Abstract
It is difficult to separate Streptococcus pneumoniae from the genotypically similar species Streptococcus mitis and Streptococcus oralis, which are commensals of the human oral cavity. A novel nucleic acid amplification technique, loop-mediated isothermal amplification (LAMP), which amplifies DNA under isothermal conditions (63°C) with high specificity, efficiency, and rapidity, was examined regarding its applicability for detecting S. pneumoniae. An S. pneumoniae-specific LAMP primer targeting the lytA gene was designed. The primer specificity was validated using 10 Streptococcus and 7 non-Streptococcus species. Within 60 min, the assay could detect 10 or more copies of purified S. pneumoniae DNA with a sensitivity 1,000 times that of conventional PCR. Clinical isolates of 21 other strains (3 S. oralis, 17 S. mitis, and 1 Streptococcus species) that harbor virulence-factor-encoding genes (lytA or ply) were tried to differentiate S. pneumoniae. The detection of S. pneumoniae in clinical isolates was more selective using the LAMP method than using conventional PCR. Therefore, LAMP appears to be a sensitive and reliable means of diagnosing S. pneumoniae infection.
Streptococcus pneumoniae is a common major human pathogen associated with community-acquired pneumonia, septicemia, meningitis, and otitis media (2). While traditional antimicrobial therapy has proven to be an effective treatment, the emergence of antimicrobial-resistant strains has resulted in an increasing number of cases (10). The isolation and identification of pneumococcus is complicated by contamination with alpha-hemolytic streptococci belonging to the normal flora. Pneumococcal detection using classical techniques, including growth-based assays, colonial morphology, optochin susceptibility, bile solubility, and serology, can be time-consuming and can produce equivocal results. Sensitive and specific assays that can be completed promptly in the clinical laboratory are essential for early diagnosis and effective antibiotic therapy. Molecular assays are inherently valuable because detection can be achieved with enhanced sensitivity and specificity and detection is not diminished with nonviable organisms.
The molecular methods that have been used to date (3, 6, 17) are expensive and complicated to perform. Varying degrees of success have been realized using PCR-based assays for detecting S. pneumoniae with primers specific to repetitive regions or to genes encoding rRNA (7, 9, 12), pneumolysin (18, 19, 23), or autolysin (5, 14, 18). Autolysin and pneumolysin, which are encoded by the lytA and ply genes, respectively, represent potential targets for the specific detection of S. pneumoniae. Both genes are essential for pathogenesis and are well-characterized virulence markers (1). The restricted allelic variation of lytA (4, 21) makes this gene an ideal target for specific identification in clinical and epidemiological studies. For example, the gene may allow the differentiation of S. pneumoniae from the genotypically similar species Streptococcus mitis and Streptococcus oralis, which are commensals of the human oral cavity (11).
The application of this strategy was complicated by the recent report of organisms that are genetically and phenotypically related to S. mitis yet harbor pneumolysin and autolysin genes (22), which were previously thought to be associated with pneumococci. This could result in the possible overestimation of S. pneumoniae (20). However, there are several variations in the autolysin gene sequences of nonpneumococcal organisms compared to the sequences of S. pneumoniae strains. Clarifying and using the consensus sequence for S. pneumoniae strains could lead to the more highly specific and sufficiently reliable molecular diagnosis of S. pneumoniae infection.
In seeking such diagnostic specificity, we investigated the applicability of a novel nucleic acid amplification method, loop-mediated isothermal amplification (LAMP) (16). LAMP amplifies DNA with high specificity, efficiency, and speed under isothermal conditions. The LAMP reaction requires a DNA polymerase with strand displacement activity and a set of four specially designed inner and outer primers that recognize six distinct sequences on the target DNA. Only simple, cost-effective equipment amenable for use in hospital laboratories is required. This method also exhibits extremely high amplification efficiency, owing, in part, to its isothermal nature; no time is lost as a result of changes in temperature, and the reaction can be conducted at the optimal temperature for enzyme function. Moreover, the inhibition reaction that occurs at later stages of amplification, which typically confounds PCR, is less likely to occur. Therefore, LAMP constitutes a potentially valuable tool for the rapid diagnosis of infectious diseases, such as those caused by S. pneumoniae, in commercial or hospital laboratories.
