Abstract
A Saccharomyces cerevisiae-expressed nucleocapsid (N) polypeptide of the M41 strain of infectious bronchitis virus (IBV) was used as antigen in a recombinant yeast-expressed N protein-based enzyme-linked immunosorbent assay (Y-N-ELISA). The Y-N-ELISA was rapid, sensitive, and specific for detecting chicken serum antibodies to IBV, and it compared favorably with a commercial ELISA.
Rapid diagnosis and immune status determination are critical to controlling outbreaks of infectious bronchitis virus (IBV) among chickens, which result in severe economic losses of populations of egg layers and broiler flocks. With appropriate standards, enzyme immunoassays are accurate indicators of anti-IBV antibody levels and facilitate immune status monitoring in large flocks (2, 4, 7, 10). Currently, inactivated and purified whole virus particles are used as the coating antigen in commercially available IBV enzyme-linked immunosorbent assay (ELISA) kits. Whole-virus purification requires the propagation of large quantities of virus in eukaryotic systems and depends on difficult and expensive processes.
The nucleocapsid (N) protein, a major structural protein of IBV, is the preferred protein to use in development of group-specific serologic assays. It has highly conserved sequences, which share 91 to 96.5% identity among various strains, is produced abundantly during infection, and has high immunogenicity, readily inducing antibodies as well as cytotoxic T-lymphocyte immunity in chickens (1, 8, 9, 11).
Recombinant proteins from avian coronaviruses have been produced in expression systems using prokaryotic or eukaryotic host organisms (3, 5). Although yeast combines the ease, simplicity, and low cost of bacterial expression systems with the authenticity of the far more expensive and less convenient animal tissue culture systems (6), there are no reports of studies using IBV recombinant proteins produced in yeast as antigens for ELISA.
In the present study, the N protein of the M41 strain of IBV (M41 IBV) was expressed in Saccharomyces cerevisiae and was purified and used as a coating antigen in an ELISA for diagnosis of IBV infection in chickens.
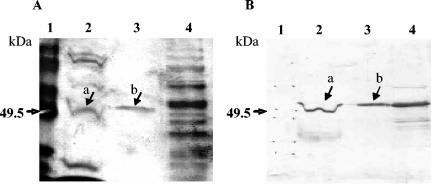
The N protein gene was amplified by reverse transcription-PCR using primers P1a (5′TTGTCATGGCAAGCGGTAAG3′) and P2a (3′AAGTTCATTCTCTCCTAGAGC5′), which were designed according to the nucleotide sequence of M41 IBV (GenBank accession number M28566). The purified PCR product containing the entire open reading frame (1,227 bp) was inserted in the vector pYES2.1/V5-His-TOPO (Invitrogen, Carlsbad, Calif.) and transformed into TOP10F′ Escherichia coli-competent cells, following the manufacturer's instructions (Invitrogen manual). The pYES2.1/V5-His-TOPO-derived expression plasmids carrying the N protein-encoding sequences were retransformed into the S. cerevisiae INVSc-1 strain (Invitrogen). Transformed yeast colonies were picked on selective plates and grown in synthetic complete medium without uracil to an optical density at 600 nm of approximately 1.2. Expression of the N gene was induced with galactose (2%), and maximum levels were obtained at 20 h postinduction. After lysis under native conditions, the N protein was affinity-purified with a nickel-chelating agarose column (HisTrap kit; Amersham Biosciences, Uppsala, Sweden). The yeast-expressed IBV N protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by silver staining and Western blotting with a chicken anti-IBV polyclonal antiserum and a rabbit antichicken immunoglobulin G-peroxidase conjugate, which showed high purity and molecular mass and antigenicity similar to the natural viral N protein (Fig. 1).
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (A) and Western blot analysis (B) of recombinant nucleocapsid protein of M41 IBV expressed in S. cerevisiae. Lanes: 1, molecular size marker; 2, proteins of M41 IBV; 3, recombinant IBV N protein purified by nickel-agarose column chromatography; 4, crude protein extract of transformed S. cerevisiae. Arrows indicate the natural IBV N protein (a) and yeast-expressed recombinant N protein (b).
Optimal dilutions of the N recombinant protein preparation and IBV-positive and IBV-negative chicken sera for the recombinant yeast-expressed N protein ELISA (Y-N-ELISA) were determined by checkerboard titration. The Y-N-ELISA followed the general guidelines of Ndifuna et al. (5), except that it was used in volumes of 50 μl and with rabbit anti-chicken immunoglobulin G (Sigma, St. Louis, Mo.). The mean optical density of each test sample (ODMTS) was expressed as a test sample to positive sample ratio (S/P) in relation to the mean OD of the positive reference serum (ODMPRS) and the negative reference serum (ODMNRS) according to the formula S/P = (ODMTS − ODMNRS)/(ODMPRS − ODMNRS). The cutoff point (0.027) corresponded to 3 standard deviations above the mean S/P values for 12 negative sera collected from chickens that were known to be free of IBV infection.
