Abstract
To look for a possible correlation between the virulence of Rhodococcus equi and its cytokine-inducing capacity, we evaluated intracellular survival and measured cytokine induction by mouse macrophages infected with a virulent strain containing an 85-kb plasmid and expressing VapA (103+), its avirulent plasmid-cured derivative (103−), and heat-killed 103+ (HK). After incubation with similar numbers of bacteria, macrophages infected with 103− contained significantly more organisms than those infected with 103+ or HK. The number of bacteria in the macrophages infected with 103− and HK decreased progressively, whereas the 103+ numbers remained constant over 48 h. Interleukin 1β (IL-1β), IL-6, IL-10, IL-12 p40, and tumor necrosis factor alpha (TNF-α) mRNA induction peaked at 4 h and returned to baseline between 12 and 48 h postinfection. IL-1β, IL-6, IL-10, and TNF-α concentrations assessed by enzyme-linked immunosorbent assay generally agreed well with mRNA expression; IL-12 could, however, not be detected. For all the cytokines detected, mean concentrations in the supernatants were consistently higher in the 103−-infected monolayers than in those infected with 103+, although, with the exception of IL-1β, the differences were not statistically significant. R. equi HK was a poor inducer of cytokine production. In conclusion, virulent and avirulent R. equi strains induced similar levels of cytokine synthesis. The slightly greater induction of most cytokines observed following infection with 103− is likely secondary to greater uptake by macrophages rather than to a direct role of VapA or another plasmid-encoded product in downregulating cytokine induction.
Rhodococcus equi, a gram-positive facultative intracellular pathogen of macrophages, is one of the most important causes of disease in foals between 1 and 5 months of age and has emerged as a significant opportunistic pathogen in human immunodeficiency virus-infected people (1, 10, 16). Infection in either species is most commonly characterized by a life-threatening pyogranulomatous pneumonia. Although R. equi strains isolated from the intestinal tracts and environments of horses often do not possess virulence plasmids, virulence in pneumonic foals has been associated with the presence of 85- or 90-kb plasmids which encode a highly immunogenic 15- to 17-kDa surface-expressed virulence-associated protein (VapA) (38, 40, 41, 45, 46). Plasmid-cured derivatives of R. equi lose their virulence for both mice and foals (44, 49). Plasmid-containing strains replicate efficiently in mouse and equine macrophages cultured in vitro, while plasmid-negative strains or plasmid-cured derivatives do not replicate appreciably (20, 30).
In mice, functional CD4+ T lymphocytes are required for pulmonary clearance of virulent R. equi (21). Immunocompetent BALB/c mice experimentally infected with virulent R. equi developed a Th1 cytokine response and progressively cleared the infection (22). In contrast, mice in which a Th2 cytokine response was induced by administration of monoclonal antibodies against gamma interferon (IFN-γ) failed to clear the infection and developed pulmonary granulomas (22). The cytokine environment that is present as Th cells differentiate is important in determining the subset that will subsequently develop (6). Because of its importance as an innate defense mechanism, the macrophage may be the key to the development of either a Th1 or a Th2 response by the nature of the cytokines produced shortly after infection. Interleukin 10 (IL-10), a cytokine produced mainly by monocytes/macrophages and T lymphocytes, can downregulate the progression of Th cells toward the Th1 cytokine profile (29). In contrast, IL-12, a heterodimer produced mainly by phagocytic cells, selects for the development of a Th1 response (47). Bacteria or their products can also induce macrophages to produce proinflammatory cytokines such as IL-1β, tumor necrosis factor alpha (TNF-α), and IL-6. These cytokines often play an important role in host defense against infection but may also contribute to lung pathology (34). The ability of some strains of intracellular pathogens to survive in macrophages in vitro has been associated with their capacity to induce particular cytokines (14, 24, 37).
We hypothesized that VapA or other plasmid-encoded products are important in regulating early cytokine induction by R. equi-infected macrophages. To address this hypothesis, we investigated the early events in the course of R. equi-macrophage interaction by evaluating intracellular survival and by measuring IL-1β, IL-6, IL-10, IL-12, and TNF-α induction by mouse resident peritoneal macrophages infected with a virulent strain of R. equi containing an 85-kb plasmid and expressing VapA, its avirulent plasmid-cured derivative, and heat-killed bacteria.
MATERIALS AND METHODS
Bacteria.
R. equi 103+, originally isolated from a pneumonic foal, which contains an 85-kb plasmid and produces VapA, and its plasmid-cured VapA-negative derivative (strain 103−) were used (5, 46). Strain 103+ induced severe granulomatous pneumonia in foals whereas strain 103− did not induce gross or histologic lesions and was cleared rapidly following intrabronchial challenge (15). Aliquots of the two strains were stored at −70°C. Prior to use, the aliquots were grown on Trypticase soy agar plates for 48 h at 37°C. Bacteria were harvested with 4 ml of sterile phosphate-buffered saline (PBS), pH 7.4, per plate, the optical density of the resulting suspension was read at 540 nm, and the bacterial concentration was estimated from a standard curve. The concentration of the inoculum actually derived was determined retrospectively by counting CFU. Killed R. equi (HK) was obtained by heating strain 103+ at 90°C for 45 min, and killing was confirmed by viability testing.
Macrophage culture.