Our goal was to establish a highly sensitive and species-specific LAMP-based S. pneumoniae DNA amplification method and to examine its reliability in discriminating among species.
MATERIALS AND METHODS
Preparation of chromosomal DNA.
Genomic DNA was extracted using Dr. GenTLE for Yeast (TaKaRa Bio Inc., Tokyo, Japan) and was purified using a QIAamp DNA mini kit (QIAGEN Inc., Valencia, Calif.) in accordance with the manufacturers' instructions. The genomic DNA used to evaluate primer specificity was prepared from 32 strains representing 10 Streptococcus species and 7 non-Streptococcus species (Table 1). For the sensitivity study, purified DNA from S. pneumoniae ATCC 6305 was isolated as described above and quantified using an Ultrospec 3300 Pro spectrophotometer (Amersham Pharmacia Biotech, Cambridge, United Kingdom). The number of genomic copies for the LAMP mixture was calculated by assuming a molecular size of 2 Mbp.
TABLE 1.
Specificity of the LAMP primers used to identify S. pneumoniae
| Bacterial strain | Amplification by LAMP primersa |
|---|---|
| Streptococcus mitis ATCC 903b | − |
| Streptococcus oralis ATCC 9811b | − |
| Streptococcus oralis ATCC 10557b | − |
| Streptococcus gordonii ATCC 12396 | − |
| Streptococcus agalactiae IID1625 | − |
| Streptococcus milleri NCTC10703 | − |
| Streptococcus sobrinus NIDR6715b | − |
| Streptococcus sobrinus OMZ176 | − |
| Streptococcus mutans XC47 | − |
| Streptococcus mutans PK1b | − |
| Streptococcus mutans JC2b | − |
| Streptococcus sanguinis ATCC 10556b | − |
| Streptococcus salivarius ATCC 7073b | − |
| Streptococcus salivarius ATCC 9222b | − |
| Streptococcus salivarius HHTb | − |
| Streptococcus pneumoniae R6 | + |
| Streptococcus pneumoniae ATCC 6305 | + |
| Streptococcus pneumoniae GTC261 (NCTC7465)c | + |
| Streptococcus pneumoniae IID553 (NYSDH DP-2)d | + |
| Streptococcus pneumoniae IID554 (NYSDH DP-3, -5A)d | + |
| Haemophilus influenzae RD | − |
| Escherichia coli DH5-α | − |
| Actinobacillus actinomycetemcomitans Y-4 | − |
| Porphyromonas gingivalis ATCC 33277 | − |
| Porphyromonas gingivalis 381b | − |
| Porphyromonas gingivalis ATCC 49417b | − |
| Actinomyces naeslundii ATCC 12104b | − |
| Actinomyces naeslundii T14b | − |
| Actinomyces naeslundii WVU627b | − |
| Prevotella intermedia ATCC 25611b | − |
| Prevotella nigrescens ATCC 25261b | − |
| Prevotella nigrescens ATCC 33563b | − |
+, amplification was seen after a 35-min incubation; −, amplification was not seen after a 60-min incubation.
Source: Department of Bacteriology, Nihon University School of Dentistry.
Source: Department of Microbiology, Gifu University School of Medicine.
Source: Institute of Medical Science, The University of Tokyo.
Primer design.
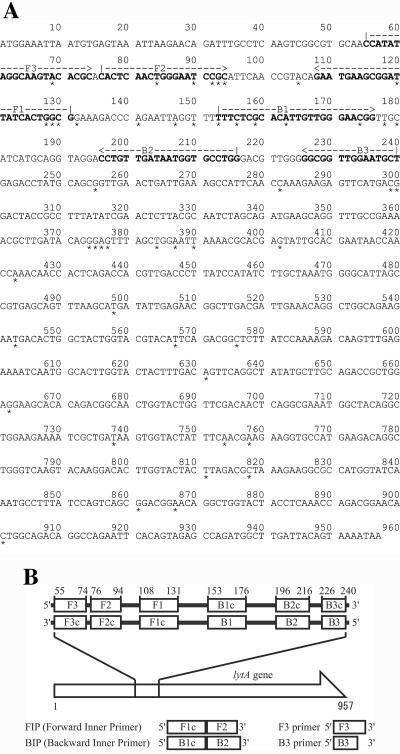
Four S. pneumoniae-specific LAMP primers were designed from the published sequence of strain R6 (GenBank accession number AE008540) by using the LAMP primer support software program (Net Laboratory, Kanagawa, Japan). We compared the lytA sequences of four S. pneumoniae strains (GenBank accession numbers AE008540, AE007483, M13812, and AF467249) and nine other organisms that harbored the autolysin gene, namely, Streptococcus mitis lytA (EMBL accession numbers AJ617815 and AJ617816) and Streptococcus species lytA (EMBL accession numbers AJ252190, AJ252191, AJ252192, AJ252193, AJ252194, AJ252195, and AJ252196). The consensus sequence among the S. pneumoniae strains was elucidated (Fig. 1), and S. pneumoniae-specific LAMP primers were designed using the alignment analysis. These comprised the outer primers (F3 and B3), a forward inner primer, and a backward inner primer (Fig. 1).
