Abstract
CAP18 (an 18-kDa cationic antimicrobial protein) is a granulocyte-derived protein that can bind lipopolysaccharide (LPS) and inhibit various activities of LPS in vitro. The present study examined the protective effect of a synthetic 27-amino-acid peptide (CAP18109–135) from the LPS-binding domain of CAP18 against antibiotic-induced endotoxin shock, using highly LPS-sensitive d-(+)-galactosamine (d-GalN)-sensitized C3H/HeN mice. The antibiotic-induced endotoxin (CAZ-endotoxin) was prepared from the culture filtrate of Pseudomonas aeruginosa PAO1 exposed to ceftazidime (CAZ). Injection of CAP18109–135 protected the mice injected with LPS or CAZ-endotoxin from death and lowered their tumor necrosis factor (TNF) levels in serum in a dose-dependent manner. Treatment with CAZ caused death of the d-GalN-sensitized P. aeruginosa PAO-infected mice within 48 h, while injection with CAP18109–135 rescued the mice from death. In the mice rescued from death by injection with CAP18109–135, endotoxin levels in plasma and TNF production by liver tissues were decreased but the numbers of viable infecting bacteria in their blood were not decreased significantly and remained at the levels in CAZ-treated mice. These results indicate that CAP18109–135 is capable of preventing antibiotic-induced endotoxic shock in mice with septicemia and that the effect is due to its LPS-neutralizing activity rather than to its antibacterial activity.
Endotoxin, i.e., lipopolysaccharide (LPS), associated with proteins in the outer membrane of gram-negative bacteria (18), is strongly implicated in the pathogenesis of gram-negative bacterial sepsis, including fever, shock, disseminated intravascular coagulation, multiple organ failure, and death (35, 39). The septic shock induced by bacteremia due to gram-negative bacteria is thought to be due to the massive release of endotoxin from the infecting organisms by spontaneous release or bacterial lysis. The released endotoxin activates macrophages, endothelial cells, and fibroblasts to produce and release potent inflammatory mediators, including tumor necrosis factor alpha (TNF), interleukin (IL)-1β, IL-6 and nitric oxide (34). The mediators, especially TNF, cause endotoxic shock syndrome (6, 28). Endotoxic shock may occur in the process of therapy with inappropriate antibiotics. Although antibiotics kill bacteria, they do not neutralize endotoxin. Sudden release of an excess amount of endotoxin from the antibiotic-disrupted bacteria can result in endotoxic shock (22).
A number of host-derived proteins, such as low-density lipoprotein, high-density lipoprotein (10), LPS-binding protein (41) and bactericidal permeability-increasing protein (9, 12, 42), can bind to endotoxin and modulate endotoxic activity negatively or positively. Lower concentrations of LPS complexed with LPS-binding protein may activate macrophages through their surface protein CD14 (41, 46). In contrast, high-density lipoprotein or bactericidal permeability-increasing protein bound to LPS inhibits the ability of LPS to elicit cellular responses (10, 30).
CAP18 is an 18-kDa cationic protein capable of binding to LPS. It was isolated from rabbit granulocytes as a protein with antimicrobial, LPS-binding, and LPS-neutralizing properties and purified by using its capacity to agglutinate LPS-sensitized sheep erythrocytes (13, 16, 26). CAP18 and its C-terminal 37-amino-acid fragment inhibit the LPS-induced production of tissue factor by murine macrophages and human monocytes (13) and of TNF, IL-1β, IL-6, and nitric oxide by murine macrophages in vitro (26). The peptide has potent activity against both gram-negative and gram-positive bacteria in vitro (25). A more recent study demonstrated that treatment with a C-terminal 37-amino-acid fragment of rabbit CAP18 neutralized the deleterious effects of LPS in pigs (45). Rabbit CAP18 cDNA has been cloned (27). Based on the nucleotide sequence of the rabbit CAP18 cDNA, human CAP18 cDNA was also cloned (24). The human CAP18 cDNA encodes a protein composed of a 30-amino-acid signal peptide, a 103-amino-acid N-terminal domain of unknown function, and a 37-amino-acid C-terminal domain that binds to LPS, neutralizes LPS-mediated activation of monocytes, and reduces the lethal toxicity of LPS in mice (15, 17, 24). The C-terminal 27-amino-acid fragment was identified as the LPS-neutralizing and antimicrobial domain (15). This peptide was synthesized and was designated CAP18109–135.
Recently, investigators have recognized that the use of antibiotic chemotherapy to treat infections with gram-negative microbes may result in the release of microbial constituents that might, in turn, exacerbate the pathophysiological manifestations of disease. Although the actual clinical importance of this phenomenon to patients with sepsis due to gram-negative bacteria is unclear, both in vitro and in vivo animal experiments have provided evidence in support of this concept (22, 31, 32). CAP18 may neutralize the toxicity of antibiotic-induced endotoxin in vivo and prevent death from septic shock. The present study was designed to determine the protective effect of CAP18109–135 against antibiotic-induced endotoxic shock in a murine model of Pseudomonas aeruginosa infection, and the results demonstrate that CAP18109–135 is capable of reducing the mortality of the mice due to endotoxic shock through neutralization of endotoxin and reduction of TNF production in the mice.
