Abstract
We sought a possible correlation between the clinical manifestations of staphylococcal scalded-skin syndrome (SSSS) and the serotype of exfoliative toxins (ET) by PCR screening of the eta and etb genes in Staphylococcus aureus strains isolated from 103 patients with generalized SSSS and 95 patients with bullous impetigo. The eta gene and the etb gene were detected in, respectively, 31 (30%) and 20 (19%) episodes of generalized SSSS and 57 (60%) and 5 (5%) episodes of bullous impetigo. Both genes were detected in 52 (50%) episodes of generalized SSS and 33 (35%) episodes of bullous impetigo. To explain this link between etb and generalized SSSS, we examined the distribution of ETA- and ETB-specific antibodies in the healthy population (n = 175) and found that the anti-ETB antibody titer was lower than the anti-ETA titer. Thus, ETA is associated with bullous impetigo and ETB is associated with generalized SSSS, possibly owing to a lower titer of anti-ETB neutralizing antibodies in the general population.
Staphylococcal scalded-skin syndrome (SSSS) refers to a spectrum of blistering skin diseases caused by Staphylococcus aureus exfoliative toxins (ETs) (7, 12, 15). These toxins cause intraepidermal splitting through the granular layer by specific cleavage of desmoglein 1, a desmosomal cadherin protein that mediates cell-cell adhesion of keratinocytes in the granular layer (2).
SSSS can take on two main clinical forms, depending on the patient's age (7, 12). Bullous impetigo, the localized form of SSSS, is the most common and can occur at any age. Its begins with skin infection by an S. aureus strain producing ETs; the infecting strain is generally recovered from bullae (13), and the toxin is produced and acts locally. By contrast, the generalized form of SSSS affects newborns (Ritter's disease), infants, and children, but rarely adults. In this form, fluid from intact bullae is generally sterile, and the infecting strain is usually recovered from distant sites, such as the throat and/or nose (13). Generalized SSSS is thought to arise from systemic ET absorption from these sites followed by bloodstream diffusion to the cutaneous target (14). According to a recent review (11), the higher incidence of generalized SSSS in children may be due to less-efficient renal clearance of the toxin and to immunological immaturity (low anti-ET antibody titers).
There are three serological forms of staphylococcal ETs (ETA, ETB, and ETD), all of which cleave human desmoglein 1. Only ETA and ETB have been firmly linked to human SSSS (2, 3, 26). In recent reviews, Ladhani et al. have suggested that ETB is more frequently isolated than ETA in children with generalized SSSS (11, 12).
The aim of this study was to investigate a possible correlation between the ET serotype and the clinical form of SSSS and to investigate the distribution of ETA- and ETB-specific antibody titers in the general population.
MATERIALS AND METHODS
Characteristics of the patients.
The cases of all children (<18 years) with generalized SSSS or with bullous impetigo spontaneously reported to the French National Staphylococcal Reference Center (Lyon, France) between 1 January 1985 and 31 December 2002 by hospitals throughout France were considered for the study. Cases were identified primarily by review of the individual records if the patient was from the Lyon area or by review of notes sent to the Reference Center. All cases fulfilled the previously described definition of generalized SSSS or bullous impetigo (13). Generalized SSSS was diagnosed in patients with fever, erythroderma progressing within 1 to 2 days to spontaneous exfoliation with a positive Nikolsky's sign followed by secondary whole-body discolored desquamation, with lack of oral and genital findings; S. aureus was recovered from various distant sites. Bullous impetigo was diagnosed in patients with localized bullous lesions and exfoliation who were afebrile; S. aureus was recovered from bullae.
S. aureus isolates.
S. aureus was isolated from patients with generalized SSSS by swab culture of the skin surface and/or distant sites, such as the throat, nose, eyes, and ears. Isolates from patients with bullous impetigo were generally cultured from bullous lesions and rarely from distant sites. S. aureus was identified on the basis of Gram staining and its ability to coagulate citrated rabbit plasma (bioMérieux, Marcy-l' Etoile, France) and to produce a clumping factor (Staphyslide test; bioMérieux). All isolates were studied for the presence of eta and etb when multiple S. aureus isolates were recovered from the same patient.
