves direct interaction of rickettsiae with the inactive pool of NF-κB dimers and that this mechanism contributes to activation which occurs during infection of the intact host cell.
MATERIALS AND METHODS
Cell culture.
Human umbilical vein endothelial cells were cultured as described previously (4, 32). Cells were grown in McCoy’s 5a medium (Flow Laboratories, McLean, Va.) containing 20% fetal bovine serum, EC mitogen (50 μg/ml; Collaborative Research Inc., Bedford, Mass.), and heparin (100 μg/ml; Sigma Chemical Co., St. Louis, Mo.). Cells were used at passage 2 unless stated otherwise and plated so as to achieve 80 to 90% confluence after 5 to 7 days in culture. T24 bladder carcinoma cells and human embryo fibroblasts (HEF cells) were cultured in RPMI 1640 and Dulbecco’s modified Eagle medium (Gibco BRL Life Technologies, Grand Island, N.Y.), respectively. The media were supplemented with 10% heat-inactivated fetal bovine serum. IκBα mutants were generated by stably transfecting these cell types with a transdominant-negative mutant IκB as described previously (17, 30). Selection was maintained by including 400 and 800 μg of G418 (Gibco) per ml in growth medium for T24 and HEF cells, respectively (30).
Isolation of cytoplasm.
Cytoplasmic extracts were prepared as described by Shirakawa and Mizel (20). In brief, cells from six to eight confluent 75-cm2 tissue culture flasks were washed twice with phosphate-buffered saline (PBS) containing 1 mM EDTA and scraped into prechilled PBS-EDTA. The cell pellet was suspended in 2 ml of chilled buffer A (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol). After 20 min of incubation on ice, cells were disrupted by 20 strokes in a loose-fitting Dounce homogenizer and the homogenate was centrifuged at 600 × g for 10 min. The supernatant fraction was then recentrifuged to remove any remaining nuclear debris, mixed with 0.11 volumes of buffer B (0.3 M HEPES [pH 7.9], 1.4 M KCl, 0.03 M MgCl2), and centrifuged for 1 h at 105,000 × g to remove membranes. The supernatant from this step was used in most experimental protocols unless otherwise stated. Protein concentration in these extracts was determined by the Bradford protein assay and typically ranged from 0.3 to 0.5 mg/ml. For the preparation of proteasome-deficient cytoplasmic extracts, the final centrifugation step was carried out at 105,000 × g for 6 h (7, 11).
Purification of R. rickettsii.
Vero (African green monkey kidney) cells (American Type Culture Collection, Rockville, Md.) from two to four 150-cm2 cell culture flasks, which were heavily infected with R. rickettsii (Sheila Smith strain), were harvested by scraping into PBS, pelleted by centrifugation (17,000 × g for 20 min), resuspended in K 36 buffer (0.1 M KCl, 0.15 M NaCl, 0.05 M potassium phosphate buffer [pH 7.0]), and subjected to purification as described by Eremeeva et al. (3), with slight modifications. Cell suspensions were sonicated with a single burst of 30 s at 25% output in an ice bath, using a Virsonic 60 ultrasonic processor equipped with a microprobe (VirTis Company, Gardiner, N.Y.). Host cell debris was removed by low-speed centrifugation (250 × g for 10 min), and the supernatant obtained was centrifuged at 18,500 × g for 30 min. The pellet was resuspended in K 36 buffer by passage through an 18-gauge needle, layered over a cushion of 30% sucrose–7.6% Renografin-76 (Squibb Diagnostics, New Brunswick, N.J.) density gradient at a ratio of 1:5, and centrifuged at 25,000 × g for 30 min. The pellet from this step (partially purified R. rickettsii) was washed twice with K 36 buffer and finally reconstituted in K 36 buffer. This preparation was used in all experiments unless otherwise specified. All steps in this procedure were performed at 4°C with prechilled buffers. Further analysis of rickettsial preparations on discontinuous Renografin density gradients (20 to 45%) revealed that the majority of organisms (about 90%) were present in the band representing viable organisms. Viability of purified rickettsial preparations was confirmed by infecting endothelial cells cultured on Thermanox coverslips (Ted Pella Inc., Tustin, Calif.) and subsequent detection of rickettsiae by immunofluorescence staining using a monospecific polyclonal anti-R. rickettsii antibody (Centers for Disease Control and Prevention, Atlanta, Ga.) as previously described (27, 32). Titers of partially purified rickettsial preparations were estimated based on the level of endothelial cell infection achieved (percent infection and number of organisms per cell) compared with rickettsial preparations of known titer (PFU/milliliter).
Infection of cultured endothelial cells and preparation of nuclear extracts.
Infection of cultured endothelial cells was carried out as previously described (29) and monitored on cells cultured in parallel on Thermanox coverslips. Following infection, endothelial cell nuclei were isolated from 0.5 × 107 to 1 × 107 cells, and nuclear proteins were extracted as described previously (29). Protein concentrations in nuclear extracts, as determined by the Bradford assay, ranged from 1 to 2 mg/ml.
EMSA.