MATERIALS AND METHODS
Mice.
Female C3H/HeN mice and male C57BL/6 mice aged 6 to 7 weeks were obtained from Nippon Clea Co., Tokyo, Japan, and Charles River Japan, Tokyo, Japan, respectively. The C3H/HeN mice were maintained in the Animal Facility of the Jichi Medical School under standard care and used at 8 to 9 weeks of age, when they had attained an average body weight of 18 g. The C57BL/6 mice were maintained in the Animal Facility of the Iwate Medical University and used at 8 weeks of age, when they had attained an average body weight of 23 g.
Bacterial strains.
P. aeruginosa PAO and PAO1 were kindly provided by Y. Hirakata, Nagasaki University, Nagasaki, Japan. The bacteria, suspended in 10% skim milk, were stored at −80°C. When needed for an experiment, the frozen bacteria were thawed and cultured overnight on a nutrient agar plate. The bacteria in a single colony arbitrarily selected from colonies on the plate were cultured overnight in a defined Pseudomonas basal mineral medium (4) and used for the experiment. The number of CFU of the organisms was determined by quantitative cultivation on nutrient agar plates.
CAP18-derived peptides.
CAP18 is composed of two domains: a highly conserved N-terminal domain with an unknown function and a less highly conserved C-terminal domain with anti-LPS and antimicrobial functions (13, 24). The C-terminal domain was synthesized, and the truncated human peptide (CAP18104–135) was more active than the full-length human domain CAP18104–140 (24). Four different truncated CAP18-derived peptides were synthesized by Peptide Institute, Inc., Minoh, Hyogo, Japan. Their amino acid sequences are shown in Table 1. When their LPS-binding activities were assessed by the agglutination test with erythrocytes coated with LPS (16), they all showed LPS-binding activities, although peptides CAP18114–135 and CAP18109–132 showed significantly less activity than peptides CAP18106–135 and CAP18109–135 did. The purity of CAP18109–135 was 97.3% when assessed by analytical high-pressure liquid chromatography. The peptides were dissolved in saline, distributed into tubes, and stored at −80°C. Immediately before use, a sample of the frozen peptide solution was thawed. Endotoxin contamination of the CAP18109–135 used in the present study was examined by the rabbit pyrogen test. The exact minimum pyrogenic dose could not be determined because of the limited availability of the test synthetic peptide. Three Kbs male Japanese White rabbits (3.77 ± 0.10 kg; Kitayama Lab. Co., Iyo, Japan) were injected intravenously (i.v.) with 100 μg of CAP18109–135 per kg in 1 ml of pyrogen-free phosphate-buffered saline per kg. The temperature of each rabbit was measured every 5 min for 3 h with a rectal thermometer (model EP76-12; Iio Electric Co., Tokyo, Japan). The temperature of the control rabbits was 39.1 ± 0.1°C. The change in rectal temperature after i.v. CAP18109–135 administration (100 μg/kg) was +0.37 ± 0.06°C. No rabbit showed an individual rise in temperature of 0.6°C or more. The sum of the three individual maximum temperature increases (1.0°C) did not exceed 1.4°C. These results indicate that the CAP18109–135 solution does not induce a significant febrile reaction in rabbits.
TABLE 1.
Sequences and LPS-binding activity of CAP18 peptides
| CAP18 sequencesa | LPS-binding activity (μg/ml)b |
|---|---|
| 100 110 120 130 140 | |
| DNK RFALL GDFFR KSKEK IGKEF KRIVQ RIKDF LRNLV PRTES | |
| CAP18106–135 (30 AA) | 1.6 |
| CAP18109–135 (27 AA) | 3.2 |
| CAP18114–135 (22 AA) | 12.5 |
| CAP18109–132 (24 AA) | 25 |
The C-terminal 37-amino-acid fragment of CAP18 (amino acids [AA] 98 to 140) is shown, and the extents of the peptides used in this study are indicated.
Minimal concentration of peptide for hemagglutination of S. minnesota R595 LPS-sensitized sheep erythrocytes (see the text).
LPS.
LPS from wild-type Salmonella minnesota purchased from Sigma Chemical Co., St. Louis, Mo., and LPS from S. minnesota R595 (Re-chemotype), purchased from List Biologicals Co., Campbell, Calif., were dissolved at 1 mg/ml in endotoxin-free H2O as a stock solution and stored at 4°C. The LPS solution was diluted appropriately in endotoxin-free saline immediately before use in an experiment. Wild-type S. minnesota LPS was used in in vivo studies unless indicated otherwise. Escherichia coli O111:B4 (S-form) LPS, obtained from Seikagaku Corp., Tokyo, Japan, was dissolved in endotoxin-free H2O at concentrations recommended by the manufacturer and used as a reference LPS in the LPS-specific chromogenic test. A 1-ng portion of E. coli LPS is equivalent to 2.9 endotoxin units.
Antibiotic-induced endotoxin preparation.