FIG. 1.
(A) Nucleotide sequences of S. pneumoniae R6 autolysin gene used for LAMP primer. The sequences used for LAMP primers are shown in boldface. *, consensus sequence among S. pneumoniae strains. (B) Schematic representation of primers used in this study.
LAMP reaction.
Twenty-five microliters of the reaction mixture contained 1.6 μM each of forward inner primer and backward inner primer, 0.2 μM each of F3 and B3, 8 U of the Bst DNA polymerase large fragment (New England Biolabs Inc., Beverly, Mass.), 1.4 mM concentrations of deoxynucleoside triphosphates, 0.8 M betaine, 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 8 mM MgSO4, 0.1% Tween 20, and template DNA (2 μl). The mixture was incubated at 63°C for 35 or 60 min and then heated at more than 80°C for 2 min to terminate the reaction.
Analysis of LAMP products.
The LAMP reaction causes turbidity in the reaction tube proportional to the amount of amplified DNA. Therefore, we observed the turbidity in the reaction tube with the naked eye. For further confirmation, the amplified products were also detected using electrophoresis in 3% agarose gels, followed by ethidium bromide staining. To confirm the sensitivity and the possibility of real-time LAMP quantification of S. pneumoniae, a Loopamp real-time turbidimeter (LA-200; Teramecs, Kyoto, Japan) was used.
To confirm the structures of the amplified products, some of the amplified products were digested with the restriction enzyme TasI (Fermentas Inc., Hanover, Md.) and their sizes were analyzed by electrophoresis in 3% agarose gels, followed by staining with ethidium bromide. Furthermore, to confirm the structure of the amplified LAMP products, the amplified products were sequenced using a BigDye Terminator V3.1 cycle sequencing kit (Applied Biosystems, Foster City, Calif.) and an ABI PRISM 377 DNA sequencer (Applied Biosystems) in accordance with the manufacturers' instructions. The primers used to sequence the target region (between F1 and B1) were as follows: F2 primer, 5′-CACTCAACTGGGAATCCGC-3′, and B2 primer, 5′-CCAGGCACCATTATCAACAGG-3′ (Fig. 1).
PCR assay.
Template DNA from clinical isolates was used at identical concentrations for the LAMP and conventional PCR assays. Sensitivity was compared using autolysin (lytA)-encoding PCR primer sets (14), and selectivity was compared using autolysin (lytA)- and pneumolysin (ply)-encoding PCR primer sets (14, 19). The sequences of the PCR primers were as follows: for lytA, 5′-CAACCGTACAGAATGAAGCGG-3′ and 5′-TTATTCGTGCAATACTCGTGCG-3′, and for ply, 5′-ATTTCTGTAACAGCTACCAACGA-3′ and 5′-GAATTCCCTGTCTTTTCAAAGTC-3′.
The PCR mixture (10 μl) consisted of 0.2 mM concentrations of each deoxyribonucleoside triphosphate, 10 mM Tris-HCl buffer (pH 8.3), 50 mM KCl, 2 mM MgCl2, 1 U of Ex Taq DNA polymerase (TaKaRa Bio Inc.), 0.5 μM concentrations of each primer, and 1 μl of template DNA. PCR was performed with a thermal cycler (MJ Research, Waltham, Mass.) for 30 cycles. Each cycle consisted of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C for the ply gene and 15 s at 94°C, 15 s at 53°C, and 15 s at 72°C for the lytA gene. Samples (2 μl) of the PCR amplification products were subjected to electrophoresis on a 3% agarose gel.