Activated NF-κB was assayed by electrophoretic mobility shift assay (EMSA) as previously described (29). A double-stranded oligonucleotide containing consensus NF-κB sequence (5′-AGT TGA GGG TTT CCC AGG C-3′) was end labeled with [γ-32P]ATP (3,000 Ci/mmol, 10 mCi/ml), using T4 polynucleotide kinase as instructed by the manufacturer (Promega Corporation, Madison, Wis.). Binding reaction mixtures of 10 to 20 μl typically contained 5 to 8 μg of protein from cytoplasmic extracts and 100,000 to 150,000 cpm of 32P-labeled DNA, and the reactions were carried out in 10 mM Tris-HCl buffer (pH 7.5) containing 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol, 50 mM NaCl, poly(dI-dC) (0.05 μg/ml), and 4% glycerol. Reaction mixtures were incubated at 37°C for 30 min and analyzed on 4% nondenaturing polyacrylamide gels or precast 6% DNA retardation gels (Novex, San Diego, Calif.) in 0.5× TBE buffer (89 mM Tris-HCl [pH 8.0], 89 mM boric acid, 2 mM EDTA). The gels were electrophoresed for 2 to 3 h at 100 V and dried at 80°C under vacuum, and DNA-protein complexes were visualized by autoradiography for 12 to 18 h. For competition assays, a 10-fold molar excess of the unlabeled, double-stranded oligonucleotide probe was added before the addition of radiolabeled oligonucleotide.
Antibody supershift assays.
Polyclonal antisera against the NF-κB subunits p50 and p65 (Santa Cruz Biotechnology Inc.) were used to determine the subunit composition of gel-shifted NF-κB complexes. One microgram of antibody was added to the binding reaction 20 min (37°C) prior to addition of labeled oligonucleotide probe. The reaction mixtures were then analyzed on 4% nondenaturing polyacrylamide gels as described above.
Measurement of cytoplasmic ATP.
ATP concentration of cytoplasmic preparations was determined by using a bioluminescence ATP assay kit (Calbiochem Corporation, San Diego, Calif.). The luciferin-luciferase was diluted to 5 mg/ml with 0.1 M HEPES buffer (pH 7.75) and kept in the dark on ice for at least 1 h prior to use to allow for enzyme stabilization. For assay, 50 to 100 μl of endothelial cell cytoplasmic extract was combined with HEPES buffer in a luminometer cuvette so as to achieve a total volume of 400 μl. To initiate the photoreaction, 100 μl of luciferin-luciferase solution was added to the cuvette and mixed gently, and the luminescence intensity was recorded by an LKB Wallac 1250 luminometer connected to a recorder. The readings were recorded 30 s after addition of the enzyme. Samples were run in triplicate, and values were averaged (error among triplicate values ranged between 10 and 15%). ATP levels were obtained from a standard curve constructed by using pure ATP (Calbiochem).
Western blot analysis.
The levels of IκBα and phospho-IκBα antigen were analyzed by using a Phosphoplus IκBα (Ser32) antibody kit (New England Biolabs, Beverly, Mass.) as instructed by the manufacturer. In brief, aliquots containing equal amounts of cytoplasmic protein from control and R. rickettsii-treated cytoplasmic extracts were denatured by boiling for 5 to 10 min in 1× sodium dodecyl sulfate sample buffer (62.5 mM Tris-HCl [pH 6.8] containing 2% [wt/vol] sodium dodecyl sulfate, 10% glycerol, 50 mM dithiothreitol, and 0.1% [wt/vol] bromophenol blue). The proteins were then separated on a 10% denaturing polyacrylamide gel and electrotransferred onto a nitrocellulose membrane. Blocking of the membrane and antibody incubations were performed as specified by the manufacturer, and proteins were detected by a chemiluminescence reaction followed by exposure of the blot to an X-ray film for various lengths of time. Untreated and tumor necrosis factor alpha (TNF-α)-treated HeLa cell extracts (New England Biolabs) were used as controls for the detection of IκBα and phospho-IκBα, respectively. Prestained molecular weight markers (Bio-Rad Laboratories, Hercules, Calif.) were loaded on each gel to verify effective transfer of proteins to membranes. Biotinylated protein markers and antibiotin antibody (New England Biolabs) were used to determine the molecular weights of electrophoresed proteins.
Densitometric scanning.
Volume analysis of autoradiograms for EMSA gels was performed on a Bio-Rad model GS-700 imaging densitometer using Molecular Analyst software, version 1.5. The gels were scanned in the transmittance mode at a resolution setting of 150 dpi, using a gray filter. The intensities of bands were compared on the basis of adjusted volume (mean optical density × area in square millimeters).
RESULTS
NF-κB is activated in cytoplasmic extracts from cultured endothelial cells by R. rickettsii.