PCR detection of eta and etb.
Genomic DNA was extracted from staphylococcal cultures and used as a template for PCR amplification with primers and thermal profiles specific for eta and etb as described previously (9). The PCR products were resolved by electrophoresis through 1.5% agarose gels (Sigma, Saint Quentin Fallavier, France), followed by ethidium bromide staining. DNA from the control S. aureus strains RN-450 (negative control), TC-7 (eta+), and TC-146 (etb+) were used to check the specificity of PCR amplification (13). Multiple isolates recovered from the same patient with similar eta and etb profiles were considered identical.
Expression and purification of recombinant toxins.
The DNA fragment of the eta gene encoding the open reading frame corresponding to the mature form of ETA was amplified by PCR with primers 5′-TGA CCA TGG CAG AAG TTT CAG CAG AAG AAA TAA AAA AAC ATG-3′ and 5′-TGC CTG CAG TTA CTC ATT TTT CTC GTT TAT AAT GCG C-3′ and cloned into the NcoI/PstI site of the pIVEX2.4d expression vector (Roche Applied, Meylan, France) in order to express the mature ETA with an N-terminal His6 tag sequence in Escherichia coli. The DNA fragment of the etb gene encoding the open reading frame corresponding to the mature form of ETB was amplified by PCR with primers 5′-GCA TGC AAG AAT ACA GCG CAG AAG AAA TC-3′ and 5′-AGA TCT ATG AGA CTG TAA TTC AGC TCT-3′ and cloned into the BamHI/SphI site of the pQE 70 vector (QIAGEN, Valencia, Calif.) in order to express mature ETB with a C-terminal His6 tag sequence in E. coli. The integrity of the open reading frame of each construct was verified by DNA sequencing.
The recombinant ETs were purified from E. coli culture supernatants as follows. E. coli cells growing exponentially in Luria-Bertani medium were inoculated into 1 liter of the same fresh medium and incubated with continuous rotary shaking for 24 h at 37°C. The medium was supplemented with ampicillin (100 μg/ml) for ETA (pIVEX2.4d vector) and ampicillin plus kanamycin (30 μg/ml) for ETB (pQE 70 vector). The cultures were then centrifuged at 4,000 × g for 20 min at 4°C and were sonicated on ice after adding lysozyme. Then, the lysates were centrifuged at 10,000 × g for 30 min at 4°C and the supernatants were collected. The His-tagged ETs were purified on Ni-nitrilotriacetic acid columns (QIAGEN), as recommended by the manufacturer, and then dialyzed against phosphate-buffered saline (PBS). ET protein concentrations were determined according to the Bradford method (4), using bovine serum albumin (BSA) as the standard. The activities of recombinant ETA and ETB were tested against recombinant desmoglein 1 and in newborn mice (data not shown). Using double immunodiffusion with intravenous immunoglobulin G (IVIgG) solution, we have verified that our recombinant proteins were antigenically similar to either ETA or ETB produced by the TC-7 (eta+) strain and ETB produced by the TC-146 (etb+) S. aureus strain, respectively (data not shown).
ELISA detection of antibodies to ETA and ETB.
Anti-ETA and anti-ETB antibodies were detected in a commercial IVIg, Tégéline LBF (Laboratoire Français du Fractionnement et des Biotechnologies [LBF], Les Ulis, France) and in serum samples by using specific solid-phase enzyme-linked immunosorbent assays (ELISAs) and with a peroxidase-conjugated antiimmunoglobulin antibody. The procedure was adapted from Current Protocols in Molecular Biology (4). Briefly, 96-well ELISA plates (Sigma) were coated with 50 μl of (5 μg/ml) recombinant ETA or ETB/well diluted in PBS, pH 7.4, containing 0.1% sodium azide and were incubated overnight at room temperature. The plates were then blocked with 5% skimmed milk in PBS, pH 7.4, for 30 min at room temperature. Unbound ETA and ETB were removed by five washes with 0.05% Tween 20 in PBS, pH 7.4. Serial twofold dilutions of IVIg (positive control) and serum samples were added to duplicate wells for 1 h at 37°C. Antigen-antibody complexes were detected by adding peroxidase-conjugated polyclonal antibodies against human IgG diluted 1:30,000 for 1 h at 37°C. After washing, the plates were developed by adding 75 μl of 3,3′,5,5′-tetramethylbenzine substrate (Sigma) to each well for 30 min at room temperature. The reaction was stopped with 1 M H2SO4. The plates were read at 450 nm in a model 680 microplate reader (Bio-Rad). Results were expressed in blanked optical density units. The specificity of the antibody reaction was examined by preincubating IVIg (125 mg/liter) for 1 h with BSA, ETA, or ETB (0 to 20 mg/liter).