Murine peritoneal macrophages were washed from the peritoneal cavities of 18- to 20-g BALB/c mice with cold PBS supplemented with penicillin G and streptomycin (100 U/ml and 80 μg/ml, respectively). The cells were centrifuged at 200 × g for 5 min, counted, suspended at 4 × 106 cells/ml in Dulbecco’s modified Eagle’s medium (DMEM) containing 2 mM glutamine and 10% fetal calf serum (FCS), and supplemented with penicillin G, streptomycin, and gentamicin (100 U/ml, 80 μg/ml, and 20 μg/ml, respectively). A 1-ml volume of the suspension was placed in each well of two-well glass chamber slides (Nunc, Naperville, Ill.), and the slides were incubated for 2 h at 37°C in a humidified atmosphere containing 7% CO2. Nonadherent cells were removed by washing the slides three times with warm DMEM-FCS, and the adherent cells were incubated overnight in antibiotic-free DMEM-FCS. Following overnight incubation and subsequent washing, approximately 2.5 × 106 cells remained attached to each coverslip. Approximately 95% of the adherent cells were macrophages as determined by Wright-Giemsa stain (Diff-Quik; Dade Diagnostics, Aquada, Puerto Rico).
In vitro infection and bacterial intracellular survival assay.
Bacterial cultures were resuspended in DMEM-FCS supplemented with 5% normal mouse serum as the complement source. The bacterial suspension containing either 103+, 103−, or HK was added to the monolayers at a multiplicity ratio of two to five bacteria per macrophage. Noninfected macrophage monolayers cultured under the same conditions were used as controls. The slides were incubated for 40 min to allow phagocytosis. The monolayers were then washed three times and incubated in DMEM-FCS supplemented with 10 μg of gentamicin per ml to kill remaining extracellular bacteria and to prevent extracellular growth with continuous reinfection of macrophages (20). At 0, 4, 12, 24, and 48 h postinfection, monolayers were fixed and stained with Wright-Giemsa stain (Diff-Quik) to enumerate R. equi organisms. The number of bacteria associated with 200 macrophages was determined. Because of the difficulty in reliably counting large numbers of bacteria in a macrophage, any cell containing 10 or more bacteria was scored as having 10 bacteria. At each time point, the number of macrophages containing ≥10 bacteria was also determined. In parallel monolayers, the supernatants were removed, centrifuged at 400 × g for 10 min, aliquoted, and stored at −70°C until used for measurement of cytokine concentrations. In these monolayers, the macrophages were used for RNA extraction. For each group (103+, 103−, HK, and negative control), three independent experiments were performed. Throughout the study, aliquots of the supernatant from each well were cultured to confirm the absence of bacteria other than R. equi.
Evaluation of endotoxin concentration.
Endotoxin concentrations of all media and reagents at the concentrations used in this study were less than the lower detectable limit of 0.5 ng/ml as determined by the Limulus amebocyte lysate assay (BioWhittaker, Walkersville, Md.).
RNA isolation and cDNA synthesis.
At the times indicated above, the monolayers were washed three times with PBS and total RNA was isolated by a modification of the single-step guanidinium thiocyanate procedure (Micro-Scale Total RNA Separator Kit; Clontech, Palo Alto, Calif.) (4).
cDNA was synthesized with the Clontech 1st-Strand cDNA synthesis kit by the protocol of the manufacturer. Briefly, 1 μg of total RNA was mixed with 1 μl of oligo(dT)18 primer (20 μM) and heated at 70°C for 2 min. After the mixture was cooled to room temperature, the following reagents were added: 4 μl of 5× reaction buffer (containing 250 mM Tris-HCl [pH 8.3], 375 mM KCl, and 15 mM MgCl2), 1 μl of deoxynucleoside triphosphates (10 mM each), 0.5 μl of RNase inhibitor (40 U/μl), and 1 μl of Moloney murine leukemia virus reverse transcriptase (200 U/μl). The mixture was incubated at 42°C for 1 h, heated at 94°C for 5 min, diluted to a final volume of 100 μl, and stored at −70°C until used for PCR analysis.
PCR analysis.
PCR primer pairs specific for mouse glyceraldehyde 3-phosphate dehydrogenase (G3PDH), IL-1β, IL-6, IL-10, and TNF-α (Clontech) and IL-12 p40 (BioSource International, Camarillo, Calif.) were purchased. cDNA as prepared above (2 μl) was amplified in a 50-μl PCR in the presence of 0.4 μM (each) primer, 0.2 mM (each) deoxynucleoside triphosphates, 5 μl of 10× reaction buffer (containing 10 mM Tris-HCl [pH 8.3] and 50 mM KCl), 1.5 mM MgCl2, and 2 U of Taq DNA polymerase (AmpliTaq; Perkin-Elmer, Branchburg, N.J.). PCR was performed with an initial denaturation step at 94°C for 2 min and with 35 cycles of amplification followed by a 7-min extension at 72°C. Each cycle included denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 2 min. Amplified PCR products were visualized by electrophoresis of 10 μl of the reaction mixture on a 1.6% agarose gel followed by ethidium bromide staining. The specificities of the amplified bands were confirmed by their predicted sizes based on a molecular weight standard. Samples without cDNA were always included in the amplification reactions to check for contamination.
Quantification of mRNA by competitive PCR.
mRNA expression of G3PDH, IL-1β, IL-6, IL-10, IL-12 p40, and TNF-α was determined quantitatively by competitive PCR. Equal amounts of cDNA were amplified in the presence of 2 μl of fourfold serially diluted nonhomologous DNA fragments (Mimic) competing for the same primers (39, 48). Mimics for G3PDH, IL-1β, IL-6, and TNF-α were purchased (Clontech) or prepared (IL-10 and IL-12 p40) with the PCR Mimic construction kit (Clontech). Following gel electrophoresis and ethidium bromide staining, densitometric analysis of the bands corresponding to the target and the competitor product was performed with a gel video system (Molecular Analyst; Bio-Rad, Hercules, Calif.). The densitometric analysis results were used to plot a standard curve from which the amount of target cDNA was determined. To account for variation in the amount of starting material, all the results were corrected to the mean G3PDH value.
