Abstract
Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has recently emerged in the southwestern Pacific, North America, and Europe. These S. aureus isolates frequently shared some genetic characteristics, including the SCCmec type IV and lukS-lukF genes. In this paper we show that typical CA-MRSA isolates have spread to South America (Brazil).
Staphylococcus aureus is the leading cause of hospital-acquired infections worldwide. All methicillin-resistant S. aureus (MRSA) isolates tested so far harbored the mobile element staphylococcal cassette chromosome mec (SCCmec). The SCCmec carries the mec gene complex, encoding methicillin resistance, and the ccr gene complex, encoding recombinases (7). This accessory element can display different combinations of mec gene and ccr gene complexes. As determined on the basis of this diversity, five types of SCCmec and their variants have been reported previously (5, 7).
Most hospital-acquired infections caused by MRSA (HA-MRSA) are associated with a relatively small number of epidemic clones spread over different continents, including Brazilian epidemic/Hungarian (SCCmec type IIIA/III), Iberic (SCCmec I), New York/Japanese (SCCmec II), and pediatric (SCCmec IV) clones (1, 3, 10, 13).
More recently, reports coming from North America (United States), the southwestern Pacific (Australia, New Zealand, and Samoa), and Europe (France, Switzerland, and The Netherlands) have indicated that community-acquired MRSA (CA-MRSA) has emerged as a new pathogen (14, 16). It is notable that these CA-MRSA isolates were not clonally related to HA-MRSA international clones. Moreover, they were more susceptible to antimicrobial drugs. It was suggested that the acquisition of the SCCmec IV genes (which confer β-lactam resistance only) and acquisition of the lukS-lukF genes (which encode the Panton-Valentine leukocidin [PVL]) were important genetic events for the fitness of the MRSA for spreading and causing diseases in the community (14). Recently, a fifth allotype of SCCmec was found on the chromosome of a CA-MRSA strain from Australia (5).
Surprisingly, CA-MRSA has been shown to cause infections in children and young adults who did not present with classical risk factors for nosocomial infections (CA-MRSA without risk factors) (12). Frequently, CA-MRSA isolates are associated with skin and soft-tissue diseases. However, more-severe infections, including highly lethal necrotizing pneumonia, have also been reported previously (4, 16).
In this paper we describe the first report of infections caused by CA-MRSA isolates in South America (Brazil).
MRSA isolates (WB49 and WB57) were obtained from two patients from the community (a 23-year-old male and a 34-year-old female, respectively) presenting with skin- and soft-tissue-associated infections, including furunculosis. Another isolate (WB69) was obtained from the joint fluid of a male, 56 years old, who presented with septic arthritis. All three were outpatients, and no healthcare-associated infection was evident, since they had no history of previous MRSA isolation, hospitalization, or surgery in the year before the MRSA isolation. In addition, there was no report of percutaneous lines or indwelling devices present at the time of cultures. Following these strict criteria, previously suggested by D. W. Dietrich et al. (2), these three cases were defined as representing community-acquired (CA) infections.
In addition, two other MRSA isolates (WB45 and WB72), displaying phenotypic and genotypic characteristics similar to those described for CA-MRSA, were also determined to have been the cause of infections in two outpatients. However, the lack of hospital records made it impossible to analyze whether these cases met the criteria for characterizing CA infections. Attempts to contact these patients afterward were unsuccessful. All the MRSA isolates were obtained from June 2002 to September 2003, in the ambulatory sections of two different hospitals located in Porto Alegre city, Rio Grande do Sul, Brazil.
The isolates were classified by Microscan and Vitek identification systems as MRSA, and the classification was confirmed by the amplification of an internal fragment of the mecA gene by PCR (11) and by a free-coagulase test. In addition, all five MRSA isolates displayed low oxacillin MICs (8 to 32 μg/ml), which is compatible with the heterogeneous resistance phenotype for methicillin-oxacillin. However, they were all susceptible to clindamycin, erythromycin, gentamicin, sulfamethoxazole-trimethoprim, ciprofloxacin, and vancomycin by a disk diffusion test, carried out as recommended by the NCCLS (9). It is important that HA-MRSA isolates displaying the heterogeneous resistance phenotype and a wide spectrum of drug susceptibility patterns are uncommon in our hospitals, where multiresistant isolates of the Brazilian epidemic clone (BEC) predominate (13).