Sera and pooled human Ig.
Serum samples were collected from French adults and children (72 and 103 individuals, respectively) coming for routine physical examinations and free of active S. aureus infection, and samples were stored at −70°C until use. Human IVIg (Tégéline LBF) was purchased from LBF. This polyclonal antiserum is prepared from pooled sera from 20,000 healthy blood donors and contains polyclonal IgG.
Statistical analysis.
We used the chi-square test to compare proportions according to ET serotype, the log rank and t tests for age, and the t test for optical density values obtained in ETA- and ETB-specific ELISAs. All analyses were done using SPSS software version 11.5 (SPSS Inc., Chicago, Ill.).
RESULTS
Pediatric cases of SSSS reported to the French National Staphylococcal Reference Center.
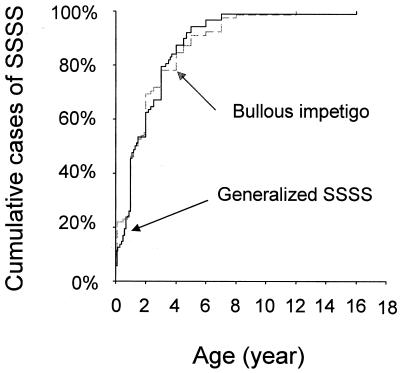
One hundred ninety-eight of the 693 cases of pediatric S. aureus infection and toxemia reported to the Center unambiguously met the case definition of SSSS and were included in the study. They comprised 103 cases of generalized exfoliative syndrome and 95 cases of bullous impetigo. The male/female sex ratio was 0.5 for generalized SSSS and 0.46 for bullous impetigo. Median age was 1 year (range, 23 days to 16 years) in patients with generalized SSSS and 1 year (range, 12 days to 13 years) in patients with bullous impetigo. The age distributions were not significantly different between the two diagnostic groups (P = 0.8, Kaplan-Meier test) (Fig. 1).
FIG. 1.
Age distribution of pediatric cases of generalized SSSS (n = 103) and bullous impetigo (n = 95) reported to the French National Staphylococcal Reference Center.
Distribution of eta and etb in SSSS isolates.
In all pediatric cases of SSSS reported, isolates were confirmed as S. aureus and harbored at least one ET gene. Eighty-eight isolates were positive for eta alone, 25 were positive for etb alone, and 85 were positive for both genes. Among the 103 patients with generalized SSSS, 31 (30%) were infected with isolates that carried eta alone, 20 (19%) carried etb alone, and 52 (51%) carried both genes. Among the 95 from patients with bullous impetigo, 57 (60%) isolates carried eta alone, 5 (5%) carried etb alone, and 33 (35%) carried both genes. We found that eta was significantly associated with bullous impetigo (P = 0.002), and etb was significantly associated with generalized SSSS (P < 0.001).
Distribution of ETA- and ETB-specific antibody titers in IVIg and in the general population.