Measurement of IL-1β, IL-6, IL-10, IL-12, and TNF-α concentrations.
Cytokine concentrations in the supernatants of macrophage monolayers were measured with enzyme-linked immunosorbent assay kits (BioSource International). Each sample was assayed in duplicate. The lower detection limits were 7, 8, 13, 2, and 3 pg/ml for IL-1β, IL-6, IL-10, IL-12, and TNF-α, respectively. The IL-12 assay recognizes both natural IL-12 and the free p40 subunit.
Statistical analysis.
Significant differences in bacterial numbers between monolayers infected with 103+ and those infected with 103− were determined at each time point by the two-tailed Student t test. Significant differences in cytokine production among the four different bacterial groups (103+, 103−, HK, and negative control) over a 48-h period were tested for each cytokine by use of a two-way analysis of variance with bacterial groups and time as factors. A significance value of ≤0.05 was used.
RESULTS
Differences in uptake and intracellular survival between virulent and avirulent R. equi.
The virulent R. equi strain 103+, its plasmid-cured derivative 103−, and heat-killed 103+ (HK) were used to infect BALB/c mouse resident peritoneal macrophages with a multiplicity ratio of approximately three bacteria per macrophage. Following 40 min of incubation with R. equi and subsequent washing (time zero), approximately 60% of the macrophages in the monolayers were infected with averages of nearly four, six, and two bacteria per infected cell for 103+, 103−, and HK, respectively. At that time, 10 μg of gentamicin per ml was added to the cell culture medium to kill remaining extracellular bacteria. At various times following infection, the number of bacteria associated with 200 macrophages was counted. R. equi cells were seen as pleomorphic coccobacilli enclosed by a clear zone in the cytoplasm, presumably a phagocytic vacuole. At time zero, the macrophages infected with 103− contained significantly more organisms than those infected with either 103+ or HK (Fig. 1A). The numbers of bacteria in the macrophages infected with 103− and HK decreased progressively, whereas 103+ numbers remained constant over 48 h. There were no differences in the morphologies of macrophages infected with either 103+ or 103− at any time.
FIG. 1.
Uptake and intracellular survival of R. equi within murine resident peritoneal macrophages. (A) The number of bacteria associated with 200 macrophages was determined at 0, 4, 12, 24, and 48 h by visual counting, with light microscopy. Macrophages containing 10 or more bacteria were scored as having 10 organisms. (B) The number of macrophages containing 10 or more bacteria (200 macrophages were counted) was recorded at various times postinfection. Values are shown as the means ± standard errors of the means of three independent experiments. ∗, P ≤ 0.05 compared with results for 103+.
Bacterial quantification was hampered because intracellular R. equi grew in clusters, making the bacteria difficult to count precisely. Therefore, heavily infected cells were scored as having 10 bacteria even though the actual number of organisms was likely much higher. For this reason, the number of macrophages containing 10 or more bacteria was also counted at each time. At 0, 4, and 12 h postinfection, the number of macrophages containing ≥10 bacteria was significantly higher for the 103−-infected monolayers than for those infected with either 103+ or HK (Fig. 1B). The numbers of macrophages containing ≥10 103− or HK bacteria decreased progressively, whereas the numbers remained constant in the 103+-infected cells. The greater number of bacteria and heavily infected cells at time zero in the monolayers infected with 103− was the result of enhanced uptake of 103− since there were no significant differences in the numbers of viable 103+ and 103− bacteria used for infection.
Induction of cytokine mRNA expression in macrophages infected with virulent and avirulent R. equi.
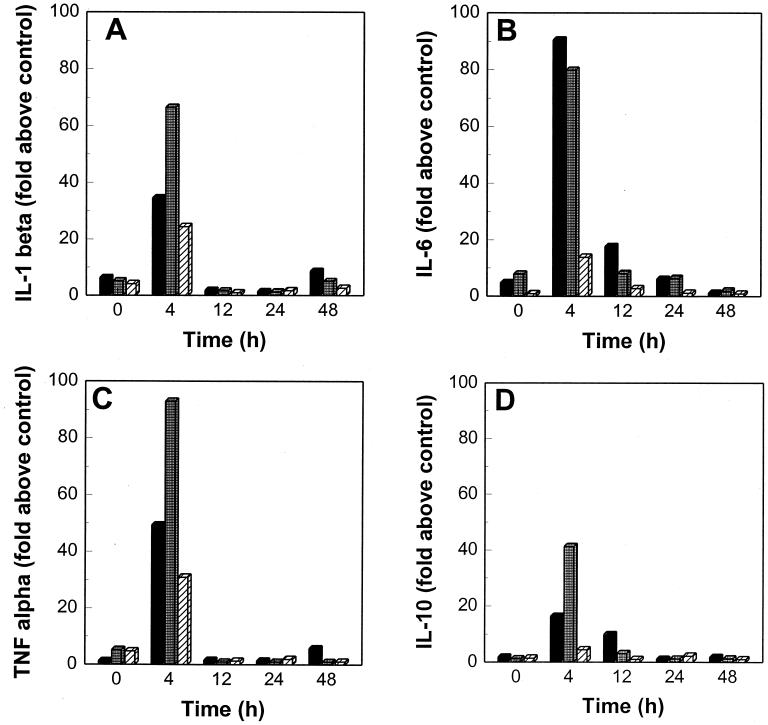
To investigate the role of macrophages in the induction of inflammation and regulation of immune response in rhodococcal infections, we infected BALB/c mouse resident peritoneal macrophages with the R. equi strains and quantitated mRNA levels at 0, 4, 12, 24, and 48 h postinfection by competitive reverse transcriptase PCR. Low concentrations of IL-1β, IL-6, IL-10, IL-12 p40, and TNF-α mRNA were detected in noninfected control macrophages at all times, suggesting that glass adherence itself or other factors present in the supernatant stimulated low levels of cytokine mRNA expression. Therefore, the results were expressed as the fold of cytokine mRNA expression above that of noninfected cells. Cytokine mRNA expression at each time was measured from a pool of macrophages obtained from two independent experiments. IL-1β, IL-6, IL-10, IL-12 p40, and TNF-α mRNA induction peaked at 4 h and returned to baseline between 12 and 48 h postinfection (Fig. 2). Monolayers infected with 103− induced approximately twice as much IL-1β, TNF-α and IL-10 as those infected with 103+ (Fig. 2A, C, and D). IL-6 and IL-12 p40 mRNA expression was slightly greater in the 103+-infected monolayers (Fig. 2B and E). For all the cytokines evaluated, HK induced considerably less mRNA expression than did live bacteria.