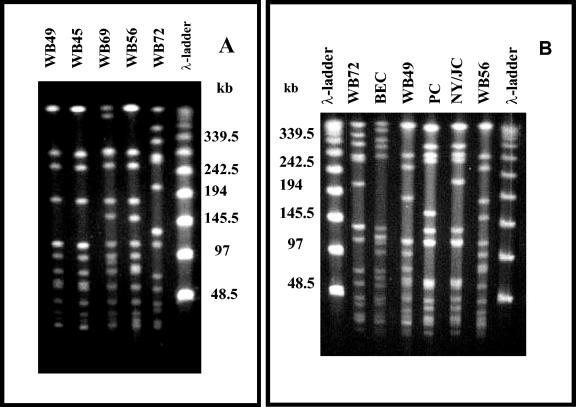
Pulsed-field gel electrophoresis (PFGE) of the total DNA was performed as described previously (13). Four outpatient isolates of MRSA had quite similar PFGE patterns, differing by only four PFGE bands. On the basis of the criteria suggested by Tenover et al. (15), isolates differing by from 1 to 6 PFGE bands should be grouped in a PFGE type (pulsotype) by assigning the same capital letter with different numeric subscripts for indicating subtypes. Accordingly, the outpatient isolates were classified in the A1 (strains WB45 and WB49), A2 (WB56), and A3 (WB69) subtype groups. The other isolate (WB72) had a totally different profile, since it differed by more than six PFGE bands, and was classified as pulsotype B (Fig. 1A). Classifications of PFGE patterns were also verified when isolates from Porto Alegre were compared with representatives of international clones of HA-MRSA prevalent in the American continent (BEC, Brazilian epidemic clones; NY/JC, New York/Japanese clones; PC, pediatric clones; Fig. 1B).
FIG. 1.
(A) Pulsed-field gel electrophoresis (PFGE) of the SmaI-fragmented genomic DNA obtained from MRSA isolates from Porto Alegre. Results for strains WB49 and WB45 (PFGE pattern A1), WB69 (pattern A2), WB56 (pattern A3), and WB72 (pattern B) are shown. (B) Comparison of the patterns exhibited by MRSA isolates from Porto Alegre city with those displayed by representatives of the international MRSA clones spread over the American continent. Results for BEC (strain BMB9393; a representative Brazilian epidemic clone), PC (strain HC563; a representative pediatric clone), and NY/JC (strain 17594; a representative New York/Japanese clone) are shown.
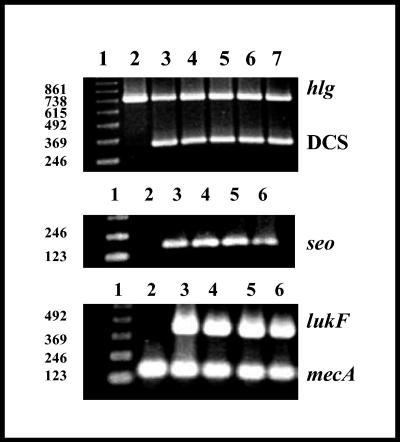
The SCCmec typing was carried out following the methodology described by Oliveira and de Lencastre (11). All the isolates from Porto Alegre carried SCCmec type IV, since a band of 342 bp was amplified for DCS primers and no amplification was verified for any of the other primers tested (Fig. 2).
FIG. 2.
Detection of γ-hemolysin genes, lukF, mecA, and seo by PCR. The primers used for amplifying accessory elements are described in Materials and Methods. The multiplex PCR for SCCmec typing (11) only amplified an expected band of 342 bp for DCS primers. (Top panel) Lane 1, 123 molecular size marker; lane 2, strain BMB9393 (a negative control); lane 3, WB69; lane 4, WB45; lane 5, WB57; lane 6, WB49; lane 7, WB72. (Center and bottom panels) Lane 1, 123 molecular size marker; lane 2, WB72; lane 3, WB69; lane 4, WB45; lane 5, WB57; lane 6, WB49.
The occurrence of the PVL-encoding gene lukF was tested by PCR using the primers P1pvl (5′ ATCCGAGAGACTATTTTGTGC 3′) and P2pvl (5′ CATCAACCTTTTTCTCACTTAC 3′), expected to amplify a fragment of 406 bp. The specific primers were designed on the basis of a sequence of the lukF gene deposited in GenBank (AB006796). γ-Hemolysin genes were also detected by PCR using the primers P1γ-H (5′ TTCGTTCCAGACAGTGAGTTA 3′) and P2γ-H (5′ TAAGTCCACCAGATAAACCATT 3′), which were expected to amplify a specific fragment of 757 kb present in both the hlg (hlgB-hlgC) and hlgV (lukS-lukF) sequences. These primers were made on the basis of the conserved regions of the sequences of the γ-hemolysin genes (S65052, X81586, and L01055) deposited in the GenBank. In addition, the primers P1seo (5′ TAGAGAGTTTGTGTAAGAAGTC 3′) and P2seo (5′ TTAAATTCAGCAGATATTCCATCT 3′), expected to generate a fragment of 180 kb, corresponding to an amplified region of the staphylococcal enterotoxin O-encoding gene (seo) were also used. These primers were based on the sequence of the complete genome of S. aureus strain N315 (AP003135), also deposited in GenBank. The specificity of all primers was tested by a nucleotide sequence alignment search using megaBLAST (http://www.ncbi.nlm.nih.gov/BLAST/megablast.shtml). mecA or DCS primers were also used as PCR internal controls. Strain HC563 (a representative of the pediatric clone; SCCmec type IV) was used as a positive control for the amplification of the seo, mecA, and γ-hemolysin genes, and the isolate WB49 was used as a positive control for the lukF gene.