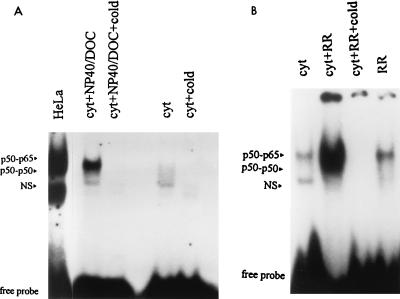
Prior to the study of R. rickettsii-induced activation, the presence of activatable NF-κB species in the endothelial cell cytoplasmic extracts was confirmed by using the detergents sodium deoxycholate (DOC; 0.6%) and Nonidet P-40 (NP-40; 1%). In vitro exposure of NF-κB to this detergent mixture has been shown previously to result in dissociation of the inhibitory subunit (IκB) (16), resulting in the exposure of DNA binding domains within the NF-κB species with which it is noncovalently complexed. As shown in Fig. 1A, endothelial cell cytoplasmic extracts prepared from unstimulated cells contained very low levels of activated NF-κB, although this background varied among cytoplasmic preparations with regard to the levels of both activation and subunit composition. Detergent exposure resulted in the appearance of a single gel-shifted NF-κB complex corresponding to the p50-p65 heterodimer.
FIG. 1.
EMSA of NF-κB activation in cytoplasmic extracts of endothelial cells. (A) Aliquots of cytoplasmic extracts (cyt) were treated with 1.0% NP-40 and 0.6% DOC (NP40/DOC) at 37°C for 30 min prior to assay of NF-κB activation with a double-stranded NF-κB oligonucleotide probe. Specificity of complex formation was confirmed by addition of a 10-fold molar excess of unlabeled oligonucleotide (+cold). Gel shift with nuclear extract from HeLa cells (5 μg of protein) was used as a positive control for DNA binding activity of NF-κB. (B) Cytoplasmic extracts (20-μl aliquots) were incubated alone (cyt) or in the presence of approximately 4 × 105 PFU of partially purified R. rickettsii (cyt+RR) for 30 min prior to assay. Rickettsial preparations alone (RR; 4 × 105 PFU) were assayed to control for the presence of contaminating, activated NF-κB. The relative positions of gel-shifted complexes p50-p65 and p50-p50 are shown; NS represents a nonspecific complex. Autoradiographic exposures were from 12 to 18 h with Kodak intensifying screens. Excess, unbound 32P-labeled oligonucleotide migrated to the bottom of the gel and is indicated as free probe.
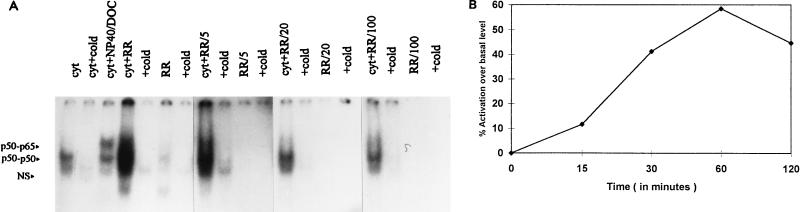
Addition of approximately 4 × 105 PFU of partially purified R. rickettsii to cytoplasmic extracts (20-μl volume) resulted in appearance of two gel-shifted NF-κB species corresponding to the p50-p65 heterodimer and the p50-p50 homodimer (Fig. 1B) (29), which was indicative of exposure of DNA binding domains. The increase in intensity of gel-shifted complexes formed following incubation with R. rickettsii for 30 min at 37°C ranged from three- to fivefold over the baseline levels present in endothelial cell cytoplasmic extracts and was inhibited by the inclusion of a 10-fold molar excess of unlabeled consensus oligonucleotide. The contribution of contaminating, activated NF-κB from Vero cells used to propagate R. rickettsii was minimal and diminished to undetectable levels when R. rickettsii preparations were serially diluted prior to addition to cytoplasmic extracts (Fig. 2A). Addition of approximately 1 × 105 to 2 × 105 PFU of R. rickettsii organisms to 20-μl aliquots of cytoplasmic extracts (prepared from approximately 4 × 105 cells) resulted in dramatic activation, which was reduced to near-baseline levels by addition of less than 1 × 103 to 2 × 103 PFU. Activation in this experiment appeared to involve predominantly the p50-p50 homodimer; however, p50-p65 heterodimer was also activated. Lipopolysaccharide from Escherichia coli serotype O111:B4 at concentrations up to 2 μg/ml had no effects on NF-κB under similar experimental conditions (data not shown). Time dependence studies (Fig. 2B) revealed that R. rickettsii-induced activation was readily detectable at 30 min, maximal at 60 min, and diminished slightly from its peak level at 120 min, likely due to nonspecific dissociation and proteolysis.
FIG. 2.
Concentration (PFU) and time dependence of in vitro NF-κB activation in endothelial cell cytoplasmic extracts. (A) Aliquots of cytoplasmic extracts from unstimulated endothelial cells (cyt) were incubated at 37°C for 30 min with undiluted R. rickettsii (RR) along with R. rickettsii diluted 1:5, 1:20, and 1:100 (RR/5, RR/20, and RR/100, respectively), followed by EMSA. Lanes marked +cold indicate the presence of a 10-fold molar excess of unlabeled probe in the binding reaction mixture. Cytoplasmic extract was also treated with NP-40 and DOC as described for Fig. 1A. NS, nonspecific complex. (B) Endothelial cell cytoplasmic extracts were incubated with approximately 105 PFU of partially purified R. rickettsii for 15, 30, 60, and 120 min, followed by EMSA. The autoradiogram of the gel was scanned as described in Materials and Methods, and band intensities were corrected by subtraction of baseline levels of NF-κB in cytoplasmic extracts and R. rickettsii preparations alone.
