Abstract
Irpex lacteus, a wood-decaying basidiomycete, was isolated from a pulmonary abscess of an immunosuppressed child. This medical strain was compared morphologically and by sequencing of the ribosomal intergenic spacers with specimens from both culture collections and herbarium desiccated material. The patient was treated successfully with amphotericin B.
CASE REPORT
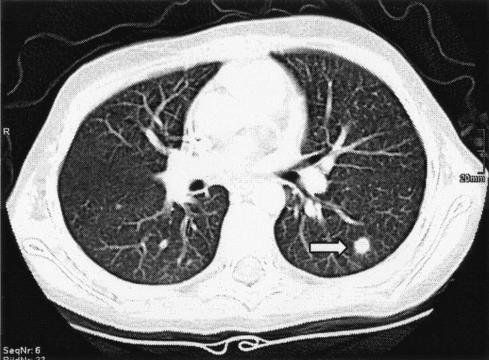
A 9-year-old girl with acute lymphoblastic leukemia (FAB-l) was admitted to the University Hospital of Innsbruck, Department of Pediatric Oncology. For chemotherapy she received vincristine (1.5 mg/m2 on days 8, 15, 22, and 29), doxorubicin (30 mg/m2 on days 8, 15, 22, and 29), 1-asparaginase (10,000 IU/m2 on days 8, 11, 15, and 18), and dexamethasone (10 mg/m2 on days 1 to 21) according to the acute lymphoblastic leukemia-BFM 2000, protocol II. Four months later she presented with fever (up to 40°C), impaired overall state of health, and aplasia. Her white blood cell count was 0.4 g/liter, hemoglobin was 129 g/liter, thrombocytes were 86 g/liter, and C-reactive protein was 3.69 mg/dl. The patient was treated empirically with imipenem/cilastatin (Zienam; MSD, Vienna, Austria), gentamicin (Refobacin; Biochemie, Vienna, Austria), and filgastrim (Neupogen; Amgen, Vienna, Austria). The blood cultures and urine samples yielded Escherichia coli. Antimicrobial treatment and voriconazole (Vfendt; Pfizer, Vienna, Austria) were administered at a dosage of 6 mg/kg of body weight/dose every 12 h for the first 24 h and then 4 mg/kg/dose every 12 h for 4 days; yet, fever persisted and the patient was started on amphotericin B at 1 mg/kg/day (amphotericin B; Bristol-Myers Squibb, Vienna, Austria). The chest X-ray showed no suspicious lesions, and multiple PCR samples for Aspergillus from blood remained negative (7). At that time the patient developed dacryocystitis of the right eye with painful eyelid and soft tissue swelling. The computed tomography (CT) scan of the sinus demonstrated pansinusitis with orbital cellulitis. Concurrently, intrapulmonary nodules were visible on the CT scan (Fig. 1), consistent with fungal abscesses. A CT-guided biopsy was performed utilizing an 18-gauge, coaxial cutting needle technique as described by Lucidarme et al. (8). Direct microscopy of a part of the obtained material after calcofluor white staining showed septate hyphae.
FIG. 1.
CT scan of the lung showing intrapulmonary nodule (arrow).
The other part of the material was aseptically transferred to Sabouraud glucose agar and Sabouraud broth (Merck, Vienna, Austria) and incubated at 37°C. The resulting cultures showed fast-growing white aerial mycelium which was covering the entire surface of the agar plates within 4 to 6 days. In older cultures the color of the mycelium changed slowly into beige to fawn, and orange droplets of exudation were produced. Microscopy showed septate branching hyphae only; no conidia, chlamydospores, or clamp connections were visible in any preparations. The patient received amphotericin B for 6 weeks; fever and intrapulmonary nodules disappeared.
Laboratory studies.
To identify the clinical isolate, the internal transcribed spacer (ITS) region of the rRNA genes was investigated. Total DNA was extracted using the commercial kit DNeasy Plant Mini Kit (Qiagen, Venlo, The Netherlands) following the manufacturer's instructions. PCR was carried out with the panfungal primer pair ITS1f (5) and ITS4 (10) as described recently (2). The resulting amplicons of 636 bp were sequenced using the ABI PRISM BigDye Terminator Cycle sequencing kit (Applied Biosystems, Foster City, Calif.).
GenBank search with the aid of Fasta 33 (http://www2.ebi.ac.uk/fasta3/) showed a homology of 99.5% (three-base difference) with the sequence AB079265, which was an environmental isolate (from Japan) of Irpex lacteus Fries, also known as Polyporus tulipiferae Schwein.