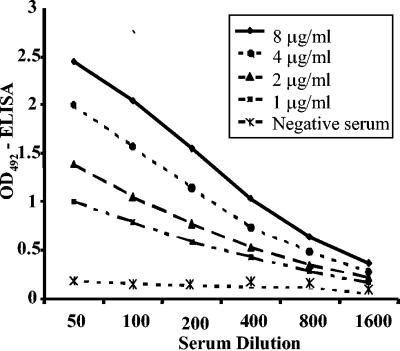
The antiserum to M41 IBV reacted strongly and in a dose-dependent manner with the recombinant N protein in the Y-N-ELISA (Fig. 2). IBV-positive and -negative chicken sera could be distinguished at coating concentrations of recombinant N protein as low as 1 μg/ml when the serum dilution was 1:100, and differentiation was markedly enhanced from 1-μg/ml to 8-μg/ml coating concentrations. The antigen concentration of 2 μg/ml and a single serum dilution of 1:100 were selected for subsequent analysis of chicken sera in Y-N-ELISA, considering that OD values were between 1.000 and 1.500 for the positive serum and less than 0.150 for the negative serum (Fig. 2).
FIG. 2.
Detection of IBV-specific antibodies in hyperimmune chicken serum by Y-N-ELISA. Microplates were coated with different concentrations of the recombinant IBV N protein and reacted with serial twofold dilutions of M41 IBV-positive and -negative chicken serum.
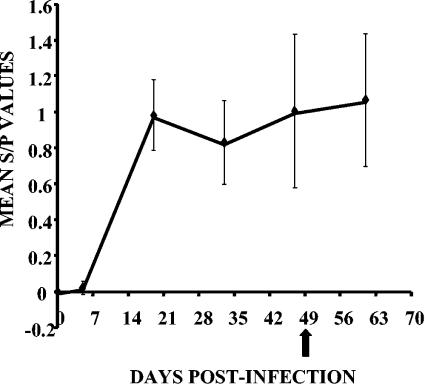
The Y-N-ELISA was used to determine serum anti-IBV antibody levels from a group of 12 chickens subjected to experimental infection and reinfection with M41 IBV over a period of 63 days. The antibody curve revealed a typical seroconversion profile after primary infection (Fig. 3); i.e., antibody levels were very low at 5 days postinfection (p.i.), increased markedly at 21 days p.i., and remained high until 47 days p.i. Serum antibody levels increased slightly after the birds were reinfected at 47 days p.i. Thus, serum antibodies of chickens subjected to experimental infection with IBV were highly cross-reactive with the recombinant yeast-expressed N protein, as demonstrated by the time course evaluation of p.i. humoral immune responses.
FIG. 3.
IBV antibody levels (mean S/P values) detected by Y-N-ELISA in sera of chickens infected with M41 IBV and infected 7 weeks later (arrow) with homologous virus.
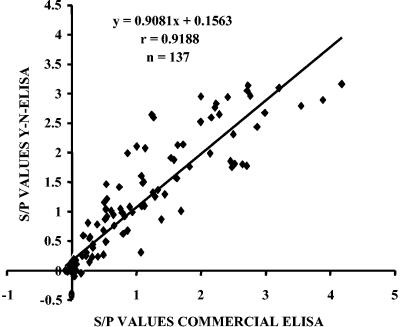
The sensitivity and specificity of the Y-N-ELISA were evaluated in comparison to a commercial ELISA kit (Kirkegaard & Perry Laboratories [KPL], Gaithersburg, Md.) using a collection of 137 serum samples from chickens suspected of being infected with IBV. The relative sensitivity of Y-N-ELISA was 98.79%, the specificity was 83.33%, and the accuracy was 92.70%. A total of 82 tested serum samples were positive, and 45 were negative in both ELISAs (Table 1). Ten sera gave discordant results, nine of which were positive only in Y-N-ELISA and one of which was positive only in the commercial kit. The nine sera that were positive only in Y-N-ELISA were collected shortly after the onset of IBV clinical signs in the flock and had low S/P values, although the S/P values were higher than the cutoff point. Y-N-ELISA results were confirmed by Western blot analysis using IBV antigens; the nine sera showed moderate to weak reactions to N protein, and the negative serum had no reactivity to IBV (data not shown). The correlation between the Y-N-ELISA and the commercial ELISA kit was high (r = 0.9188) and significant (P < 0.0001) (Fig. 4). Discordant positive results might be ascribed to some differences in the viral antigen preparations adsorbed to ELISA microplates and to the predominant antigen in Y-N-ELISA (N protein), which is more immunogenic and cross-reactive and which reacts with antibodies produced earlier during a humoral immune response p.i. (8, 9).