Both p50-p65 heterodimers and p50-p50 homodimers are activated by R. rickettsii.
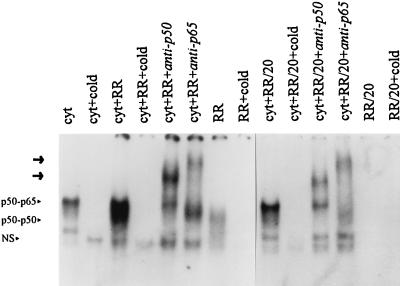
Gel supershift analysis was used to verify the subunit composition of the gel-shifted DNA-protein complexes. In cytoplasmic extracts incubated with R. rickettsii, preincubation with polyclonal antisera against the NF-κB subunits p50 and p65 prior to addition of 32P-labeled oligonucleotide probe led to the formation of slower-migrating bands (supershifted complexes) (Fig. 3). A gel-shifted complex reactive with both anti-p50 and anti-p65 and corresponding to the p50-p65 heterodimer was present in all cytoplasmic extracts, even in the absence of R. rickettsii, and the intensity of this complex increased slightly following incubation with R. rickettsii. In contrast, the faster-migrating complex reactive only with anti-p50 (p50-p50 homodimer) was virtually absent from untreated cytoplasmic extracts but was clearly evident following incubation with R. rickettsii.
FIG. 3.
Antibody supershift analysis of activated NF-κB species. Endothelial cell cytoplasmic extracts incubated with R. rickettsii undiluted (RR) and diluted 1:20 (RR/20) at 37°C for 30 min were subjected to gel supershift analysis using antibodies against the NF-κB subunits p50 and p65. Antibody (2 μg) was incubated with R. rickettsii-treated cytoplasmic extracts at 37°C for 20 min prior to the addition of radiolabeled oligonucleotide probe. Supershifted complexes on the gel are indicated by arrows, and the original locations of gel-shifted complexes are indicated by the arrowheads. NS, nonspecific complex. Lanes are labeled as for Fig. 2.
In vitro activation of NF-κB occurs independently of the presence of cellular membranes.
To study the dependence of in vitro activation on the presence of endothelial cell membranes, endothelial cell homogenates were fractionated by differential centrifugation as shown in Table 1. A postnuclear supernatant fraction (600 × g supernatant) that contained cellular membranes, as well as a 105,000 × g fraction lacking cellular membranes, was generated. A proteasome-deficient fraction was also prepared by prolonged (6-h) centrifugation at 105,000 × g. All of these cellular extracts were adjusted to equalize protein concentrations prior to incubation with R. rickettsii. NF-κB activation occurred following addition of R. rickettsii to all of these cellular preparations. R. rickettsii-induced NF-κB activation as well as background levels increased as cellular components were removed, likely because the effective concentration of NF-κB was enhanced with increasing refinement of cell homogenates. The ratios of R. rickettsii-induced activation to baseline levels, however, were similar in all cellular preparations (Table 1).
TABLE 1.
Membrane independence of R. rickettsii-induced NF-κB activation in endothelial cell extractsa
| Cellular fraction | Basal level of NF-κB in unstimulated cellsb | % Activation over basal level after incubation with R. rickettsii |
|---|---|---|
| 600 × g supernatant (cytoplasmic extract with membranes) | 1 | 178 |
| 105,000 × g supernatant, centrifuged for: | ||
| 1 h (membrane-free cytoplasmic extract) | 1.9 | 129 |
| 6 h (proteasome-free cytoplasmic extract) | 3.3 | 127 |
Cellular preparations were incubated with R. rickettsii for 30 min at 37°C and subsequently analyzed for DNA binding activity by EMSA.
Values for NF-κB pools in different subcellular fractions, with 600 × g supernatant contents assigned a value of 1.
In vitro activation of NF-κB by R. rickettsii occurs independently of proteasome activity.