FIG. 2.
IL-1β, IL-6, IL-10, IL-12 p40, and TNF-α mRNA expression at various time points in murine resident peritoneal macrophages infected in vitro with R. equi 103+ (solid bars), 103− (shaded bars), and HK (striped bars). mRNA expression was quantified by competitive reverse transcriptase PCR. The results are expressed as the fold of cytokine mRNA expression above that of noninfected cells cultured under the same conditions. The results shown were measured from a pool of macrophages obtained from two independent experiments.
Cytokine concentrations in the culture supernatants of macrophages infected with virulent and avirulent R. equi.
To confirm that cytokine mRNA expression induced by virulent and avirulent strains of R. equi reflected cytokine release, we measured concentrations of IL-1β, IL-6, IL-10, IL-12, and TNF-α in the supernatants of the same R. equi-infected monolayers as those used for cytokine mRNA quantification. Cytokine concentrations in the supernatants of noninfected monolayers cultured under the same conditions were used to establish basal cytokine production.
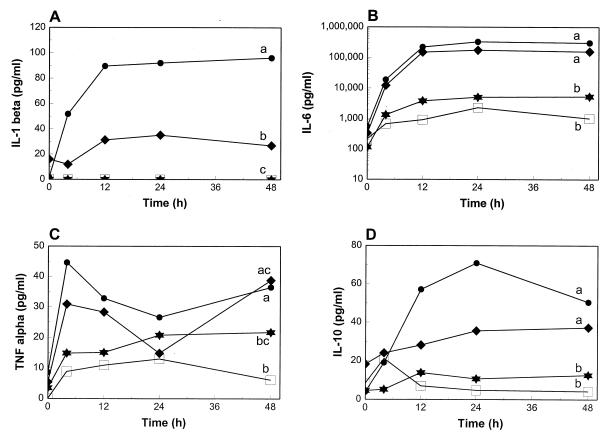
IL-1β production over a 48-h period was significantly greater in the monolayers infected with 103− than in those infected with 103+ (Fig. 3A). Although HK-infected monolayers induced 25 times more IL-1β mRNA expression than noninfected cells, this cytokine could not be detected in the supernatants of either group. Mean IL-6, IL-10, and TNF-α concentrations were higher in the supernatants of monolayers infected with 103− than in those from monolayers infected with 103+, although differences over the 48-h period were not statistically significant (Fig. 3B, C, and D). 103+- and 103−-infected monolayers produced significantly more IL-1β, IL-6, IL-10, and TNF-α than noninfected cells and more IL-1β, IL-6, and IL-10 than monolayers infected with HK. The difference in TNF-α production between 103+- and HK-infected monolayers was not statistically significant. Although mean IL-6, IL-10, and TNF-α concentrations were consistently higher in the HK-infected monolayers than in noninfected cells, the differences were not statistically significant. IL-12 or IL-12 p40 could not be detected in the supernatants even though 103+ and 103− induced up to 39-fold IL-12 p40 mRNA expression. For all the other cytokines measured, however, there was concordance between mRNA expression and the amount of cytokine in the supernatant.
FIG. 3.
IL-1β, IL-6, IL-10, and TNF-α concentrations at various time points in the supernatants of murine resident peritoneal macrophages infected in vitro with R. equi 103+ (diamonds), 103− (circles), and HK (stars). Noninfected macrophages cultured under the same conditions (open squares) were used as a baseline for cytokine production. Values are shown as the means of three independent experiments. Letters differing between two groups indicate a statistically significant difference in cytokine production over a 48-h period (P ≤ 0.05). When at least one letter is common between two groups, the difference is not statistically significant.
DISCUSSION
It was shown here for the first time that both a virulent VapA+ strain of R. equi and its avirulent plasmid-cured derivative induce expression of IL-1β, IL-6, IL-10, IL-12 p40, and TNF-α mRNA by resident macrophages. In order to determine if differences in cytokine induction between virulent and avirulent strains could be explained by variations in the extent of phagocytosis, uptake and intracellular survival of the two strains were evaluated with a resident macrophage population. Hondalus and Mosser (20) recently demonstrated the similarity of R. equi intracellular survival between BALB/c mouse peritoneal macrophages and equine alveolar macrophages, supporting the validity of the murine system as an in vitro model for studying early R. equi-macrophage interactions. Because of errors in using CFU to evaluate R. equi killing by macrophages (20), we evaluated intracellular survival by counting morphologically intact bacteria after methanol fixation and Wright-Giemsa staining. Although this technique did not allow us to differentiate between intra- and extracellularly located bacteria, it has been shown that the majority of R. equi bacteria that are cell associated under light or immunofluorescence microscopy are intracellular (20, 51).