The three well-characterized CA-MRSA strains WB49, WB57, and WB69 and the MRSA WB45 strain (pulsotypes A1 to A3) share some genotypic characteristics with CA-MRSA isolates described in the United States, the southern Pacific, and a few countries in Europe, in view of the fact that they harbored SCCmec type IV-, Panton-Valentine leukocidin-, enterotoxin-, and γ-hemolysin-encoding genes (Fig. 2), including the ability to cause infections in immunocompetent patients, in the case of strains WB49, WB57, and WB69. Despite the fact that we do not have sufficient data from the medical records to characterize the isolates WB45 and WB72 as cases of CA infections, the isolate WB45 (pulsotype A1) was classified as CA-MRSA on the basis of these phenotypic and genotypic results. The MRSA isolate displaying pulsotype B (strain WB72), also isolated from an outpatient, carried the SCCmec type IV and had a PFGE pattern totally different from that of HA-MRSA isolates commonly associated with nosocomial infections in our continent. Thus, this strain was considered to be a possible CA-MRSA strain. Strain WB72 is unusual, given that it does not carry the lukF gene (Fig. 2). However, CA-MRSA infections by SCCmec type IV isolates that do not produce PVL have also been reported (14). PVL production has frequently been associated with S. aureus strains involved in primary skin infections and necrotizing pneumonia (4, 12, 16). In another study, PVL genes were detected in about 90% of the strains associated with furunculosis and 50% of those associated with cellulitis or cutaneous abscess. In contrast, these genes were absent from isolates associated with folliculitis and impetigo (8). In contrast to CA-MRSA isolates, HA-MRSA isolates generally do not carry lukS-lukF genes.
It was verified that the seo gene in S. aureus is located in the enterotoxin gene cluster (egc locus), carrying the seg, sei, sem, sen, and seo genes (6). The egc locus is a genetic marker present in 100% of the isolates belonging to the Oceania Southwest Pacific clone (OSPC), sequence type 30 (ST-30) (14). The CA-MRSA isolates from Porto Alegre, displaying pulsotypes A1 to A3, also carried the seo gene (Fig. 2). Because these isolates had many genetic characteristics similar to those of OSPC (14), we compared their PFGE patterns by capturing PFGE images from the web and calculating the band sizes of the SmaI-fragmented genomic DNA by the use of a λ-ladder molecular marker (http://jac.oupjournals.org/cgi/content/full/50/6/825). We verified that the Brazilian CA-MRSA (pulsotypes A1 to A3) isolates and representatives of the OSPC displayed identical restriction fragment length polymorphisms. Multilocus sequence typing (MLST) was performed for a representative of the Brazilian isolates displaying pulsotype A. Sequence types were assigned with reference to the MLST database (http://www.mlst.net), and it was verified that the WB49 isolate (pulsotype A1) displayed the MLST allelic profile 2-2-2-2-6-3-2 (ST-30), indicating that OSPC has spread and is causing CA infections in our country.
On the basis of these results, we suggest that all outpatient isolates of MRSA displaying low oxacillin-methicillin MICs and a wide range of antimicrobial susceptibility should be considered indicative of possible cases of CA-MRSA, and genetic studies including PFGE, SCCmec typing, detection of PVL genes, and MLST should be employed for further characterization of these bacteria. We believe that these approaches can be useful for the presumptive identification of a strain from a community source in the absence of complete medical records, as was the case for the CA-MRSA WB45 strain.