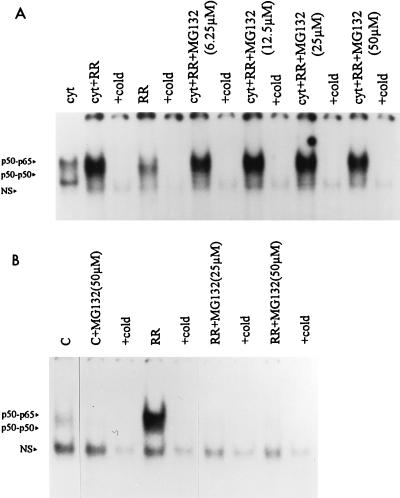
To gain insight into the mechanism(s) involved in R. rickettsii-induced in vitro activation, the involvement of the ubiquitin-proteasome pathway in this event was explored. The peptide-aldehyde proteasome inhibitors MG 132 (carbobenzoxyl-leucinyl-leucinyl-leucinal-H) and MG 115 (carbobenzoxyl-leucinyl-leucinyl-norvalinal-H) had no effect on in vitro activation of NF-κB over a range of concentrations tested (Fig. 4A). MG 132 and MG 115 did, however, inhibit the early phase of NF-κB activation during infection of cultured endothelial cells (Fig. 4B). None of these compounds had any effect on DNA binding of NF-κB from endothelial cells or HeLa cell extracts when added directly to the complexing reaction (not shown). Similarly, pyrrolidine dithiocarbamate, an antioxidant and potent inhibitor of NF-κB activation in intact cells, did not inhibit the activation of NF-κB when the cytoplasmic extracts were preincubated with micromolar amounts of the inhibitor prior to the addition of R. rickettsii (not shown) but had pronounced inhibitory effects on the early phase of activation observed in infected endothelial cells (19).
FIG. 4.
Effect of proteasome inhibitor MG 132 on R. rickettsii-induced activation of NF-κB in cytoplasmic extracts and intact cells. (A) Endothelial cell cytoplasmic extracts were preincubated with MG 132 at 37°C for 30 min prior to incubation with R. rickettsii (cyt+RR+MG132) or with dimethyl sulfoxide (used as solvent) prior to addition of R. rickettsii (cyt+RR). EMSA was then performed to visualize activated NF-κB complexes. Treatment with MG 115 yielded similar results. NS, nonspecific complex. (B) Nuclear extracts were prepared from uninfected cultures (C), uninfected cultures treated with 50 μM MG 132 for 1 h (C+MG132), cultures infected with R. rickettsii for 3 h (RR), or cultures treated with MG 132 (25 and 50 μM) for 1 h prior to and incubation with R. rickettsii (RR+MG132); 5 μg of nuclear protein was used in each gel shift reaction. Similar inhibition was seen with MG 115 (not shown).
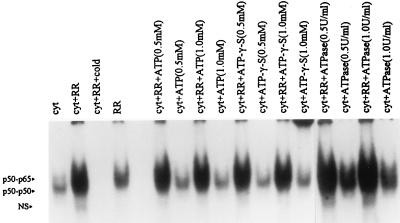
Since the proteasome-dependent pathway for the degradation of regulatory proteins, including IκB and inhibitory prosequence from p105, in intact cells requires ATP (5), we explored whether the R. rickettsii-induced activation of NF-κB subunits in endothelial cell cytoplasmic extracts was influenced by either the elimination or supplementation of ATP. Measurements of ATP by using a biochemiluminescence assay indicated that concentrations of ATP present in cytoplasmic extracts ranged from 10 to 15 μM. Hydrolysis of this endogenous ATP with ATPase (0.5 or 1.0 U/ml) had no effect on NF-κB activation. Addition of the unhydrolyzable ATP analog ATPγS to the activation mixture at 50- to 100-fold molar excess also resulted in no inhibition of activation. Furthermore, R. rickettsii-induced activation was not affected by supplementation of the cytoplasmic extracts with exogenous ATP (0.5 and 1 mM) (Fig. 5).
FIG. 5.
ATP independence of R. rickettsii-induced NF-κB activation. Endothelial cell cytoplasmic extracts (cyt) were preincubated at 37°C for 30 min with ATP, ATPγS, and ATPase individually, at concentrations indicated, prior to incubation with R. rickettsii (RR) or buffer alone. The samples were analyzed by EMSA for comparison between the levels of activated NF-κB species.
In vitro activation of NF-κB by R. rickettsii does not involve phosphorylation of IκBα at position serine 32.
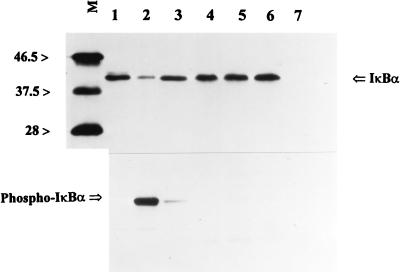
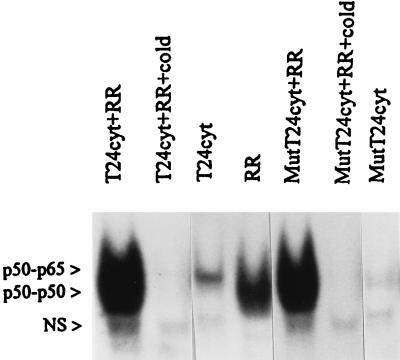
Since agents such as TNF-α, interleukin-1β, and endotoxin are known to initiate a phosphorylation-dependent degradation of IκBα from NF-κB, levels of IκBα and phospho-IκBα in control and R. rickettsii-activated cytoplasmic extracts were measured by Western blot analysis. Readily detectable basal levels of IκBα with comparatively low levels of its phosphorylated form (phospho-IκBα) were detected in cytoplasmic extracts from untreated endothelial cells. Activation of NF-κB by R. rickettsii (measured in parallel by gel shift analysis) resulted in no detectable depletion of IκBα antigen levels or appearance of phospho-IκBα (Fig. 6). Lack of involvement of phosphorylation at known sites was further supported by our finding that NF-κB in cytoplasmic extracts from cells stably transfected with phosphorylation mutant IκBα (IκBαM), in which serines 32, 36, 283, 288, and 293 and threonines 291 and 296 were replaced by alanines, was activated by R. rickettsii. Comparable increases in the DNA binding activity of NF-κB over basal values were observed upon addition of partially purified R. rickettsii to cytoplasmic preparations from both IκBαM-transfected T24 cells and T24 cells transfected with empty LXSN vector (Fig. 7). Activation of NF-κB was also achieved in assays using cytoplasmic preparations from wild-type and IκBαM-transfected HEF cells (not shown).