The avirulent plasmid-cured derivative was phagocytized to a greater extent than the parent strain but was progressively cleared by macrophages. In contrast, bacterial numbers in the monolayers infected with the parent strain remained constant over the 48-h period (Fig. 1). These results differ from those of Hondalus and Mosser (20, 30), who found that virulent, plasmid-containing strains replicated extensively in mouse peritoneal macrophages, whereas plasmid-negative or plasmid-cured strains persisted but did not replicate. Differences in extents of phagocytosis between plasmid-positive strains and plasmid-cured derivatives were not reported (20, 30). Our results are, however, similar to those of Takai et al. (43), who found that mouse virulent strains were somewhat resistant to phagocytosis, resisted intracellular killing, and failed to multiply significantly. In contrast, the avirulent strains were phagocytized to a greater extent but were progressively cleared by mouse macrophages (43). In mice, functional T lymphocytes are absolutely required for in vivo clearance of R. equi (32). T-lymphocyte-deficient athymic nude mice developed severe pulmonary granulomas after experimental infection with virulent R. equi, whereas the plasmid-cured derivative bacteria failed to induce granulomas and were totally cleared from the lungs within 1 week of infection (26). Immunocompetent BALB/c mice seroconverted following infection with virulent R. equi, but no antibody response could be detected after challenge with the plasmid-cured derivative (42). These results suggest that clearance of avirulent plasmid-negative strains in mice does not require functional T lymphocytes and depends mainly on innate defense mechanisms such as macrophages. Taken together, these in vivo studies support in vitro findings reported here that the plasmid-cured derivatives are progressively cleared by macrophages rather than being able to persist intracellularly.
Optimal adherence of R. equi to macrophages in vitro requires host complement, and binding has been shown to be mediated exclusively by Mac-1, the macrophage complement receptor type 3 (19). However, others have found that adherence to macrophages or HeLa cells correlates with hemagglutination and hydrophobicity (2, 5, 13). In one study, strain 103− was found to associate better with HeLa cells than its parent strain, presumably due to a more hydrophobic surface (5). The greater hydrophobicity of this strain may have contributed to the greater uptake observed in this study. Our results and those of others show that the virulence plasmid is required for survival of R. equi in macrophages and perhaps also may contribute to reducing phagocytosis. However, because we have not excluded a chromosomal mutation in the plasmid-cured derivatives, definitive proof awaits reintroduction of the plasmid into plasmid-cured derivatives with full restoration of virulence and intracellular survival.
Macrophages infected with the avirulent strain induced almost twice as much IL-1β, TNF-α, and IL-10 but slightly less IL-6 and IL-12 p40 mRNA expression compared with those infected with the parent strain (Fig. 2). In most cases, mRNA expression concurred well with cytokine production and reflected the early bacterial numbers associated with macrophages. Mean concentrations in the supernatants for all the cytokines detected were also consistently higher in the 103−-infected monolayers, although with the exception of IL-1β, the differences were not statistically significant (Fig. 3). Cytokine mRNA induction occurred maximally at 4 h postinfection and declined markedly by 12 h, whereas the cytokines persisted in the supernatant over 48 h. The greater induction of cytokines following infection with 103− is likely secondary to the greater uptake of this strain by macrophages (Fig. 1). VapA has recently been found to be lipid modified, giving it hydrophobic properties (46). Lipoproteins from various bacterial species can induce cytokine release by macrophages (18). The present study shows that VapA or other plasmid-encoded products are, however, not required for cytokine induction in the early phase of R. equi-macrophage interactions in mice. Other components such as capsular polysaccharide, peptidoglycan, lipoglycans, teichoic and lipoteichoic acids, or extracellular products must be responsible for this cytokine induction.
Activation of resident macrophages is one of the earliest events in the cellular host response to microbial invasion, and macrophage-derived cytokines play a key role in regulation of the immune response as well as initiation and amplification of the inflammatory process. TNF-α has been shown to play a fundamental role in the microbicidal activity against various organisms (12, 25). TNF-α also mediates granuloma formation in mycobacterial inflammation (23). In mice, administration of anti-TNF-α antibody inhibited the formation of Mycobacterium bovis BCG-induced granulomas, an effect associated with a decrease in antimycobacterial activity (23). The role of TNF-α in induction of pulmonary granulomas in R. equi infections has never been addressed, but treatment of immunocompetent mice with anti-TNF-α antibody significantly increased bacterial numbers in tissues following intravenous challenge with R. equi (31). The capacity of virulent Mycobacterium avium isolates to grow well in murine macrophages resides either in their ability to downregulate secretion of TNF-α (14, 37) or in their ability to upregulate soluble p75 TNF receptor (11). Our results demonstrate that, as opposed to that of M. avium, the capacity of R. equi to survive in murine macrophages is not related to early TNF-α production. However, concentrations of soluble TNF receptors were not addressed in the present study. IL-1 has also been shown to play an essential role in the early resistance against various intracellular bacterial pathogens (8, 25), but our results also show no correlation between IL-1β production and intracellular survival. However, IL-1 activity may also depend on soluble IL-1 receptor and IL-1 receptor antagonist concentrations. The role of IL-6 during infection by intracellular pathogens is more controversial and may be pathogen specific. In vitro, IL-6 enhanced the bactericidal activity of murine macrophages infected with Listeria monocytogenes (7). However, IL-6 acted as a growth factor for intracellular survival of M. avium in vitro and impaired the ability of human macrophages to respond to stimulation with recombinant TNF-α (9). In our study, IL-6, a potent hepatocyte-stimulating factor and a key player in the acute-phase response, was produced in very high concentrations in the monolayers infected with viable bacteria. High IL-6 concentrations, if also present in vivo, may contribute to the prominent and consistent rise in levels of acute-phase proteins such as fibrinogen during rhodococcal infections in foals.