Antibiotic-induced endotoxin was prepared as described previously (36). Briefly, P. aeruginosa PAO1 organisms (107/ml) were inoculated into synthetic M9 medium (4) and cultured in the presence of twice the MIC of ceftazidime (CAZ) at 37°C for 8 h. After incubation, the culture supernatant was filtered through a membrane filter (Sterifil D-GV; pore size, 0.22 μm; Millipore Corp., Bedford, Mass.) and the filtrate was used as the CAZ-induced endotoxin preparation (CAZ-endotoxin). The preparation contained 5.4 μg of LPS per ml as measured by the chromogenic endotoxin-specific assay (Endospecy; Seikagaku Corp., Tokyo, Japan).
LPS/endotoxin assay.
The Endospecy kit reagent consists of coagulation factors (factor C, factor B, and proclotting enzyme) from the horseshoe crab (Tachypleus tridentatus) and a chromogenic substrate, t-butyloxycarbonyl-Leu-Gly-Arg-p-nitroanilide (Boc-Leu-Gly-Arg-pNA). Since the reagent does not contain a protease, factor G, it does not react with (1 → 3)-β-d-glucan, a component of fungus (43). To avoid nonspecific reaction, the plasma sample to be assayed (5 μl) was previously treated with 20 μl of a solution containing 0.1% (wt/vol) Triton X-100, 0.07% (wt/vol) ethyleneimine polymer, 0.01 M CaCl2, 0.1 M KOH, 0.03 M N,N′-bis(2-hydroxyethyl)glycine (Bicine), and 0.1% (wt/vol) Polybrene (solution A) at 37°C for 10 min in a 96-well plate (Seikagaku Corp.). Then the LPS assay was conducted as specified in the manual accompanying the kit, and the dose of LPS in the specimen was determined by measuring the absorbance at 405 nm.
To examine the influence of CAP18109–135 on the LPS assay, E. coli LPS (100 μg/ml, 2 μl) was added to 18 μl of the coagulation factors and the mixture was incubated at 37°C for 30 min. Then CAP18109–135 (400 μg/ml, 75 μl) was added to the mixture, and the mixture was incubated at 37°C for 60 min. Finally, the chromogenic substrate was added to the mixture, and the mixture was further incubated at 37°C for 30 min. The absorbance of the reaction mixture was measured at 405 nm.
Reagents.
The (1 → 3)-β-d-glucan from Alcaligenes faecalis subsp. myxogenese IFO13140 was obtained from Seikagaku Corp. Murine recombinant TNF-α was provided by the Institute for Biomedical Research, Suntory Co., Osaka, Japan. d-(+)-galactosamine (d-GalN) was obtained from Sigma. CAZ was obtained from Glaxo Japan Co., Tokyo, Japan. The CAZ solution (100 mg/ml in endotoxin-free saline) was distributed into tubes and stored at −80°C, and a sample was thawed immediately before use. During the study period, CAZ did not lose antimicrobial activity as determined by measuring its MICs for P. aeruginosa PAO and PAO1, both of which were 1 μg/ml. Endotoxin-free saline and water were obtained from Ohtsuka Pharmaceutical Co., Naruto, Japan.
Detection of glucan.
A (1 → 3)-β-d-glucan-specific chromogenic test kit (G-test) was obtained from Seikagaku Corp. The kit contains a reagent consisting of factor G and proclotting enzyme derived from the horseshoe crab and Boc-Leu-Gly-Arg-pNA. CAP18109–135 (400 μg/ml in 25 μl) was added to a solution of (1 → 3)-β-d-glucan (96.8 pg/ml), and the mixture was incubated at 37°C for 60 min. Then 100 μl of the G-test reagent was added to the mixture, the mixture was further incubated at 37°C for 30 min, and the absorbance was measured at 405 nm.
Infection.
P. aeruginosa PAO organisms were grown in 10 ml of defined Pseudomonas basal mineral medium (4) in a 50-ml conical tube (Falcon 2070; Becton Dickinson Labware, Lincoln Park, N.J.) at 37°C overnight with shaking. Then the bacterial suspension (1 ml) was diluted in the medium (10 ml) and cultured for 2 h with shaking. The number of log-phase bacteria in the culture was determined by measuring the optical density, and the culture was diluted in endotoxin-free saline until it contained the appropriate number of bacteria. The bacterial suspension thus prepared (0.2 ml/mouse) was injected intraperitoneally (i.p.) into mice.
Determination of the protective effect of CAP18109–135 against lethal activities of LPS and P. aeruginosa infection.
d-GalN-sensitized mice are highly susceptible to LPS (4). We used d-GalN-sensitized mice in an experiment to determine the protective effect of CAP18-derived peptides against the lethal activity of LPS or CAZ-endotoxin. CAP18109–135 (0.2 ml), either S. minnesota LPS (100 ng/0.2 ml) or CAZ-endotoxin (0.2 ml/mouse), and d-GalN (18 mg/0.5 ml of saline) were sequentially injected i.p. into the mice. Injections of all reagents were performed within 15 s. In some experiments, LPS and d-GalN were injected i.p. into the mice together, and immediately after the injection CAP18109–135 was injected i.v. into the mice. In other experiments, equal volumes (0.1 ml) of LPS and CAP18-derived peptides were mixed in vitro and incubated at 37°C for 30 min and then the mixture (0.2 ml) and d-GalN were injected i.p. into the mice.