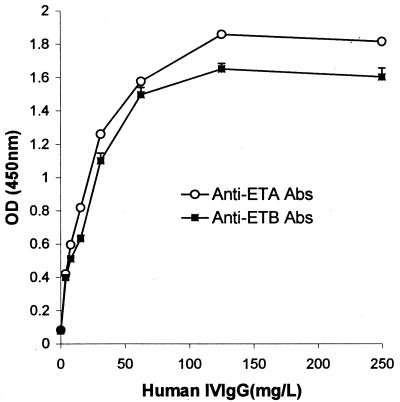
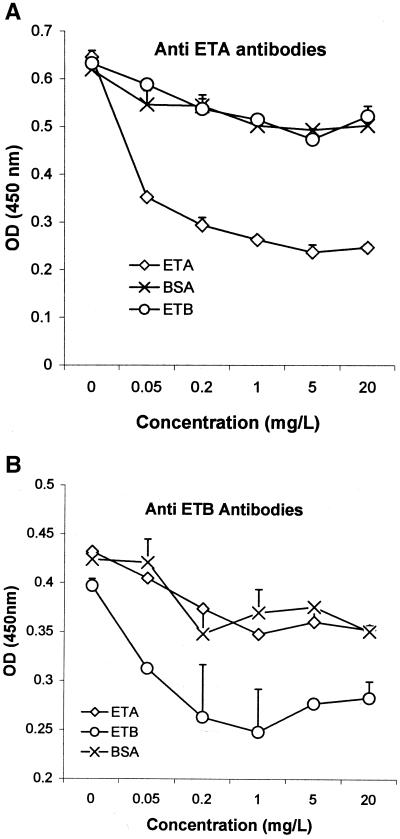
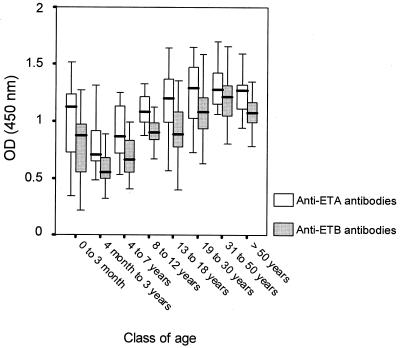
As shown in Fig. 2, anti-ETA and anti-ETB antibodies were detected in the commercial IVIg preparation (Tégéline). Signals were IVIg concentration-dependent from 0 to 50 mg/liter. The specificity of the ETA and ETB ELISAs was confirmed by the stronger signal reduction after IVIg preincubation with soluble ETA or ETB than with BSA (Fig. 3). IVIg consistently yielded slightly stronger signals with ETA than with ETB. ETA- and ETB-specific antibody titers in the control population of subjects free of S. aureus infection (Fig. 4) were similarly age dependent, falling from birth to 3 years, gradually rising until age 8 years, and then remaining stable. ETA-specific antibody titers were higher than ETB-specific antibody titers in 164 of the 174 sera tested (P < 0.001).
FIG. 2.
Reactivity of commercial IVIg to ETA and ETB. IgG antibodies to ETA and ETB were detected by ELISA at the indicated dilutions (see Materials and Methods). Results are expressed as absorbance at 450 nm (means ± standard deviations of duplicate experiments).
FIG. 3.
Specificity of anti-ETA and anti-ETB antibodies detected by ELISA. The reactivity of commercial IVIg was examined by ELISA (as described in the legend for Fig. 1) after preincubation for 1 h with BSA, ETA, or ETB at the indicated concentrations.
FIG. 4.
Distribution of specific anti-ETA and anti-ETB antibody titers in the different age classes of healthy control subjects (n = 22, 25, 19, 20, 17, 20, 25, and 27, respectively).
DISCUSSION
We retrospectively compared the distribution of the eta and etb genes in S. aureus isolates associated with pediatric cases of SSSS reported to the French National Staphylococcal Reference Center in order to identify a possible association with the clinical form (generalized SSSS versus bullous impetigo). The age distribution and sex ratio did not differ between the two diagnostic groups. All the S. aureus isolates contained at least one ET gene. The presence of eta was significantly associated with bullous impetigo, and etb was significantly associated with generalized SSSS.
The rare cases of generalized SSSS reported in young immunocompetent adults were also associated with etb-harboring strains (18) (unpublished data). This link between ETB and generalized SSSS might be due to increased virulence of ETB or to more abundant ETB release. Plano et al. recently demonstrated in an experimental model that high ET serum concentrations were associated with generalized SSSS (21). Differential passage of ETA and ETB through the epithelium could also contribute to this link between ETB and generalized SSSS. As eta is chromosome borne and etb is plasmid borne, it is possible that multiple copies of the etb gene could lead to higher ETB production (8, 22). However, levels of ETA and ETB are quite similar in vitro (19) (unpublished data).