The role of IL-10 in antibacterial resistance is also complex and perhaps influenced by the timing of its production. In vitro evidences indicate that IL-10 downregulates the progression of Th cells toward the Th1 cytokine profile (29). Therefore, IL-10 decreases the resistance of mice to various intracellular pathogens (29). Paradoxically, administration of anti-IL-10 antibody to mice just before challenge with L. monocytogenes enhanced resistance to infection whereas administration 1 to 3 days after infection impaired resistance (50). Our results demonstrate no relation between IL-10 production and intracellular survival. IL-12 is a heterodimer composed of two covalently linked chains, a heavy chain or p40 and a light chain or p35. The message for the IL-12 p40 gene is expressed only in the cell types producing the biologically active heterodimer whereas the message for the p35 subunit is expressed, often constitutively, in almost every cell type (47). In response to IL-12, IFN-γ is rapidly produced first by NK cells and then by T cells. The IL-12-induced IFN-γ enhances phagocytosis and bactericidal activity by phagocytic cells (47). In this study, R. equi induced IL-12 p40 mRNA expression, but IL-12 concentrations in the supernatant, if present, were below the limit of detection. In view of the central role of IL-12 in selecting for the development of a Th1 response, it was perhaps surprising that none was detected in the supernatant of macrophages. BALB/c mice have been previously shown to develop a Th1-based immunity to R. equi, even though BALB/c mice are recognized to be generally deficient in their ability to generate a Th1 response to intracellular pathogens (17, 27).
We found that R. equi HK is a poor inducer of cytokine production by murine peritoneal macrophages compared to live bacteria. However, Pece et al. (33) found that killed R. equi induced significant TNF-α, IL-6, and IL-8 production by human peripheral blood mononuclear cells. As opposed to live bacteria, killed L. monocytogenes did not induce TNF-α or IL-6 mRNA in a murine macrophage cell line (24). Similarly, viable L. monocytogenes stimulated high levels of IL-1 release while killed bacteria did not (28). In the same study, the failure of heat-killed L. monocytogenes to induce IL-1 release was not due to heat treatment because mechanically disrupted bacteria also failed to induce IL-1 release (28). In the present study, although R. equi HK induced a 25-fold rise in IL-1β mRNA expression, this cytokine could not be detected in the supernatant, suggesting either posttranscriptional regulation or release of IL-1β concentrations below the limit of sensitivity of the test. IL-1 plays a key role in T-lymphocyte activation, and it can be hypothesized that the ineffectiveness of killed R. equi vaccine (32, 35) compared to live vaccine (3, 36) in both mice and foals may be due to insufficient production of IL-1 in the initial phase of the immune response in vivo.
This study demonstrated that live R. equi can induce resident macrophages to produce several cytokines likely to play an important role in inflammation and regulation of the immune response. The virulence of R. equi has been shown to correlate with its ability to survive in macrophages, but the virulence of R. equi did not affect early cytokine release by macrophages. Further studies are necessary to examine the role of these cytokines in the pathogenesis of and immunity to R. equi infections.
ACKNOWLEDGMENTS
This work was supported by the Natural Sciences and Engineering Research Council of Canada and by the Ontario Ministry of Agriculture, Food and Rural Affairs. S. Giguère was supported in part by the Fonds pour la formation de chercheurs et l’aide à la recherche du Québec and is the recipient of a fellowship from the Medical Research Council of Canada.
We thank Vivian Nicholson for technical assistance.