Clinical isolates with ply or lytA genes.
From 15 oral mucosa swab samples of healthy children (5 to 6 years old), we isolated 25 alpha-hemolytic streptococci in which genes encoding pneumolysin (ply) or autolysin (lytA) were detected using conventional PCR with the primers described above (14, 19). The identification of these isolates involved standard criteria, as follows. The optochin sensitivity test used a 6.5-mm-diameter disk containing 5 μg of optochin (Eiken Chemical Co., Ltd., Tochigi, Japan) in an atmosphere of 5% CO2. The absence of an inhibition zone at least 13 mm in diameter was interpreted as a negative result, whereas a zone of inhibition at least 13 mm in diameter constituted a positive result. Bile solubility was tested, as previously described (8). A commercial system, API 20 Strep (bioMérieux, Lyon, France), was used according to the manufacturer's instructions.
RESULTS
The LAMP assay successfully amplified the 186-bp target sequence of S. pneumoniae lytA at 63°C in 60 min (Tables 1 and 2). The product was evident upon agarose gel electrophoresis (Fig. 2) as a ladder-like pattern on the gel, which is characteristic of the LAMP reaction and indicates the production of stem-loop DNA with inverted repeats of the target sequence. The LAMP reaction revealed that 103 copies of S. pneumoniae could be detected in 35 min (Table 2).
TABLE 2.
Sensitivities of the LAMP and PCR assays for S. pneumoniae ATCC 6305
| Assay | Resultsa for no. of copies:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1,000,000 | 100,000 | 10,000 | 1,000 | 100 | 10 | 1 | 0 | |
| PCRb | + | + | + | − | − | − | − | − |
| LAMPc | ||||||||
| 35 min | + | + | + | + | − | − | − | − |
| 60 min | + | + | + | + | + | + | − | − |
+, amplification occurred; −, amplification did not occur.
Results were obtained using electrophoretic analysis.
Results were determined by eye.
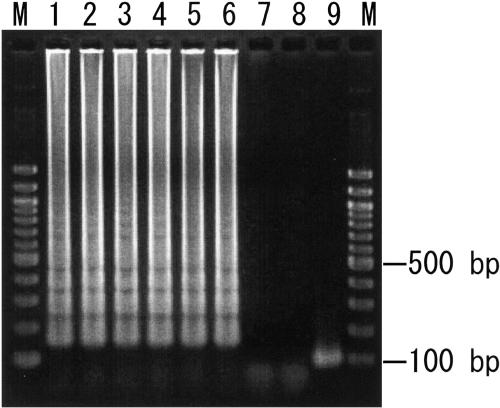
FIG. 2.
Sensitivities of electrophoretic analysis of LAMP-amplified products. Lanes M, 100-bp ladder used as a size marker; lane 1, 106 copies of genomic DNA from S. pneumoniae ATCC 6305; lane 2, 105 copies; lane 3, 104 copies; lane 4, 103 copies; lane 5, 102 copies; lane 6, 10 copies; lane 7, 1 copy; lane 8, no template; lane 9, LAMP product from lane 1 after TasI digestion. The digested fragments were 102 and 111 bp.
To ascertain the detection limit of the LAMP assay for S. pneumoniae, serial 10-fold dilutions of genomic DNA were tested and the results were compared with those of conventional PCR. The detection limits for the LAMP assay and PCR were 10 and 10,000 copies, respectively (Table 2 and Fig. 2). Therefore, the sensitivity of the LAMP assay was 1,000 times that of conventional PCR. No amplification was apparent when the sample tube did not contain target DNA.
To evaluate the species specificity of the LAMP primers, we tested 10 Streptococcus species and 7 non-Streptococcus species (Table 1). Significant amplification of the DNA originating from S. pneumoniae was observed after a 35-min incubation. By contrast, the other strains were not amplified, even after a 60-min incubation. The specificity of the amplification was confirmed by TasI digestion to ensure that the amplification product had sequences corresponding to the selected target. The resulting digestion products were 102 and 111 bp, in good agreement with the predicted size (Fig. 2). The structures of the amplified products were confirmed by sequencing, and the obtained sequences were compared to sequences in the targeted region in the lytA gene (bases 132 to 152 in the original sequence) of S. pneumoniae strains (between F1 and B1) (Fig. 1). These results matched the expected nucleotide sequences perfectly (data not shown).