Another possible explanation relates to differences in protective antibody titers against the two toxins in the general population. Host antibody status is critical in SSSS. Indeed, small inocula of toxigenic S. aureus injected into neonatal mice fail to induce exfoliation if the animal has previously been immunized with the toxin (14). In addition, anti-ET antibodies are generally absent during the acute phase of generalized SSSS but are present in 62% of patients with localized SSSS (14). Finally, human antibodies against ETs are thought to have neutralizing properties (16, 23, 25).
We developed specific ELISAs to determine the distribution of antibodies against ETA and ETB in the healthy population. IVIg was used as a positive control, as previous studies have demonstrated the presence of antibodies against the two toxins in most human sera tested (23, 25). We found that anti-ETA and anti-ETB antibody titers were age dependent, falling from 1 to 3 years and then gradually increasing to a plateau at about age 8 years. A similar kinetic pattern has already been reported for anti-ETA antibodies, but with the increase beginning at age 2 years (14); the authors postulated that this observation was due to passive transfer of maternal antibodies, followed by immunization due to natural exposure to ETs. The origin and nature of these antibodies are not clear, because only 5% of clinical isolates contain eta and etb genes. But all S. aureus isolates produce serine protease, which possesses 32% sequence homology to ETA and 27% to ETB. We suspect that immunization against serine protease is at the origin of the antibodies against ETA and ETB that we have detected.
Interestingly, anti-ETA antibody titers were higher than anti-ETB antibody titers in the general French population. This could be due to the higher homology of serine protease to ETA than ETB, a higher prevalence of the eta gene in staphylococci, or a greater immunogenicity of ETA (17). In Europe (6, 20), North America (5), and Africa (1), where fewer than 5% of human S. aureus isolates produce an ET, ETA is predominant. ETB-producing strains are more frequent in Japan, yet anti-ETA antibodies also predominate in healthy Japanese subjects (10, 24).
In conclusion, we observed an association between the ET serotype and the clinical severity of SSSS. The link between ETB and generalized SSSS may be explained, at least in part, by lower levels of anti-ETB antibodies than anti-ETA antibodies in the general population. This relation should be confirmed in patients with generalized SSSS by correlating the serotype of the ET with the titers of anti-ETA and anti-ETB antibodies of patients.
Acknowledgments
We thank C. Courtier, C. Gardon, C. Spinelli, C. Badiou, and F. Couzon for technical assistance and D. Young for editing the manuscript.
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- 1.Adesiyun, A., W. Lenz, and K. Schaal. 1991. Exfoliative toxin production by Staphylococcus aureus strains isolated from animals and human beings in Nigeria. Microbiologica 14:357-362. [PubMed] [Google Scholar]
- 2.Amagai, M., N. Matsuyoshi, Z. Wang, C. Andl, and J. Stanley. 2000. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat. Med. 6:1275-1277. [DOI] [PubMed] [Google Scholar]
- 3.Amagai, M., T. Yamaguchi, Y. Hanakawa, K. Nishifuji, M. Sugai, and J. Stanley. 2002. Staphylococcal exfoliative toxin B specifically cleaves desmoglein 1. J. Investig. Dermatol. 118:845-850. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel, F., R. Brent, R. Kingston, B. Moore, J. Seidman, J. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Greene Publishing Associates and Wiley Interscience, New York, N.Y.