d-GalN-sensitized mice were also used in an experiment to determine the protective effect of CAP18109–135 against death from P. aeruginosa infection. Mice were injected i.p. with CAZ (20 mg/kg in 0.2 ml per mouse), P. aeruginosa PAO (3 × 106 CFU in 0.2 ml per mouse), and d-GalN (18 mg in 0.5 ml per mouse). All injections were given within 15 s. Then the mice received i.p. injections of CAP18111–137 (80 μg in 0.2 ml per mouse) at 30, 90, and 150 min after infection. Death from infection was recorded every 24 h until day 7 of the infection. All deaths occurred within 48 h of infection. All mice that did not die within 48 h survived for the entire 7 days.
Bacterial counts in blood.
Blood was withdrawn from infected mice by cardiac puncture with heparinized syringes at 120 min of infection. The number of viable bacteria in the blood was determined by quantitative cultivation on nutrient agar plates.
Bactericidal assay.
P. aeruginosa PAO was plated on nutrient agar. Bacterial cultures were collected at the logarithmic phase of growth, washed twice with phosphate-buffered saline (pH 7.2), and adjusted to a final concentration of 5 × 103 to 1 × 104 cells per ml. To 450 μl of bacterial suspension was added 50 μl of peptide, and the mixture was incubated at 37°C for 30 min; 100 μl of the reaction mixture was then plated onto the agar plate. After 24 h of incubation at 37°C, the numbers of CFU were counted.
Plasma and serum samples.
Blood was withdrawn from the mice by cardiac puncture with heparinized or nonheparinized syringes. The heparinized blood was immediately centrifuged to separate the plasma, and the plasma was stored at −80°C until determination of the endotoxin level. The blood taken without heparin was allowed to clot, and the serum thus obtained was stored at −80°C for the TNF assay.
TNF assay.
TNF-sensitive L929 cells were donated by M. J. Parmely, University of Kansas Medical Center. TNF activity was determined by a functional cytotoxic assay with the L929 cells as target cells, as described previously (21). The viability of the cells was determined by quantitative colorimetric staining with tetrazolium salt [3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT); Sigma]. TNF activity was expressed as units per milliliter. One unit is the amount of TNF causing 50% lysis of L929 cells, normalized by the activity of murine recombinant TNF-α.
Determination of TNF secretion in liver tissue.
Liver slices were prepared as previously described (20). Briefly, the left lobe of the mouse liver was removed after 120 min of infection and 10-mm-diameter cylindrical liver core specimens were taken. The specimens were placed in a Krumdieck tissue slicer (Alabama Research and Development Corp., Munford, Ala.), and 200-μm-thick sections (about 15 mg [wet weight]) were cut and collected in cold Hanks balanced salt solution. Each slice was incubated in a 24-mm transwell culture dish (pore size, 3.0 μm; Costar, Cambridge, Mass.) containing 1 ml of RPMI 1640 medium (ICN Pharmaceuticals, Inc., Amsterdam, The Netherlands) supplemented with 5% heat-inactivated fetal bovine serum (JRH Biosciences, Inc., Lenexa, Kans.), 4 mM of l-glutamine (Kanto Chemical Co., Tokyo, Japan), 100 U of penicillin (Meiji-Seika Co., Tokyo, Japan), 100 μg of streptomycin (Meiji-Seika), and 10 μg of bovine pancreatic insulin (Sigma). At 30 min after the incubation, the culture medium was collected and stored at −80°C until the TNF assay was performed as described in the preceding section.
Statistical analysis.
Results are expressed as means ± standard deviations for four to eight mice. Statistical significance was determined by Student’s t test for the TNF level, endotoxin level, and bacterial counts and by the χ2 test for mortality. The 50% inhibitory concentrations (IC50s) were determined by least-squares linear regression.
RESULTS
Previous incubation of LPS with CAP18 peptides attenuates lethal toxicity of LPS against d-GalN-sensitized mice.
Mice sensitized with d-GalN are quite susceptible to the lethal effect of LPS (11). Approximately two-thirds of the d-GalN-sensitized C57BL/6 mice injected i.p. with a dose of 100 ng of S. minnesota LPS died within 48 h (Table 2). However, preincubation of LPS with CAP18106–135 or CAP18109–135 at 1:10 in vitro could significantly attenuate the lethal toxicity of LPS against the d-GalN-sensitized C57BL/6 mice. A reduction in the lethal toxicity of LPS was also obtained by pretreatment with CAP114–135, although it was not statistically significant. CAP18109–132 did not confer any significant protection in this model. The LPS-neutralizing activities observed among these CAP18 peptides were well correlated with their LPS-binding capabilities. These data may lead to a conclusion that CAP18109–135 is the minimal peptide unit with effective anti-LPS activity. Our previous studies also demonstrated that CAP18109–135 has antimicrobial activities (14). We therefore selected CAP18109–135 for further in vivo experimental studies.
TABLE 2.