Previously, we isolated 25 alpha-hemolytic streptococci in which genes encoding pneumolysin (ply) or autolysin (lytA) were detected using conventional PCR. Using three standard tests (the optochin sensitivity test, the bile solubility test, and API 20 Strep [bioMérieux]), we identified 4 S. pneumoniae strains and 21 atypical strains, including 3 S. oralis, 17 S. mitis, and 1 streptococcus species (Table 3). To confirm its ability to evaluate clinical isolates, the LAMP assay was used to screen these 25 isolates (Table 3). The concentration of genomic DNA in each isolate was 106 copies per assay mixture, allowing direct comparison of the results of the PCR and LAMP assays. LAMP detected 4 positive isolates and 21 negative isolates; PCR detected 8 positive isolates and 17 negative isolates for the lytA gene and 21 positive isolates and 4 negative isolates for ply gene. Moreover, the LAMP assay detected the 4 S. pneumoniae isolates and none of the 21 other isolates while PCR produced 4 false-positive results for lytA and 17 false-positive results for ply.
TABLE 3.
Detection of Streptococcus pneumoniae in clinical isolates of oral streptococci by PCR and LAMP methods
| Isolate no. | Results of:
|
Identificationd | Results ofe:
|
||||
|---|---|---|---|---|---|---|---|
| API testa | Optochin testb | Bile testc | PCR
|
LAMP | |||
| lytA | ply | ||||||
| 1 | S. oralis | − | + | S. oralis | − | + | − |
| 2 | S. mitis | − | + | S. mitis | + | − | − |
| 3 | S. mitis | − | + | S. mitis | − | + | − |
| 4 | S. mitis | − | + | S. mitis | − | + | − |
| 5 | S. mitis | − | + | S. mitis | − | + | − |
| 6 | S. oralis | − | + | S. oralis | − | + | − |
| 7 | S. mitis | − | + | S. mitis | − | + | − |
| 8 | S. mitis | − | + | S. mitis | − | + | − |
| 9 | S. mitis | − | + | S. mitis | − | + | − |
| 10 | S. mitis | − | − | S. mitis | − | + | − |
| 11 | S. mitis | − | − | S. mitis | − | + | − |
| 12 | S. pneumoniae | + | + | S. pneumoniae | + | + | + |
| 13 | S. pneumoniae | + | + | S. pneumoniae | + | + | + |
| 14 | S. pneumoniae | + | + | S. pneumoniae | + | + | + |
| 15 | Not identified | − | − | Streptococcus species | + | − | − |
| 16 | S. mitis | − | − | S. mitis | − | + | − |
| 17 | S. mitis | − | + | S. mitis | − | + | − |
| 18 | S. oralis | − | − | S. oralis | − | + | − |
| 19 | S. pneumoniae | + | + | S. pneumoniae | + | + | + |
| 20 | S. mitis | − | − | S. mitis | + | − | − |
| 21 | S. mitis | − | + | S. mitis | − | + | − |
| 22 | S. mitis | − | + | S. mitis | − | + | − |
| 23 | S. mitis | − | + | S. mitis | − | + | − |
| 24 | S. mitis | − | + | S. mitis | − | + | − |
| 25 | S. mitis | − | − | S. mitis | + | − | − |
Classification based on the API 20 Strep test.
+, optochin sensitive; −, optochin resistant.
+, bile soluble; −, bile insoluble.
Identification via consideration of all three conventional identification methods: API 20 Strep testing, optochin sensitivity, and bile solubility.
+, amplification occurred; −, amplification did not occur.