To confirm this result, other specimens of trustworthy identity were examined (Table 1). DNA was extracted from three desiccated basidiocarps of the herbarium at the Institute of Botany, Karl-Franzens University, Graz, Austria. These herbarium samples were between 12 and 38 years old; two of them originated from Germany and one was from Canada. Another specimen was collected from the same region where the patient lives (Tyrol, Austria) and was obtained from the herbarium of the Leopold-Franzens University, Innsbruck, Austria. One strain (DSMZ 1183 = CBS 431.48) from the German Collection of Microorganisms and Cell Cultures (DSMZ) was examined as well. This strain was isolated in 1948 from an oak (Quercus sp.) in Canada. Alignment of all sequence data of the rRNA gene ITS region revealed analogies between 99.5% (clinical isolate) and 100%.
TABLE 1.
Investigated specimens of Irpex lacteus
| Specimen | Origin of specimen | Yra | Accession no. |
|---|---|---|---|
| Clinical isolate, CBS 113886b | Tyrol, Austria | 2003 | AY569561 |
| DSMZ 1183c = CBS 431.48 | Canada | 1948 | AY569564 |
| National Mycological Herbarium Canada DAOM 198628 | Canada | 1988 | AY569563 |
| Herbarium Poelt 3411d | Germany | 1966 | AY569562 |
| Herbarium Oberwinkler 12046d | Germany | 1972 | AY569565 |
| Herbarium Innsbruck 1994/0564c | Tyrol, Austria | 1994 | AY569566 |
Year of first isolation or collection.
Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany.
Institute of Botany, Karl-Franzens University, Graz, Austria.
Institute of Botany, Leopold-Franzens University, Innsbruck, Austria.
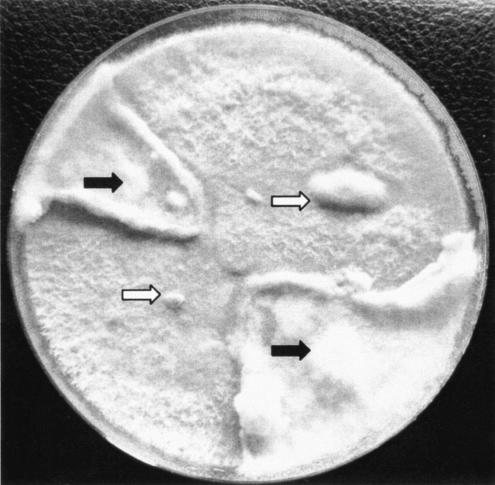
To eventually induce formation of dikaryotic mycelium or basidiocarps, mycelia of the clinical isolate and the strain DSMZ 1183 were inoculated together on the same petri dish with Sabouraud glucose agar and malt extract agar (Merck, Vienna, Austria), respectively, and incubated at room temperature in daylight for up to 4 weeks. Mycelium of the contact zone was examined by microscopy. No inhibition but rather an increase in growth at the contact zone was observed (Fig. 2). Microscopic examination of mycelium from this area showed a frequent appearance of interhyphal connections (anastomoses) between neighboring hyphae. Nevertheless, clamp connections or basidiocarps were not produced.
FIG. 2.
Mating experiment with clinical (black arrows) and reference (white arrows) strains of Irpex lacteus.
The culture of the clinical isolate was deposited at the Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands, under the strain number CBS 113886.
Conclusions.
Homobasidiomycetes occur rarely in mycotic diseases. Recently, a higher prevalence of Schizophyllum commune (Aphyllophorales, bracket fungi) in fungus balls of nasal sinuses and in patients suffering from chronic rhinosinusitis has been reported (2, 3). Another basidiomycete repeatedly reported in humans is Hormographiella aspergillata, the anamorph of the Agaricales mushroom Coprinus cinereus (9). To our knowledge, this is the first time that Irpex lacteus Fries (Hymenomycetes, Stereales) is reported to be involved in a human mycosis. This bracket mushroom is known as the milkwhite toothed polypore mushroom, one of the causal organisms of white rot in wood, and it is distributed worldwide, although its main occurrence is in the boreal region of the northern hemisphere. To our knowledge transmission from the environment to humans is unknown.
To date, there is no treatment regimen for infections due to I. lacteus. In our case, infection did not respond to systemic voriconazole, as fever did not subside. Administration of amphotericin B appears to be the most effective agent. However, no detailed information is available for I. lacteus because of the lack of applicable susceptibility tests. Therapy with new antifungal agents should be administered only to patients with identified fungal pathogens, as these agents lack effectiveness against Mucor and Rhizopus species.