REFERENCES
- 1.Arlotti M, Zoboli G, Moscatelli G L, Magnati G, Maserati R, Borghi V, Andreoni M, Libanore M, Bonazzi L, Piscina A, Ciammarughi R. Rhodococcus equi infection in HIV-positive subjects: a retrospective analysis of 24 cases. Scand J Infect Dis. 1996;28:463–467. doi: 10.3109/00365549609037941. [DOI] [PubMed] [Google Scholar]
- 2.Bern D, Lämmler C. Relationship between haemagglutination and HeLa-cell adherence of Rhodococcus equi. J Vet Med B. 1996;43:147–153. doi: 10.1111/j.1439-0450.1996.tb00299.x. [DOI] [PubMed] [Google Scholar]
- 3.Chirino-Trejo J M, Prescott J F, Yager J A. Protection of foals against experimental Rhodococcus equi pneumonia by oral immunization. Can J Vet Res. 1987;51:444–447. [PMC free article] [PubMed] [Google Scholar]
- 4.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 5.de la Pena-Moctezuma A, Prescott J F. Association with HeLa cells by Rhodococcus equi with and without the virulence plasmid. Vet Microbiol. 1995;46:383–392. doi: 10.1016/0378-1135(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 6.Del Prete G, Romagnani S. The role of Th1 and Th2 subsets in human infectious diseases. Trends Microbiol. 1994;2:4–6. doi: 10.1016/0966-842x(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 7.Denis M. Growth of Listeria monocytogenes in murine macrophages and its modulation by cytokines; activation of bactericidal activity by interleukin-4 and interleukin-6. Can J Microbiol. 1991;37:253–257. doi: 10.1139/m91-039. [DOI] [PubMed] [Google Scholar]
- 8.Denis M, Ghadirian E. Interleukin-1 is involved in mouse resistance to Mycobacterium avium. Infect Immun. 1994;62:457–461. doi: 10.1128/iai.62.2.457-461.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denis M, Gregg E O. Recombinant tumor necrosis factor-alpha decreases whereas recombinant interleukin-6 increases growth of a virulent strain of Mycobacterium avium in human macrophages. Immunology. 1990;71:139–141. [PMC free article] [PubMed] [Google Scholar]
- 10.Donisi A, Suardi M G, Casari S, Longo M, Cadeo G P, Carosi G. Rhodococcus equi infection in HIV-infected patients. AIDS. 1996;10:359–362. doi: 10.1097/00002030-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Eriks I S, Emerson C L. Temporal effect of tumor necrosis factor alpha on murine macrophages infected with Mycobacterium avium. Infect Immun. 1997;65:2100–2106. doi: 10.1128/iai.65.6.2100-2106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 13.Fuhrmann C, Lämmler C. Adherence of haemagglutinating Rhodococcus equi to murine macrophages. Med Sci Res. 1996;24:291–293. [Google Scholar]
- 14.Furney S K, Skinner P S, Roberts A D, Appelberg R, Orme I M. Capacity of Mycobacterium avium isolates to grow well or poorly in murine macrophages resides in their ability to induce secretion of tumor necrosis factor. Infect Immun. 1992;60:4410–4413. doi: 10.1128/iai.60.10.4410-4413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giguère, S., and J. F. Prescott. Unpublished data.
- 16.Harvey R L, Sunstrum J C. Rhodococcus equi infections in patients with and without human immunodeficiency virus infection. Rev Infect Dis. 1991;13:139–145. doi: 10.1093/clinids/13.1.139. [DOI] [PubMed] [Google Scholar]
- 17.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon γ or interleukin-4 during the resolution or progression of murine leishmaniasis: evidence for the expansion of distinct T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hondalus M K, Diamond M S, Rosenthal L A, Springer T A, Mosser D M. The intracellular bacterium Rhodococcus equi requires Mac-1 to bind to mammalian cells. Infect Immun. 1993;61:2919–2929. doi: 10.1128/iai.61.7.2919-2929.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hondalus M K, Mosser D M. Survival and replication of Rhodococcus equi in macrophages. Infect Immun. 1994;62:4167–4175. doi: 10.1128/iai.62.10.4167-4175.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanaly S T, Hines S A, Palmer G H. Failure of pulmonary clearance of Rhodococcus equi infection in CD4+ T-lymphocyte-deficient transgenic mice. Infect Immun. 1993;61:4929–4932. doi: 10.1128/iai.61.11.4929-4932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanaly S T, Hines S A, Palmer G H. Cytokine modulation alters pulmonary clearance of Rhodococcus equi and development of granulomatous pneumonia. Infect Immun. 1995;63:3037–3041. doi: 10.1128/iai.63.8.3037-3041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kindler V, Sappino I P, Grau G E, Piguet P F, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn M, Goebel W. Induction of cytokines in phagocytic mammalian cells infected with virulent and avirulent Listeria strains. Infect Immun. 1994;62:348–356. doi: 10.1128/iai.62.2.348-356.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langermans J A M, van Furth R. Cytokines and the host defense against Listeria monocytogenes and Salmonella typhimurium. Biotherapy. 1994;7:169–178. doi: 10.1007/978-94-011-0233-9_4. [DOI] [PubMed] [Google Scholar]
- 26.Madarame H, Takai S, Matsumoto C, Minamiyama K, Sasaki Y, Tsubaki S, Hasegawa Y, Nakane A. Virulent and avirulent Rhodococcus equi infection in T-cell deficient athymic nude mice: pathologic, bacteriologic and immunologic responses. FEMS Immunol Med Microbiol. 1997;17:251–262. doi: 10.1111/j.1574-695X.1997.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 27.Magee D M, Cox R A. Role of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect Immun. 1995;63:3514–3519. doi: 10.1128/iai.63.9.3514-3519.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitsuyama M, Igarashi K I, Kawamura I, Ohmori T, Nomoto K. Difference in the induction of macrophage interleukin-1 production between viable and killed cells of Listeria monocytogenes. Infect Immun. 1990;58:1254–1260. doi: 10.1128/iai.58.5.1254-1260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore K W, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 30.Mosser D M, Hondalus M K. Rhodococcus equi: an emerging opportunistic pathogen. Trends Microbiol. 1996;4:29–33. doi: 10.1016/0966-842x(96)81502-2. [DOI] [PubMed] [Google Scholar]