TABLE 1.
Detection of IBV antibodies in chicken sera by the Y-N-ELISA and a commercial IBV ELISA kit
| Y-N-ELISA resulta | No. of samples tested with commercial ELISA kit
|
||
|---|---|---|---|
| IBV positive | IBV negative | Total | |
| Positive | 82 | 9 | 91 |
| Negative | 1 | 45 | 46 |
| Total | 83 | 54 | 137 |
Relative sensitivity, (82/83) × 100 = 98.79%; Relative specificity, (45/54) × 100 = 83.33%; relative accuracy, [(82 + 45)/137] × 100 = 92.70%.
FIG. 4.
Correlation between antibody levels (S/P values) measured by Y-N-ELISA and a commercial ELISA kit.
Although the yeast-expressed IBV N protein has not been tested with heterologous virus strains, the potential cross-reactivity of the recombinant protein was indirectly shown by the positive results of field serum samples, since distinct strains from the reference vaccine strain (H 120) might be responsible for these outbreaks.
In conclusion, a recombinant nucleocapsid protein that resembles the native N protein in size and antigenicity can be expressed in and purified from S. cerevisiae in an economical and reproducible way. Regarding sensitivity, specificity, accuracy, and correlation with other diagnostic systems, this recombinant protein can be successfully used in Y-N-ELISA to detect IBV-specific antibodies in chicken sera.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP (proc. 01/14650-3 and proc. 02/06083-0) and by Conselho Nacional de Pesquisa CNPq (proc. 477140/2003-3).
We thank Clovis de Oliveira and Victorio Chiramonte from MERIAL Saúde Animal.
REFERENCES
- 1.Boursnell, M. E. G., M. M. Binns, I. J. Foulds, and T. D. K. Brown. 1985. Sequences of the nucleocapsid genes from two strains of avian infectious bronchitis virus. J. Gen. Virol. 66:573-580. [DOI] [PubMed] [Google Scholar]
- 2.Cavanagh, D., and S. A. Naqi. 1997. Infectious bronchitis, p. 511-526. In B. W. Calnek, C. W. Beards, L. R. McDougald, and Y. M. Saif (ed.), Diseases of poultry, 9th ed. Iowa State University Press, Ames, Iowa.
- 3.Chen, H., B. Coote, S. Attree, and J. A. Hiscox. 2003. Evaluation of a nucleoprotein-based enzyme-linked immunosorbent assay for the detection of antibodies against infectious bronchitis virus. Avian Pathol. 32:519-526. [DOI] [PubMed] [Google Scholar]
- 4.Marquardt, W. W., D. B. Snyder, and B. A. Schlotthober. 1981. Detection and quantification of antibodies to infectious bronchitis virus by enzyme-linked immunosorbent assay. Avian Dis. 25:713-722. [PubMed] [Google Scholar]
- 5.Ndifuna, A., A. K. Waters, M. Zhou, and E. W. Collisson. 1998. Recombinant nucleocapsid protein is potentially an inexpensive, effective serodiagnostic reagent for infectious bronchitis virus. J. Virol. Methods 70:37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romanos, M. A., C. A. Scorer, and J. J. Clare. 1992. Foreign gene expression in yeast: a review. Yeast 8:423-488. [DOI] [PubMed] [Google Scholar]
- 7.Saif, L. J. 1993. Coronavirus immunogens. Vet. Microbiol. 37:285-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo, H. S., L. Wang, R. Smith, and E. W. Collisson. 1997. The carboxyl-terminal 120-residue polypeptide of infectious bronchitis virus nucleocapsid induces cytotoxic T lymphocytes and protects chicken from acute infection. J. Virol. 71:7889-7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sneed, L. W., G. D. Butcher, R. Parr, L. Wang, and E. W. Collisson. 1989. Comparisons of the structural proteins of avian bronchitis virus as determined by western blot analysis. Viral Immunol. 2:221-227. [DOI] [PubMed] [Google Scholar]
- 10.Snyder, D. B., W. W. Marquardt, E. T. Mallinson, P. K. Savage, and D. C. Allen. 1984. Rapid serological profiling by enzyme-linked immunosorbent assay. III. Simultaneous measurements of antibody titers to infectious bronchitis, infectious bursal disease, and Newcastle disease viruses in a single serum dilution. Avian Dis. 28:12-24. [PubMed] [Google Scholar]
- 11.Williams, A. K., L. Wang, L. W. Sneed, and E. W. Collisson. 1992. Comparative analyses of the nucleocapsid genes of several strains of infectious bronchitis virus and other coronaviruses. Virus Res. 25:213-222. [DOI] [PMC free article] [PubMed] [Google Scholar]