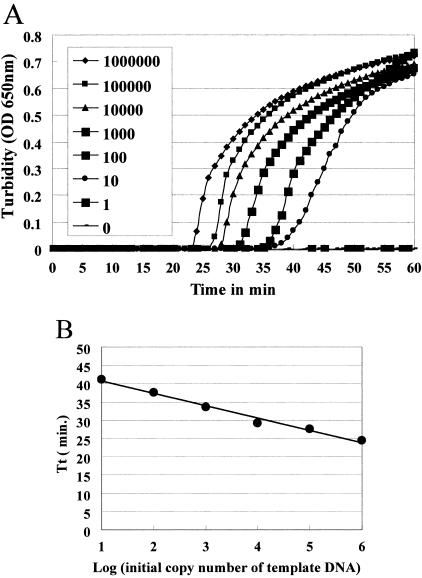
Figure 3A shows the results of real-time turbidity measurements in the LAMP reaction solutions containing 10 to 106 copies of S. pneumoniae template DNA in one reaction tube. The sensitivity of detection using a Loopamp real-time turbidimeter was the same as that using the naked eye (Table 2) or electrophoresis (Fig. 2). An increase in the quantity of initial template DNA was observed to shorten the threshold time. A plot of the amplification time required to exceed a turbidity level of 0.1 (threshold time) versus the log of the initial template DNA (Fig. 3B) showed a linear relationship, with a good correlation coefficient (r2 = 0.986).
FIG. 3.
(A) Real-time sensitivity of S. pneumoniae LAMP, as monitored by the measurement of turbidity. Shown from left to right in the figure are the curves of decreasing concentrations (1,000,000 to 1) of bacteria. The detection limit was 10 copies. (B) The relation between the threshold times (Tt) of each sample and the log of the amount of initial template DNA.
DISCUSSION
S. mitis and S. oralis are considered commensals of the human oral cavity. They are the most closely related species to S. pneumoniae on this ecological basis, and their 16S rRNA sequences share over 99% identity with S. pneumoniae. This similarity makes it a challenge for clinical laboratories to differentiate between S. pneumoniae and other alpha-hemolytic oral streptococci, especially S. mitis and S. oralis, as misidentification may influence diagnosis and treatment. While conventional methods allow the identification of the majority of pneumococcal isolates, observations of atypical pneumococci based on optochin susceptibility, bile solubility, and encapsulation (20) can be the source of considerable confusion in the clinical laboratory. Despite these problems, most diagnostic laboratories continue to use these conventional identification techniques.
PCR-based technology holds more promise. Concerning pneumococci, the putative virulence factors pneumolysin (ply) and autolysin (lytA) offer compellingly specific diagnostic targets. As yet, however, PCR has not established a routine foothold in clinical laboratories because it is a time-consuming complex and needs a high-precision thermal cycler. Reaction equipment that is much simpler and amenable for use in hospital laboratories is required.
By contrast, the LAMP assay reported here is advantageous owing to its simple operation, rapid reaction, and easy detection. LAMP operates under isothermal conditions at 63°C for 1 h. Therefore, no time is lost as a result of changes in temperature, as is the case with thermal cycling with PCR. Moreover, LAMP requires only simple reaction equipment; it can be performed using a regular laboratory bath or heat block that provides a constant temperature of 63°C. It is even possible to determine the reaction directly with the naked eye, without electrophoretic analysis, unlike PCR. Since the LAMP assay is simple and relatively easy to perform, even a clinical microbiological laboratory that has not performed PCR or molecular testing could introduce this technology.
In this study, of 25 clinical isolates that harbored virulence-factor-encoding genes (lytA or ply), 17 nonpneumococcal isolates were detected for the ply gene (Table 3). This result was concordant with a report of the presence of pneumolysin in viridans group streptococci which concluded that the ply gene is not specific for S. pneumoniae while the lytA gene is highly specific for S. pneumoniae (15). It was thought that lytA separated S. pneumoniae from the genotypically similar species S. mitis and S. oralis (11). Recently, however, the existence of organisms that appear to be genetically and phenotypically related to S. mitis, but which harbor the autolysin gene normally associated with pneumococci, was reported (22). In fact, we detected 4 nonpneumococcal isolates for the lytA gene out of 25 clinical isolates (Table 3). We must now consider how to differentiate S. pneumoniae from closely related alpha-hemolytic streptococci.