- 5.de Azavedo, J., and J. Arbuthnott. 1981. Prevalence of epidermolytic toxin in clinical isolates of Staphylococcal aureus. J. Med. Microbiol. 14:341-344. [DOI] [PubMed] [Google Scholar]
- 6.Elster, P., and A. Hartmann. 1988. Epidemiology of ETA- and ETB-producing staphylococci in dermatological patients. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 268:534. [Google Scholar]
- 7.Gemmell, C. 1995. Staphylococcal scalded skin syndrome. J. Med. Microbiol. 43:318-327. [DOI] [PubMed] [Google Scholar]
- 8.Jackson, M., and J. Iandolo. 1986. Cloning and expression of the exfoliative toxin B gene from Staphylococcus aureus. J. Bacteriol. 166:574-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson, W., S. Tyler, E. Ewan, F. Asthon, D. Pollard, and K. Rozee. 1991. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by polymerase chain reaction. J. Clin. Microbiol. 29:426-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondo, I., S. Sakurai, Y. Sarai, and S. Futaki. 1975. Two serotypes of exfoliatin and their distribution in staphylococcal strain isolated from patients with scalded skin syndrome. J. Clin. Microbiol. 1:397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladhani, S. 2003. Understanding the mechanism of action of the exfoliative toxins of Staphylococcal aureus. FEMS Immunol. Med. Microbiol. 39:181-189. [DOI] [PubMed] [Google Scholar]
- 12.Ladhani, S., C. Joannou, D. Lochrie, R. Evans, and S. Poston. 1999. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin. Microbiol. Rev. 12:224-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lina, G., Y. Gillet, F. Vandenesch, M. Jones, D. Floret, and J. Etienne. 1997. Toxin involvement in staphylococcal scalded skin syndrome. Clin. Infect. Dis. 25:1369-1373. [DOI] [PubMed] [Google Scholar]
- 14.Melish, M., F. Chen, S. Sprouse, and M. Murata. 1981. Epidermolytic toxin in Staphylococcal infection: toxin levels and host response, p. 287-298. In J. Jeljaszewicz (ed.), Staphylococci and staphylococcal infections. Gustav Fischer Verlag, Stuttgart, Germany.
- 15.Melish, M., and L. Glasgow. 1970. The staphylococcal scalded skin syndrome. N. Engl. J. Med. 282:1114-1119. [DOI] [PubMed] [Google Scholar]
- 16.Miller, M., and F. Kapral. 1972. Neutralization of Staphylococcus aureus exfoliatin by antibody. Infect. Immun. 6:561-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monday, S., G. Vath, W. Ferens, C. Deobald, J. Rago, P. Gahr, D. Monie, J. Iandolo, S. Chapes, W. Davis, D. Ohlendorf, P. Schlievert, and G. Bohach. 1999. Unique superantigen activity of staphylococcal exfoliative toxins. J. Immunol. 15:4550-4559. [PubMed] [Google Scholar]
- 18.Opal, S., A. Johnson-Winegar, and A. Cross. 1988. Staphylococcal scalded skin syndrome in two immunocompetent adults caused by exfoliatin B-producing Staphylococcus aureus. J. Clin. Microbiol. 26:1283-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piemont, Y., M. Haubensack, and H. Monteil. 1984. Enzyme-linked immunosorbent assays for Staphylococcus aureus exfoliative toxins A and B and some applications. J. Clin. Microbiol. 20:1114-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piemont, Y., D. Rasoamananjara, J. Fouace, and T. Bruce. 1984. Epidemiological investigation of exfoliative toxin-producing Staphylococcus aureus strains in hospitalized patients. J. Clin. Microbiol. 19:417-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plano, L., B. Adkins, M. Woischnik, R. Ewing, and C. Collins. 2001. Toxins levels in serum correlates with the development of staphylococcal scalded skin syndrome in a murine model. Infect. Immun. 69:5193-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenblum, E., and S. Tyrone. 1976. Chromosomal determinants for exfoliative toxin production in two strains of staphylococci. Infect. Immun. 14:1259-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarai, Y. 1975. Distribution of anti-exfoliatin hemagglutination titers in normal human population. Jikeikai Med. J. 22:227-235. [Google Scholar]
- 24.Sarai, Y., H. Nakahara, T. Ishikawa, I. Kondo, S. Futaki, and K. Hirayama. 1977. Detection of anti-exfoliatin antibodies in healthy adults and children by the passive hemagglutination test. Infect. Immun. 16:1024-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiley, B., L. Glasgow, and M. Rogolsky. 1976. Staphylococcal scalded-skin syndrome: development of a primary binding assay for human antibody to the exfoliative toxin. Infect. Immun. 13:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi, T., Y. Yokota, J. Terajima, T. Hayashi, M. Aepfelbacher, M. Ohara, H. Komatsuzawa, H. Watanabe, and M. Sugai. 2002. Clonal association of Staphylococcus aureus causing bullous impetigo and the emergence of new methicillin-resistant clonal groups in Kansai district in Japan. J. Infect. Dis. 185:1511-1516. [DOI] [PubMed] [Google Scholar]