Human peptides block LPS lethality in d-GalN-sensitized C57BL/6 micea
| Peptide | Amt of peptide (μg/mouse) | Amt of LPS (μg/mouse) | No. of mice that died/total no. | Survival (%) |
|---|---|---|---|---|
| None | 0.1 | 16/25 | 36.0 | |
| CAP18106–135 | 1.0 | 0.1 | 1/8 | 87.5b |
| CAP18109–135 | 0.1 | 0.1 | 5/10 | 50.0 |
| 1.0 | 0.1 | 5/18 | 72.2b | |
| CAP18114–135 | 0.1 | 0.1 | 7/10 | 30.0 |
| 1.0 | 0.1 | 9/19 | 52.6 | |
| CAP18109–132 | 1.0 | 0.1 | 7/8 | 12.5 |
LPS (1 μg/ml) was incubated with an equal volume of the indicated peptide (10 or 1 μg/ml) at 37°C for 30 min, and the mixture (0.2 ml) and d-GalN (20 mg/mouse) was sequentially injected i.p. into C57BL/6 mice.
P < 0.05 compared with the LPS control.
CAP18109–135 protects d-GalN-sensitized mice from the lethal toxicity of LPS/endotoxin.
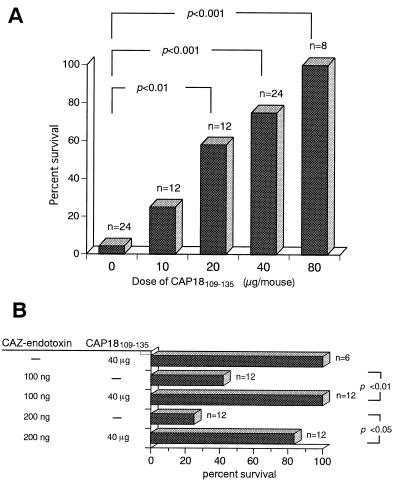
Of the d-GalN-sensitized C3H/HeN mice injected i.p. with 100 ng of S. minnesota LPS, 96% died within 48 h (Fig. 1A). In contrast, i.p. injection of CAP18109–135 protected the d-GalN-sensitized mice from death caused by 100 ng of LPS in a dose-dependent fashion, and injection of 80 μg of CAP18109–135 (4.4 mg/kg of body weight) protected 100% of the mice from death (P < 0.01). The 50% inhibitory concentration (IC50) of CAP18109–135 against the lethal toxicity of LPS was 20 μg per mouse. Furthermore, an i.v. injection of 80 μg of CAP18109–135 also protected 100% of the d-GalN-sensitized mice from death by i.v. or i.p. injection with 100 ng of LPS (n = 4), while all of the control saline-injected mice died (n = 4) (data not shown). In these experiments, all the deaths occurred within 48 h. These results clearly indicate that CAP18109–135 protects the mice from the toxic effect of LPS. CAP18109–135 itself did not cause any toxic death in d-GalN-sensitized mice, and no deaths were observed even in the mice (n = 4) injected i.p. with 160 μg of the peptide per mouse five times (at 0, 1, 2, 3, and 5 h).
FIG. 1.
Protective effect of CAP18109–135 against the lethal toxicity of LPS/endotoxin in d-GalN-sensitized mice. (A) C3H/HeN mice were injected i.p. with CAP18109–135 at the indicated doses and immediately injected i.p. with LPS (100 ng/mouse) and d-GalN (18 mg/mouse). (B) C3H/HeN mice were injected i.p. with CAP18109–135 (40 μg/mouse) or saline, CAZ-endotoxin (100 or 200 ng of LPS equivalent/mouse) or saline, and d-GalN (18 mg/mouse). Death was scored for 1 week after the injection.
It is well documented that antibiotics whose the target is the cell wall of gram-negative bacteria, such as the β-lactams, e.g., CAZ, induce the release of endotoxin from the bacteria either in vivo or in vitro (22, 36). We have demonstrated that CAZ-endotoxin obtained from the culture supernatant of P. aeruginosa cultured in the presence of CAZ was toxic to mice and that it contained large amounts of endotoxin and bacterial protein (36). We examined whether CAP18109–135 could protect mice from the lethal toxicity of the protein-rich CAZ-endotoxin. d-GalN-sensitized C3H/HeN mice were injected i.p. with CAZ-endotoxin equivalent to 100 or 200 ng of LPS and with 40 μg (2.2 mg/kg of body weight) of CAP18109–135 or saline. The treatment with 40 μg of CAP18109–135 protected the mice from the lethal toxicity of the CAZ-endotoxin (Fig. 1B), indicating that CAP18109–135 is capable of abolishing the deleterious activity of the antibiotic-induced endotoxin in vivo.
CAP18109–135 inhibits LPS/endotoxin-induced TNF production in mice.