Fortunately, there are several variations of autolysin genes in nonpneumococcal organisms to the lytA gene sequences of S. pneumoniae type strains. Therefore, by clarifying and using these consensus genes for S. pneumoniae, a more specific and sufficiently reliable molecular diagnosis of S. pneumoniae infection should be possible. In addition, the selectivity of the LAMP reaction is extremely high because it uses four primers that recognize six distinct regions of the target DNA (16). From the alignment analysis of the lytA gene sequences among four S. pneumoniae strains and nine other streptococcus strains that harbor the autolysin gene, we were able to evaluate the lytA consensus sequence specific for S. pneumoniae strains (Fig. 1A). Then, we designed LAMP primers including these S. pneumoniae-specific consensus sequences (Fig. 1A). This may have influenced the high selectivity of the LAMP assay and might be why LAMP did not detect the four nonpneumococcal strains in which PCR detected lytA (Table 3).
As the LAMP reaction progresses, the reaction by-product pyrophosphate ions bind to magnesium ions and form a white precipitate of magnesium pyrophosphate. Therefore, the results of the LAMP reactions can be judged simply by the naked eye. This characteristic feature of the LAMP reaction can be used to detect the reaction end point simply by gauging the presence of a precipitate. In 2004, Mori et al. (13) reported that real-time turbidity measurements of the LAMP reaction by using a simple, inexpensive apparatus (Loopamp real-time turbidimeter) permits quantitative analysis of the minute amounts of nucleic acids present in a sample, with a high degree of precision, over a wide range. In this study, we also tried to use the LAMP assay to quantify template S. pneumoniae in real time by reading the optical density at 650 nm every 6 s with a Loopamp real-time turbidimeter.
The curve obtained using the real-time turbidity measurements had a high linearity (Fig. 3B). This indicated that the quantity of template DNA of an unknown concentration can be determined by comparing its threshold time with the threshold times of template DNA of known concentrations. Therefore, using the real-time turbidity monitoring system, we will be able to estimate the concentration of S. pneumoniae DNA from clinical samples. In the clinical laboratory, optochin and bile reactions can be replaced by a LAMP test. Alternatively, we can utilize this technique for direct detection from clinical specimens in order to reduce laboratory time. In this case, quantitative detection using the Loopamp real-time turbidimeter is helpful for the diagnosis of pneumococcal infection.
In conclusion, we have established a LAMP-based S. pneumoniae DNA amplification method and we report that its reliability in species discrimination is high. This assay may allow more sensitive, specific, and practical detection of S. pneumoniae than previous detection methods. LAMP is potentially useful for the reliable routine diagnosis of S. pneumoniae infections.
Acknowledgments
This investigation was supported in part by the Sato Fund from Nihon University School of Dentistry, the Nihon University Research Grant for Assistants and Young Researchers (2003), the Grant-in-Aid for Scientific Research (C) (no. 16592097), and a grant to promote multidisciplinary research projects from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
We are grateful to Muneaki Tamura, Department of Bacteriology, Nihon University School of Dentistry, and Yutaka Arimoto, Department of Pediatrics, St. Marianna University School of Medicine, for their contributions to this work. We are also thankful to Kazumitu Sano, Department of Clinical Laboratory, Nerima-Hikarigaoka Nihon University Hospital, for his technical support in this study.
REFERENCES
- 1.Berry, A. M., R. A. Lock, D. Hansman, and J. C. Paton. 1989. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect. Immun. 57:2324-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, P. D., and S. A. Lerner. 1998. Community-acquired pneumonia. Lancet 352:1295-1302. [DOI] [PubMed] [Google Scholar]
- 3.Gillespie, S. H. 1999. The role of the molecular laboratory in the investigation of Streptococcus pneumoniae infections. Semin. Respir. Infect. 14:269-275. [PubMed] [Google Scholar]