FIG. 6.
Western blot analysis for levels of IκBα and phospho-IκBα (Ser32) during in vitro activation of NF-κB by R. rickettsii. Lane 1, extract from HeLa cells (control for IκBα); lane 2, extract from TNF-α-treated HeLa cells (control for phospho-IκBα); lane 3, cytoplasmic extract alone from untreated endothelial cells; lane 4, cytoplasmic extract from endothelial cells to which R. rickettsii was added at 4°C and immediately frozen; lane 5, cytoplasmic extract from endothelial cells incubated with R. rickettsii at 37°C for 30 min to activate NF-κB; lane 6, cytoplasmic extract from endothelial cells incubated with R. rickettsii at 37°C for 30 min followed by removal of R. rickettsii by centrifugation prior to gel electrophoresis; lane 7, R. rickettsii preparation alone. Equal amounts of cytoplasmic protein were loaded on lanes 3 through 6. Relative positions of size markers (lane M) are shown at the left in kilodaltons.
FIG. 7.
R. rickettsii-induced NF-κB activation in cytoplasmic extracts of T24 bladder carcinoma cells transfected with either empty LXSN vector (T24cyt) or IκBαM-containing vector (MutT24cyt). Cytoplasmic extracts (20-μl aliquots) were incubated alone or in the presence of 4 × 105 PFU of partially purified R. rickettsii (+RR) for 30 min prior to assay. Preparation of R. rickettsii (RR) was also analyzed to control for the presence of any preexisting activated NF-κB species. NS, nonspecific complex.
DISCUSSION
The majority of stimuli triggering activation of NF-κB converge upon a common pathway and involve phosphorylation of IκB (or an inhibitory prosequence), conjugation with ubiquitin, and subsequent proteasome-mediated degradation of the inhibitory protein. Such activation results in conversion to an active DNA binding form capable of nuclear translocation which allows it to exert transcriptional control over a number of genes (reviewed in references 1 and 10). NF-κB activation occurs during infection of endothelial cells with R. rickettsii and participates in infection-induced expression of certain endothelial genes (19, 29). The cell-free system of NF-κB activation described in this report was designed to explore the nature of rickettsial interactions with host cell signalling molecules resulting in activation of this important transcription factor.
NF-κB activation has been achieved under cell-free conditions in a limited number of cases by activation of enzymes or addition of key regulatory molecules in the signal transduction pathway, and activation in these systems likely involves reconstitution of the signalling pathways that occur within the intact cells. The response to TNF-α, a potent physiologic inducer of NF-κB activation, is mediated by a pathway which involves production of diacylglycerol via a TNF-responsive phosphatidylcholine-specific phospholipase C (PLC) (16). NF-κB activation in cell extracts of Jurkat cells was shown to occur by addition of exogenous phospholipase C or downstream signalling molecules including acid and neutral sphingomyelinase or ceramide. Similarly, the catalytic subunit of cyclic AMP-dependent protein kinase or protein kinase C induces NF-κB binding activity in cytosolic extracts from pre-B cells in a process that is dependent on ATP, magnesium, and cellular membranes (20). The cell-free R. rickettsii-induced activation of NF-κB described herein appears to be novel in that all evidence points toward a mechanism operating without involvement of cellular membranes or the proteasome. This activation may, in fact, derive from a direct interaction between R. rickettsii and NF-κB.
From the studies described in this report, it is clear that the in vitro NF-κB activation achieved with R. rickettsii did not involve known upstream signalling events. Many signal transduction pathways resulting in NF-κB activation culminate in serine phosphorylation of IκBα at residues 32 and 36 by an IκBα kinase (2). Several lines of evidence suggest that R. rickettsii-induced NF-κB activation in cytoplasmic extracts did not involve phosphorylation of IκBα or its subsequent degradation. Phosphorylation of serine 32 was not detected following in vitro activation, and IκBα antigen levels were maintained (Fig. 6), indicating that no degradation of this inhibitory peptide occurred. Consistent with this observation, R. rickettsii was able to activate NF-κB in cytoplasmic extracts prepared from two cell lines that were transfected with a transdominant-negative mutant IκBα in which both amino-terminal (serines 32 and 36) and carboxy-terminal phosphorylation sites were mutated. This, however, does not exclude the possibility of an alternative phosphorylation-mediated mechanism, for example, tyrosine phosphorylation of IκBα that has been described for Jurkat T cells, which represents a proteasome-independent pathway (8). Furthermore, subcellular fractionation to remove cell membranes and the proteasome had no effect on NF-κB activation. Involvement of the proteasome pathway of degradation was also ruled out in assays with specific proteasome inhibitors used to inhibit proteasome-dependent degradation of inhibitory proteins in similar cell-free systems (11) and by depletion of ATP.