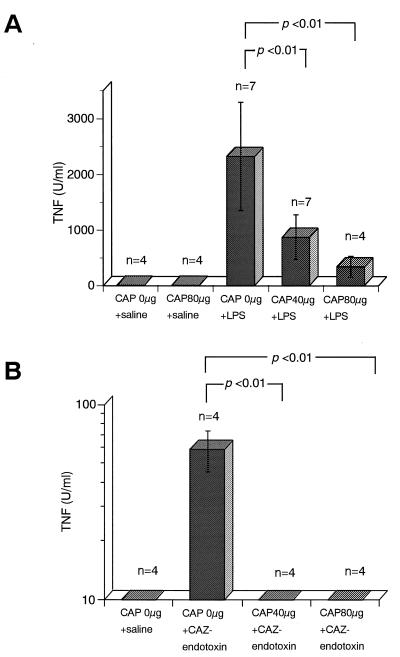
TNF is thought to be a major mediator of endotoxin shock (6, 28). To determine whether the protective effect of CAP against the lethal toxicity of LPS/endotoxin in mice is due to its suppression of induction of TNF production, TNF levels in serum were assayed in C3H/HeN mice injected with CAP18109–135 and LPS or CAZ-endotoxin. In this experiment, we used naive mice without d-GalN sensitization, since the LPS-induced TNF levels in sera of d-GalN-sensitized mice were fundamentally similar to those in sera of unsensitized mice (TNF levels in serum 1 h after injection with 5 μg of LPS were 2,100 ± 500 U/ml for d-GalN-sensitized C3H/HeN mice and 1,950 ± 400 U/ml for naive C3H/HeN mice). Although an i.p. injection of 100 ng of LPS into naive mice did not lead to any significant increase in TNF levels in serum 1 h after the injection (data not shown), injection with 5 μg of LPS or CAZ-endotoxin equivalent to 2 μg of LPS caused an obvious increase in the level of TNF in serum (Fig. 2). The induction of TNF production by LPS and CAZ-endotoxin was significantly inhibited by injection of CAP18109–135 peptide. The IC50s of CAP18109–135 against LPS-induced TNF production and CAZ-endotoxin-induced TNF production were about 40 μg and less than 40 μg, respectively.
FIG. 2.
Effect of CAP18109–135 on LPS/endotoxin-induced TNF levels in serum of mice. Mice were injected i.p. with CAP18109–135 at the indicated doses or with saline and immediately injected i.p. with LPS (5 μg/mouse) or saline (A) or CAZ-endotoxin (2 μg of LPS equivalent/mouse) (B). Blood was collected 1 h after the injections. TNF levels in serum were determined by a functional cytotoxicity assay with L929 cells as the target cells.
CAP18109–135 prevents death in P. aeruginosa-infected and CAZ-treated mice.
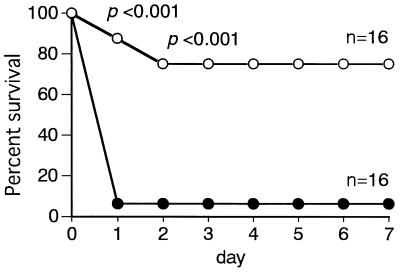
We have demonstrated that CAZ treatment did not save the lives of d-GalN-sensitized mice with P. aeruginosa infection but, rather, accelerated death, probably as a result of sudden release of a large amount of endotoxin from infecting organisms disrupted by the action of CAZ (22, 36). We examined whether the adverse effect of antibiotics in infection caused by antibiotic-induced endotoxin release can be prevented by CAP18109–135 treatment. d-GalN-sensitized C3H/HeN mice were infected with P. aeruginosa and treated with CAZ. One group of mice was given CAP18109–135, and another group of mice was left untreated. In this experiment, 80 μg of CAP18109–135 was given at 30, 90, and 150 min of the infection (4.4 mg/kg of body weight per administration) because a preliminary experiment showed that a single injection of a high dose (8.8 mg/kg of body weight) of CAP18109–135 was ineffective. In the control group of mice that did not receive CAP18109–135, 94% of the mice died within 24 h of infection. In contrast, only 25% of the mice that were treated with CAP18109–135 died (Fig. 3). The results suggested a protective effect of CAP18109–135 against antibiotic-induced endotoxic shock in bacterial infection, and this problem was explored as described in the following section.
FIG. 3.
Protective effect of CAP18109–135 against death in CAZ-treated, d-GalN-sensitized mice infected with P. aeruginosa. Mice were injected i.p. with CAZ (20 mg/kg in 0.2 ml per mouse), P. aeruginosa PAO organisms (3 × 106 CFU/mouse), and d-galactosamine (18 mg/mouse). At 30, 90, and 150 min after the infection, the mice were injected with CAP18109–135 (80 μg per mouse per injection) (○) or saline (•). Death was recorded every 24 h after the infection until day 7.
CAP18109–135 lowers the level of endotoxin in the blood but does not decrease the number of viable bacteria in the blood of P. aeruginosa-infected, CAZ-treated mice.