- 31.Nordman P, Ronco E, Guenounou M. Involvement of interferon-γ and tumor necrosis factor-α in host defense against Rhodococcus equi. J Infect Dis. 1993;167:1456–1459. doi: 10.1093/infdis/167.6.1456. [DOI] [PubMed] [Google Scholar]
- 32.Nordman P, Ronco E, Nauciel C. Role of T-lymphocyte subsets in Rhodococcus equi infection. Infect Immun. 1992;60:2748–2752. doi: 10.1128/iai.60.7.2748-2752.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pece S, Giuliani G, Fumarola D, Mastroianni C M, Lichtner M, Vullo V, Antonaci S, Jirillo E. In vitro production of tumor necrosis factor-α, interleukin-6 and interleukin-8 from normal human peripheral blood mononuclear cells stimulated by Rhodococcus equi. Vet Microbiol. 1997;56:277–285. doi: 10.1016/s0378-1135(97)00096-5. [DOI] [PubMed] [Google Scholar]
- 34.Pittet J F, Mackersie R C, Martin T R, Matthay M A. Biological markers of acute lung injury: prognostic and pathogenic significance. Am J Crit Care Med. 1997;155:1187–1205. doi: 10.1164/ajrccm.155.4.9105054. [DOI] [PubMed] [Google Scholar]
- 35.Prescott J F, Markham R J F, Johnson J A. Cellular and humoral immune response of foals to vaccination with Corynebacterium equi. Can J Comp Med. 1979;43:356–364. [PMC free article] [PubMed] [Google Scholar]
- 36.Prescott J F, Patterson M C, Nicholson V M, Morein B, Yager J A. Assessment of the immunogenic potential of Rhodococcus equi virulence associated protein (VapA) in mice. Vet Microbiol. 1997;56:213–225. doi: 10.1016/s0378-1135(97)00090-4. [DOI] [PubMed] [Google Scholar]
- 37.Sarmento A M, Appelberg R. Relationship between virulence of Mycobacterium avium strains and induction of tumor necrosis factor alpha production in infected mice and in in vitro-cultured mouse macrophages. Infect Immun. 1995;63:3759–3764. doi: 10.1128/iai.63.10.3759-3764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekizaki T, Takai S, Egawa Y, Ikeda T, Ito H, Tsubaki S. Sequence of the Rhodococcus equi gene encoding the virulence-associated 15-17-kDa antigens. Gene. 1995;155:135–136. doi: 10.1016/0378-1119(95)00009-u. [DOI] [PubMed] [Google Scholar]
- 39.Siebert P D, Larrick J W. PCR MIMICs: competitive DNA fragments for use as internal standards in quantitative PCR. BioTechniques. 1993;14:244–249. [PubMed] [Google Scholar]
- 40.Takai S, Anzai T, Sasaki Y, Tsubaki S, Kamada M. Virulence of Rhodococcus equi isolated from lesions of infected foals. Bull Equine Res Inst. 1993;30:9–14. [Google Scholar]
- 41.Takai S, Iie M, Watanabe Y, Tsubaki S, Sekizaki T. Virulence-associated 15- to 17-kilodalton antigens in Rhodococcus equi: temperature-dependent expression and location of the antigens. Infect Immun. 1992;60:2995–2997. doi: 10.1128/iai.60.7.2995-2997.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takai S, Madarame H, Matsumoto C, Inoue M, Sasaki Y, Hasegawa Y, Tsubaki S, Nakane A. Pathogenesis of Rhodococcus equi infection in mice: roles of virulence plasmids and granulomagenic activity of bacteria. FEMS Immunol Med Microbiol. 1995;11:181–190. doi: 10.1111/j.1574-695X.1995.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 43.Takai S, Michizoe T, Matsumura K, Nagai M, Sato H, Tsubaki S. Correlation of in vitro properties of Rhodococcus (Corynebacterium) equi with virulence for mice. Microbiol Immunol. 1985;29:1175–1184. doi: 10.1111/j.1348-0421.1985.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 44.Takai S, Sekizaki T, Ozawa T, Sugawara T, Watanabe Y, Tsubaki S. Association between large plasmid and 15- to 17-kilodalton antigens in virulent Rhodococcus equi. Infect Immun. 1991;59:4056–4060. doi: 10.1128/iai.59.11.4056-4060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takai S, Watanabe Y, Ikeda T, Ozawa T, Matsukura S, Tamada Y, Tsubaki S, Sekizaki T. Virulence-associated plasmids in Rhodococcus equi. J Clin Microbiol. 1993;31:1726–1729. doi: 10.1128/jcm.31.7.1726-1729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan C, Prescott J F, Patterson M C, Nicholson V M. Molecular characterization of a lipid-modified virulence-associated protein of Rhodococcus equi and its potential in protective immunity. Can J Vet Res. 1995;59:51–59. [PMC free article] [PubMed] [Google Scholar]
- 47.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukocyte Biol. 1996;59:505–511. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 48.Überla K, Platzer C, Diamantstein T, Blankenstein T. Generation of competitor DNA fragments for quantitative PCR. PCR Methods Appl. 1991;1:136–139. doi: 10.1101/gr.1.2.136. [DOI] [PubMed] [Google Scholar]
- 49.Wada R, Kamada M, Anzai T, Nakanishi A, Kanemaru T, Takai S, Tsubaki S. Pathogenicity and virulence of Rhodococcus equi in foals following intratracheal challenge. Vet Microbiol. 1997;56:301–312. doi: 10.1016/s0378-1135(97)00098-9. [DOI] [PubMed] [Google Scholar]
- 50.Wagner R D, Maroushek N M, Brown J, Czuprynski C J. Treatment with anti-interleukin-10 monoclonal antibody enhances early resistance to but impairs complete clearance of Listeria monocytogenes infection in mice. Infect Immun. 1994;62:2345–2353. doi: 10.1128/iai.62.6.2345-2353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zink M C, Johnson J A, Prescott J F, Pascoe P J. The interaction of Corynebacterium equi and equine alveolar macrophages in vitro. J Reprod Fert Suppl. 1982;32:491–496. [PubMed] [Google Scholar]