The subunit composition of the activated species, which includes both p50-p50 homodimers and p50-p65 heterodimers, suggested that an as yet uncharacterized proteolytic mechanism was likely operative. In contrast with the action of detergents, which resulted in the activation of a single dimeric species of NF-κB (p50-p65) by causing dissociation of the noncovalently bound inhibitory subunit (IκB) (11), R. rickettsii resulted in the activation of the p50-p50 homodimer as well. This molecule likely exists as a precursor (p105) held in its inactive state by its covalent linkage to an inhibitory prosequence, a notion supported by our finding that activation of this homodimer is not induced by detergent treatment (Fig. 1A). It is yet unclear whether R. rickettsii-induced activation in the cell-free system involves IκB or if its action is restricted to p105. It is possible that activated p50-p65 heterodimers, induced by R. rickettsii, derive from a p65-p105 precursor (6) present in the cytoplasmic extracts. A precedent exists for the capacity of an infectious agent to directly activate NF-κB by such a mechanism, since studies of the effects of human immunodeficiency virus type 1 infection on the processing of human p105 and p50 revealed that the viral protease can process these proteins to a 45-kDa cleavage product which is capable of DNA binding (13). It is possible that R. rickettsii possesses a protease capable of activation of NF-κB via a similar cleavage mechanism.
It remains to be determined which components of R. rickettsii are responsible for the activation or if activation requires rickettsial viability. Addition of purified lipopolysaccharide derived from E. coli to cytoplasmic extracts did not result in NF-κB activation (not shown). Lipopolysaccharide from R. rickettsii, however, may differ in some unknown critical characteristics; thus, the contribution of rickettsial lipopolysaccharide to the in vitro activation of NF-κB remains to be explored. Experiments using fractions enriched in cytoplasmic and membrane components from purified R. rickettsii will provide further insight into whether activation requires intact, metabolically active organisms.
Important questions regarding the relationship between the activation achieved in the cell-free system and that which occurs in the intact cell remain to be answered. A relatively low concentration of organisms is required to induce activation in cytoplasmic extracts. Based on our calculations, addition of fewer than 10 organisms to a volume of extract equivalent to that obtained from a single cell is sufficient to induce activation. Therefore, it appears that the rickettsia-induced stimulus in vitro is a potent one, and it is feasible that this mechanism is operative in initiating or sustaining activation during actual cellular infection. Results presented here (Fig. 4B) and in prior reports (19) have established that the early phase of R. rickettsii-induced activation in intact endothelial cells is dependent on the activity of the proteasome pathway, but it is not known whether the later, sustained phase of activation seen during infection of cultured endothelial cells involves the proteasome function. Unlike activation achieved by most soluble agonists, R. rickettsii-induced NF-κB activation in intact endothelial cells is prolonged, as is activation resulting from cellular infection with other intracellular parasites including Theileria parva and several viruses including cytomegalovirus and adenovirus type 5 (21). It is possible that these infectious agents possess a mechanism to directly activate NF-κB which could contribute to the sustained activation observed. Induction and/or maintenance of NF-κB activation by such a mechanism may provide a means by which these infectious agents adapt or preserve the intracellular environment of the host cell to suit their growth and replication requirements.
To the best of our knowledge, this study represents the first demonstration of the induction of NF-κB by a bacterial organism achieved under cell-free conditions and implicates the possible existence of a novel mechanism of activation perhaps involving a direct interaction of the organism with this transcription factor. Further investigation into the molecular mechanisms underlying this phenomenon and its relevance to the host cell response to infection and pathogenesis of rickettsial disease are in progress.
ACKNOWLEDGMENTS
We thank Loel Turpin, Lisa Domotor, and Li Hua Rong for expert technical assistance; Edward Shaw and Dawn Clifton for helpful discussions; and Carol Weed for help in the preparation of the manuscript.
This work was supported in part by grants HL 30616, AI 40689, and AI 17416 from the National Institutes of Health, Bethesda, Md.