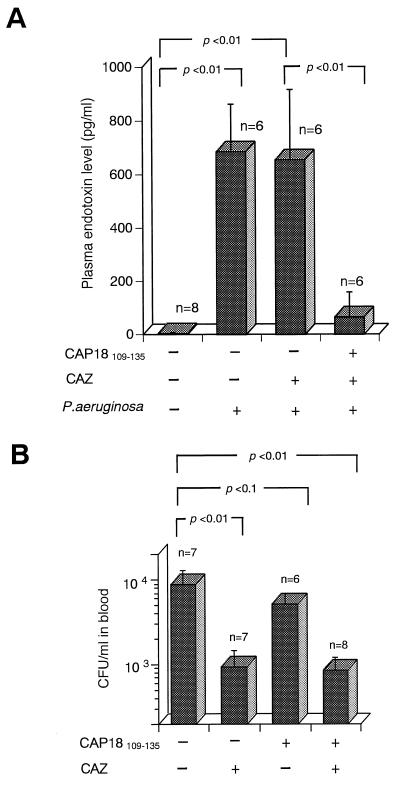
To explore whether the protective effect of CAP18109–135 against death in P. aeruginosa-infected, CAZ-treated mice depends on neutralization of CAZ-endotoxin released from infecting bacteria, the following experiments were conducted. GalN-sensitized C3H/HeN mice were infected with P. aeruginosa, treated with CAZ, and then either treated or not treated with CAP18109–135. Blood was taken from the mice 120 min after the infection for determination of the endotoxin level and the number of viable infecting bacteria.
As shown in Fig. 4, high levels of endotoxin and CFU were detected in the blood of mice infected with P. aeruginosa (control mice). Treatment with CAZ still resulted in high endotoxin levels, although the bacterial numbers were reduced to about one-10th of those in the control mice. In contrast, treatment with CAP18109–135 markedly lowered the endotoxin levels in the blood of P. aeruginosa-infected CAZ-treated mice whereas the reduction in the CFU was not accelerated in comparison with the mice without CAP18109–135 treatment. CAZ is known to induce a relatively larger amount of endotoxin from growing P. aeruginosa in vitro than does Imipenem (36). The quick reduction of the number of CFU in the mice treated with CAZ seemed to be unable to raise the endotoxin level in excess of that in control mice without CAZ treatment. The endotoxin levels in uninfected mice were lower than 10 pg/ml (5 ± 1 pg/ml). These results suggest that CAP18109–135 has the ability to neutralize endotoxin activity released from infecting organisms with or without antibiotic treatment and prevents death caused by antibiotic-induced endotoxin release, although it is not effective against in vivo infection with P. aeruginosa.
FIG. 4.
Effects of CAZ and/or CAP18109–135 on endotoxin levels in plasma and numbers of bacteria in the blood of P. aeruginosa-infected and d-GalN-sensitized mice. Mice were injected i.p. with CAZ (20 mg/kg in 0.2 ml per mouse) or saline, infected with P. aeruginosa PAO (3 × 106 CFU/mouse in an experiment for endotoxin levels and 6 × 106 CFU/mouse in an experiment for determination of the numbers of bacteria), and d-GalN (18 mg/mouse). At 30 and 90 min after the infection, the mice were injected with CAP18109–135 peptide (80 μg per mouse per injection) or saline. The endotoxin levels (A) and number of bacteria (B) in the blood were determined 120 min after the infection.
CAP18 exerts in vitro activity against various species of bacteria, including P. aeruginosa (13, 24–26). To clarify why the antibacterial activity of CAP18109–135 observed in vitro was not effective against in vivo infection with P. aeruginosa, we examined the effect of plasma components on the anti-bacterial activity of CAP18109–135. P. aeruginosa was incubated with CAP18109–135 (20 μg/ml) in the presence or absence of 10% murine plasma for 30 min, and the numbers of viable bacteria were determined. In the absence of plasma, CAP18109–135 exhibited significant antibacterial activity: the bacterial counts were 6,763 ± 935 CFU/ml for the saline control and 3,473 ± 648 CFU/ml for the CAP18109–135-treated bacteria (P < 0.01). However in the presence of plasma, CAP18109–135 did not exhibit significant antibacterial activity: the bacterial counts were 7,647 ± 818 CFU/ml for the saline control and 6,297 ± 1,756 CFU/ml for the CAP18109–135-treated bacteria. These data, indicating that plasma components inhibit the antibacterial activity of CAP18109–135, may explain why this peptide is not effective against in vivo infection with P. aeruginosa.
CAP18109–135 itself does not affect the Limulus assay.