Fungi which fail to produce unambiguous morphological features for reliable “classical” identification, as described above for this isolate (white aerial mycelium consisting of septate branching hyphae without conidia, chlamydospores, or clamp connections), have always been encountered in routine clinical work. This could be occurring more frequently because of growing numbers of mycotic diseases. Diagnosis of such isolates by comparing the sequences of the rRNA gene ITS region and other targets with entries in GenBank is a fast method and therefore increasingly used. Nevertheless, since entries in gene banks are not checked for reliability and accuracy, up to 20% of these data are incorrect (1, 4). Therefore, results obtained in this way should always be reviewed carefully. The identification of our clinical isolate was confirmed by sequencing specimens from international culture collections as well as from basidiocarps obtained from public herbarium collections. Despite the fact that the specimens compared originated from different regions of the world (Austria, Germany, Japan, and Canada) and were up to 38 years old, the sequence data differed only between 0% and 0.5% (3 bp). One of these differences in ITS1 was common to the clinical strain and a 32-year-old fruiting body from Germany (T:C); the other two changes concerned the clinical strain only and were found in ITS2 (T:C and C:T).
There was no visible difference between the case isolate and the DSMZ strain concerning cultural and hyphal features. Thermotolerance seems to be more advanced in the case isolate. It grew easily at 37°C, whereas the maximum growth temperature for the DSMZ strain was 30°C. Mating experiments with two strains of I. lacteus did not lead to the formation of basidiocarps or clamp connections. However, I. lacteus never forms the latter even in dikaryotic mycelium, as discussed by Jülich in 1984 (6).
It can be assumed that isolates obtained from clinical samples with white, cottony, rapidly growing cultures and which fail to produce distinct microscopic features for identification might represent filamentous basidiomycetes; those fungi deserve to be examined in more detail as we learn about their role as human pathogens and their occurrence in mycotic diseases.
Nucleotide sequence accession number.
The sequence data were submitted to the National Center for Biotechnology Information and were registered with accession numbers AY569561 through AY569566 (Table 1).
REFERENCES
- 1.Bridge, P. D., P. J. Roberts, B. M. Spooner, and G. Panchal. 2003. On the unreliability of published DNA sequences. New Phytol. 160:43-78. [DOI] [PubMed] [Google Scholar]
- 2.Buzina, W., D. Lang-Loidolt, H. Braun, K. Freudenschuss, and H. Stammberger. 2001. Development of molecular methods for identification of Schizophyllum commune from clinical samples. J. Clin. Microbiol. 39:2391-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buzina, W., H. Braun, K. Freudenschuss, A. Lackner, K. Schimpl, and H. Stammberger. 2003. The basidiomycete Schizophyllum commune in paranasal sinuses. Mycoses 46(Suppl. 1):23-27. [PubMed] [Google Scholar]
- 4.de Hoog, G. S., and R. Horré. 2002. Molecular taxonomy of the Alternaria and Ulocladium species from humans and their identification in the routine laboratory. Mycoses 45:259-276. [DOI] [PubMed] [Google Scholar]
- 5.Gardes, M., and T. D. Bruns. 1993. ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol. Ecol. 2:113-118. [DOI] [PubMed] [Google Scholar]
- 6.Jülich, W. 1984. Kleine Kryptogamenflora (Gams H.), Band IIb/1, Basidiomyceten/1. Teil Die Nichtblätterpilze, Gallertpilze und Bauchpilze (Aphyllophorales, Heterobasidiomycetes, Gastromycetes), 1st ed. Fischer, Jena, Germany.
- 7.Lass-Flörl, C., J. Aigner, E. Gunsilius, A. Petzer, D. Nachbaur, G. Gastl, H. Einsele, J. Löffler, M. P. Dierich, and R. Würzner. 2001. Screening for Aspergillus spp. using polymerase chain reaction of whole blood samples from patients with haematological malignancies. Br. J. Haematol. 113:180-184. [DOI] [PubMed] [Google Scholar]
- 8.Lucidarme, O., N. Howarth, J. F. Finet, and P. A. Grenier. 1998. Intrapulmonary lesions: percutaneous automated biopsy with a detachable, 18-gauge, coaxial cutting needle. Radiology 207:759-765. [DOI] [PubMed] [Google Scholar]
- 9.Surmont, I., F. Van Aelst, J. Verbanck, and G. S. de Hoog. 2002. A pulmonary infection caused by Coprinus cinereus (Hormographiella aspergillata) diagnosed after a neutropenic episode. Med. Mycol. 40:217-219. [DOI] [PubMed] [Google Scholar]
- 10.White, T. J., T. Bruns, S. Lee, and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press Inc., San Diego, Calif.