REFERENCES
- 1.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 2.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 3.Eremeeva M E, Balayeva N M, Ignatovich V F, Raoult D. Proteinic and genomic identification of spotted fever group rickettsiae isolated in the former USSR. J Clin Microbiol. 1993;31:2625–2633. doi: 10.1128/jcm.31.10.2625-2633.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimbrone M A, Jr, Cotran R S, Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974;60:673–680. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg A L. Functions of the proteasome: the lysis at the end of the tunnel. Science. 1995;268:522–523. doi: 10.1126/science.7725095. [DOI] [PubMed] [Google Scholar]
- 6.Hay R T. Control of nuclear factor-κB DNA-binding activity by inhibitory proteins containing ankyrin repeats. Biochem Soc Trans. 1993;21:926–930. doi: 10.1042/bst0210926. [DOI] [PubMed] [Google Scholar]
- 7.Hegde A N, Goldberg A L, Schwartz J H. Regulatory subunits of cAMP-dependent protein kinases are degraded after conjugation to ubiquitin: a molecular mechanism underlying long-term synaptic plasticity. Proc Natl Acad Sci USA. 1993;90:7436–7440. doi: 10.1073/pnas.90.16.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imbert V, Rupec R A, Livolsi A, Paul H L, Traenckner E B-M, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle P A, Peyron J-F. Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 9.Israel A. IκB kinase all zipped up. Nature. 1997;388:519–521. doi: 10.1038/41433. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto S, Verma I M. REL/NF-κB/IκB story. Adv Cancer Res. 1995;66:255–292. [PubMed] [Google Scholar]
- 11.Palombella V J, Rando O J, Goldberg A L, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 12.Rivett A J. Proteasomes: multicatalytic proteinase complexes. Biochem J. 1993;291:1–10. doi: 10.1042/bj2910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riviere Y, Blank V, Kourilsky P, Israel A. Processing of the precursor of NF-κB by the HIV-1 protease during acute infection. Nature. 1991;350:625–626. doi: 10.1038/350625a0. [DOI] [PubMed] [Google Scholar]
- 14.Santucci L A, Gutierrez P L, Silverman D J. Rickettsia rickettsii induces superoxide radical and superoxide dismutase in human endothelial cells. Infect Immun. 1992;60:5113–5118. doi: 10.1128/iai.60.12.5113-5118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreck R, Rieber P, Baeuerle P A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schütze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Krönke M. TNF activates NF-κB by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz E M, Van Antwerp D, Verma I M. Constitutive phosphorylation of IκBα by casein kinase II occurs preferentially at serine 293: requirement for degradation of free IκBα. Mol Cell Biol. 1996;16:3554–3559. doi: 10.1128/mcb.16.7.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi R-J, Simpson-Haidaris P J, Marder V J, Silverman D J, Sporn L A. Increased expression of plasminogen activator inhibitor-1 in R. rickettsii-infected endothelial cells. Thromb Haemostasis. 1996;75:600–606. [PubMed] [Google Scholar]
- 19.Shi R-J, Simpson-Haidaris P J, Lerner N B, Marder V J, Silverman D J, Sporn L A. Transcriptional regulation of endothelial cell tissue factor expression during Rickettsia rickettsii infection: involvement of the transcription factor NF-κB. Infect Immun. 1998;66:1070–1075. doi: 10.1128/iai.66.3.1070-1075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirakawa F, Mizel S B. In vitro activation and nuclear translocation of NF-κB catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989;9:2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 22.Silverman D J. Rickettsia rickettsii-induced cellular injury of human vascular endothelium in vitro. Infect Immun. 1984;44:545–553. doi: 10.1128/iai.44.3.545-553.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverman D J. Adherence of platelets to human endothelial cells infected by Rickettsia rickettsii. J Infect Dis. 1986;153:694–670. doi: 10.1093/infdis/153.4.694. [DOI] [PubMed] [Google Scholar]
- 24.Silverman D J, Santucci L A. Potential for free radical-induced lipid peroxidation as a cause of endothelial cell injury in Rocky Mountain spotted fever. Infect Immun. 1988;56:3110–3115. doi: 10.1128/iai.56.12.3110-3115.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverman D J, Santucci L A. A potential protective role for thiols against cell injury caused by Rickettsia rickettsii. Ann N Y Acad Sci. 1990;590:111–117. doi: 10.1111/j.1749-6632.1990.tb42213.x. [DOI] [PubMed] [Google Scholar]
- 26.Silverman D J, Santucci L A, Meyers N, Sekeyova Z. Penetration of host cells by Rickettsia rickettsii appears to be mediated by a phospholipase of rickettsial origin. Infect Immun. 1992;60:2733–2740. doi: 10.1128/iai.60.7.2733-2740.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sporn L A, Haidaris P J, Shi R-J, Nemerson Y, Silverman D J, Marder V J. Rickettsia rickettsii infection of cultured human endothelial cells induces tissue factor expression. Blood. 1994;83:1527–1534. [PubMed] [Google Scholar]
- 28.Sporn L A, Marder V J. Interleukin-1α production during Rickettsia rickettsii infection of cultured endothelial cells: potential role in autocrine cell stimulation. Infect Immun. 1996;64:1609–1613. doi: 10.1128/iai.64.5.1609-1613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sporn L A, Sahni S K, Lerner N B, Marder V J, Silverman D J, Turpin L C, Schwab A L. Rickettsia rickettsii infection of cultured human endothelial cells induces NF-κB activation. Infect Immun. 1997;65:2786–2791. doi: 10.1128/iai.65.7.2786-2791.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 31.Verma I M, Stevenson J. IκB kinase: beginning, not the end. Proc Natl Acad Sci USA. 1997;94:11758–11760. doi: 10.1073/pnas.94.22.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner D D, Olmstead J B, Marder V J. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1983;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]