Although diseases caused by CA-MRSA affecting children, adolescents, and young adults have been reported previously, only relatively few CA-MRSA carrying SCCmec IV and PVL genes have been detected to date. Searching in PubMed (http://www.ncbi.nlm.nih.gov/), we found that well-characterized CA-MRSA isolates have been described only in Australia, New Zealand, the United States, France, The Netherlands, and Switzerland. However, the emergence of well-adapted CA-MRSA can be considered a major health threat, since these strains are resistant to β-lactam antibiotics, which are intensely used for empirical treatment of a variety of community infections, including pneumonia. Also, one can predict that in the near future, additional genetic elements coding for antimicrobial resistance may be acquired by CA-MRSA.
Although most CA-MRSA-associated infections reported essentially involved skin and soft tissue (12, 14), it is important that in our study one of the patients presented with septic arthritis. Even though no evident connection was verified among these patients, CA-MRSA isolates from four of the five outpatients were clonally related (belonging to OSPC).
In conclusion, it is necessary that doctors be aware that skin and soft-tissue community-associated infections, in addition to other diseases commonly caused by S. aureus, may involve a CA-MRSA isolate even in the absence of risk factors and that these strains are quickly spreading globally. Our results point toward a realistic pandemic potential of the ST-30 Oceania Southwest Pacific clone. Therefore, more surveillance studies are necessary to evaluate the extent of the dissemination of this well-adapted pathogen in community infections in Brazil and also in other countries. Finally, an effective policy for the control of the antimicrobial usage in the community must be implemented worldwide.
Acknowledgments
The present study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Projeto Universal), Fundação de Amparo à Pesquisa Carlos Chagas Filho do Estado do Rio de Janeiro (FAPERJ), and (FAPERJ/PRONEX). A.R. had a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and F.A.F. had a fellowship from CNPq.
We thank Leonardo R. Coelho for technical advice on the experiments using MLST.
REFERENCES
- 1.da Silva Coimbra, M. V., M. C. Silva-Carvalho, H. Wisplinghoff, G. O. Hall, S. Tallent, S. Wallace, M. B. Edmond, A. M. Figueiredo, and R. P. Wenzel. 2003. Clonal spread of methicillin-resistant Staphylococcus aureus in a large geographic area of the United States. J. Hosp. Infect. 53:103-110. [DOI] [PubMed] [Google Scholar]
- 2.Dietrich, D. W., D. B. Auld, and L. A. Mermel. 2004. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 113:e347-e352. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez, M. A., H. de Lencastre, J. Linares, and A. Tomasz. 1994. Spread and maintenance of a dominant methicillin-resistant Staphylococcus aureus (MRSA) clone during an outbreak of MRSA disease in a Spanish hospital. J. Clin. Microbiol. 32:2081-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillet, Y., B. Issartel, P. Vanhems, J. C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Piemont, N. Brousse, D. Floret, and J. Etienne. 2002. Association between Staphylococcus aureus strains carrying gene for Panton Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753-759. [DOI] [PubMed] [Google Scholar]
- 5.Ito, T., X. X. Ma, F. Takeuchi, K. Okuma, H. Yuzawa, and K. Hiramatsu. 2004. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob. Agents Chemother. 48:2637-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarraud, S., M. A. Peyrat, A. Lim, A. Tristan, M. Bes, C. Mougel, J. Etienne, F. Vandenesch, M. Bonneville, and G. Lina. 2001. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 166:669-677. [DOI] [PubMed] [Google Scholar]
- 7.Katayama, Y., T. Ito, and K. A. Hiramatsu. 2000. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 44:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128-1132. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing, vol. 22, no. 1. M100-S12. NCCLS, Villanova, Pa.
- 10.Oliveira, D. C., A. Tomasz, and H. de Lencastre. 2002. Secrets of success of a human pathogen: molecular evolution of pandemic clones of methicillin-resistant Staphylococcus aureus. Lancet Infect. Dis. 2:180-189. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira, D. C., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Said-Salim, B., B. Mathema, and B. N. Kreiswirth. 2003. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging pathogen. Infect. Control Hosp. Epidemiol. 24:451-455. [DOI] [PubMed] [Google Scholar]
- 13.Teixeira, L. A., C. A. Resende, L. R. Ormonde, R. Rosenbaum, A. M. S. Figueiredo, H. de Lencastre, and A. Tomasz. 1995. Geographic spread of epidemic multiresistant Staphylococcus aureus clone in Brazil. J. Clin. Microbiol. 33:2400-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Flier, M., N. B. van Dijk, A. C. Fluit, A. Fleer, T. F. Wolfs, and J. P. van Gestel. 2003. Fatal pneumonia in an adolescent due to community-acquired methicillin-resistant Staphylococcus aureus positive for Panton-Valentine-leukocidin. Ned. Tijdschr. Geneeskd. 147:1076-1079. [PubMed] [Google Scholar]