- 4.Gillespie, S. H., T. D. McHugh, H. Ayres, A. Dickens, A. Efstratiou, and G. C. Whiting. 1997. Allelic variation in Streptococcus pneumoniae autolysin (N-acetyl muramoyl-l-alanine amidase). Infect. Immun. 65:3936-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillespie, S. H., C. Ullman, M. D. Smith, and V. Emery. 1994. Detection of Streptococcus pneumoniae in sputum samples by PCR. J. Clin. Microbiol. 32:1308-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall, L. M. C. 1998. Application of molecular typing to the epidemiology of Streptococcus pneumoniae. J. Clin. Pathol. 51:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall, L. M., B. Duke, and G. Urwin. 1995. An approach to the identification of the pathogens of bacterial meningitis by the polymerase chain reaction. Eur. J. Clin. Microbiol. Infect. Dis. 14:1090-1094. [DOI] [PubMed] [Google Scholar]
- 8.Hawn, C. V. Z., and E. Beebe. 1965. Rapid method for demonstrating bile solubility of Diplococcus pneumoniae. J. Bacteriol. 90:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendolin, P. H., A. Markkanen, J. Ylikoski, and J. J. Wahlfors. 1997. Use of multiplex PCR for simultaneous detection of four bacterial species in middle ear effusions. J. Clin. Microbiol. 35:2854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacoby, G. A. 1996. Antimicrobial-resistant pathogens in the 1990s. Annu. Rev. Med. 47:169-179. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura, Y., R. A. Whiley, S. E. Shu, T. Ezaki, and J. M. Hardie. 1999. Genetic approaches to the identification of the mitis group within the genus Streptococcus. Microbiology 145:2605-2613. [DOI] [PubMed] [Google Scholar]
- 12.Lu, J.-J., C.-L. Perng, S.-Y. Lee, and C.-C. Wan. 2000. Use of PCR with universal primers and restriction endonuclease digestions for detection and identification of common bacterial pathogens in cerebrospinal fluid. J. Clin. Microbiol. 38:2076-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori, Y., M. Kitao, N. Tomita, and T. Notomi. 2004. Real-time turbidimetry of LAMP reaction for quantifying template DNA. J. Biochem. Biophys. Methods 59:145-157. [DOI] [PubMed] [Google Scholar]
- 14.Nagai, K., Y. Shibasaki, K. Hasegawa, T. A. Davies, M. R. Jacobs, K. Ubukata, and P. C. Appelbaum. 2001. Evaluation of PCR primers to screen for Streptococcus pneumoniae isolates and β-lactam resistance, and to detect common macrolide resistance determinants. J. Antimicrob. Chemother. 48:915-918. [DOI] [PubMed] [Google Scholar]
- 15.Neeleman, C., C. H. W. Klaassen, D. M. Klomberg, H. A. de Valk, and J. W. Mouton. 2004. Pneumolysin is a key factor in misidentification of macrolide-resistant Streptococcus pneumoniae and is a putative virulence factor of S. mitis and other streptococci. J. Clin. Microbiol. 42:4355-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Notomi, T., H. Okayama, H. Masubuchi, T. Yonekawa, K. Watanabe, N. Amino, and T. Hase. 2000. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olive, D. M., and P. Bean. 1999. Principles and applications of methods for DNA-based typing of microbial organisms. J. Clin. Microbiol. 37:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudolph, K. M., A. J. Parkinson, C. M. Black, and L. W. Mayer. 1993. Evaluation of polymerase chain reaction for diagnosis of pneumococcal pneumonia. J. Clin. Microbiol. 31:2661-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salo, P., Å. Örtqvist, and M. Leinonen. 1995. Diagnosis of bacteremic pneumococcal pneumonia by amplification of pneumolysin gene fragment in serum. J. Infect. Dis. 171:479-482. [DOI] [PubMed] [Google Scholar]
- 20.Wester, C. W., D. Ariga, C. Nathan, T. W. Rice, J. Pulvirenti, R. Patel, F. Kocka, J. Ortiz, and R. A. Weinstein. 2002. Possible overestimation of penicillin resistant Streptococcus pneumoniae colonization rates due to misidentification of oropharyngeal streptococci. Diagn. Microbiol. Infect. Dis. 42:263-268. [DOI] [PubMed] [Google Scholar]
- 21.Whatmore, A. M., and C. G. Dowson. 1999. The autolysin-encoding gene (lytA) of Streptococcus pneumoniae displays restricted allelic variation despite localized recombination events with genes of pneumococcal bacteriophage encoding cell wall lytic enzymes. Infect. Immun. 67:4551-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whatmore, A. M., A. Efstratiou, A. P. Pickerill, K. Broughton, G. Woodard, D. Sturgeon, R. George, and C. G. Dowson. 2000. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: characterization of “atypical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect. Immun. 68:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheeler, J., R. Freeman, M. Steward, K. Henderson, M. J. Lee, N. H. Piggott, G. J. Eltringham, and A. Galloway. 1999. Detection of pneumolysin in sputum. J. Med. Microbiol. 48:863-866. [DOI] [PubMed] [Google Scholar]