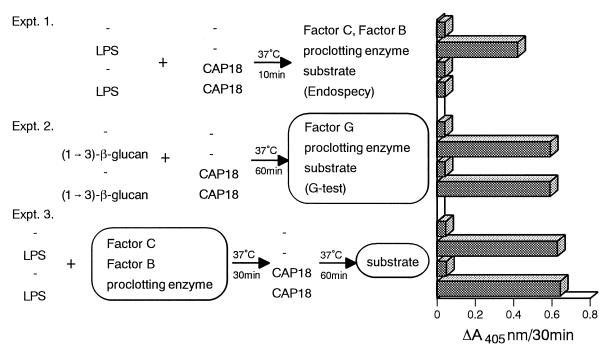
As shown in Fig. 4A, treatment with CAP18109–135 lowered the endotoxin levels detectable by the Limulus assay. To exclude the possibility that CAP18109–135 in the plasma sample directly interferes with the Limulus assay, we examined the effects of CAP18109–135 on the Limulus assay (Fig. 5). In the LPS-specific chromogenic endotoxin test (Endospecy), LPS activates factor C to the activated form. The activated factor C activates factor B, which in turn converts the proclotting enzyme to the active clotting enzyme. The clotting enzyme hydrolyzes a chromogenic substrate to release pNA (43) (Fig. 5, experiment 1). CAP18109–135 alone did not activate the LPS-specific protease cascade. Preincubation with CAP18109–135 and LPS completely inhibited LPS-induced activation of the protease cascade. The (1 → 3)-β-d-glucan-specific chromogenic test (G-test) reagents consist of the same enzymes and substrate as those of the Endospecy kit reagents except that factor G is present instead of factor C. (1 → 3)-β-d-Glucan activates factor G to the activated form, which converts the proclotting enzyme to the clotting enzyme. The clotting enzyme hydrolyzes a chromogenic substrate (43) (Fig. 5, experiment 2). CAP18109–135 alone did not activate the (1 → 3)-β-d-glucan-specific protease cascade. Preincubation with CAP18109–135 and (1 → 3)-β-d-glucan did not inhibit (1 → 3)-β-d-glucan-induced activation of the protease cascade. When LPS was incubated with factor C, factor B, and proclotting enzyme and the mixture was further incubated with CAP18109–135, CAP18109–135 did not affect LPS-induced activation of the protease cascade (Fig. 5, experiment 3). These results suggest that CAP18109–135 does not affect the LPS-specific cascade in the Limulus assay, although we cannot exclude the possibility that CAP18109–135 inactivates factor C or factor B.
FIG. 5.
Effect of CAP18109–135 peptide on the Limulus assay. In experiment 1, LPS (50 pg/ml) was mixed with CAP18109–135 (400 μg/ml) and incubated at 37°C for 10 min. Then 50 μl of the Endospecy reagent was added to the mixture, which was incubated at 37°C for a further 30 min. At the end of the incubation, the absorbance of the reaction mixture was measured at 405 nm (A405). In experiment 2, (1 → 3)-β-d-glucan (48.4 pg/ml in 25 μl) was incubated with CAP18111–137 (200 μg/ml in 25 μl) at 37°C for 60 min. Then G-test reagent was added to the mixture, and the mixture was incubated at 37°C for a further 30 min. At the end of the incubation, the absorbance of the reaction mixture was measured at 405 nm. In experiment 3, LPS (10 μg/ml) was mixed with a solution of coagulation factors prepared from horseshoe crab (factor C, factor B, and proclotting enzyme) and the mixture was incubated at 37°C for 30 min. Then CAP18111–137 (315 μg/ml) was added to the mixture, which was incubated at 37°C for a further 60 min. Finally, the substrate solution was added to the mixture, and the mixture was incubated at 37°C for a further 30 min. At the end of incubation, the absorbance of the reaction mixture was measured at 405 nm.
Treatment with CAP18109–135 suppresses TNF secretion by the liver tissue of P. aeruginosa-infected, CAZ-treated mice.
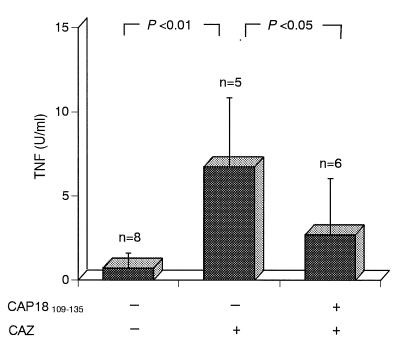
Levels in plasma of endotoxin released from infecting bacteria in CAZ-treated mice were lowered by treatment with CAP18109–135 (Fig. 4A), and levels in serum of TNF induced by LPS/endotoxin were also lowered by treatment with CAP18109–135 (Fig. 2). We attempted to determine whether treatment with CAP18109–135 lowered the levels of TNF in the sera of d-GalN-sensitized mice infected with P. aeruginosa and treated with CAZ. However, the amounts of TNF in the sera of P. aeruginosa-infected CAZ-treated mice at 60 and 120 min of infection were too low to be reliable, even in mice not treated with CAP18109–135. Therefore, we determined the effect of treatment with CAP18109–135 on TNF production by liver tissues of the mice. The livers were taken from the mice that had been injected with CAZ and d-GalN, infected with P. aeruginosa (3 × 106 cells/mouse), and either treated or not treated with CAP18109–135, and slices of the livers were prepared. The liver slices from uninfected mice treated with CAZ secreted marginal levels of TNF (less than 3 U/ml), while the liver slices from the mice that had been infected with P. aeruginosa and treated with CAZ secreted higher levels of TNF. Treatment of the P. aeruginosa-infected CAZ-treated mice with CAP18109–135 clearly lowered TNF secretion by the liver slices (Fig. 6).
FIG. 6.
Effects of CAP18109–135 on TNF secretion in the liver tissues taken from P. aeruginosa-infected, CAZ-treated, d-GalN-sensitized mice. The mice were injected with CAZ (20 mg/kg in 0.2 ml per mouse) or saline, infected with P. aeruginosa (3 × 106 CFU/mouse), and then injected with d-GalN (18 mg/mouse). At 30 and 90 min after the infection, the mice were treated with CAP18109–135 peptide (80 μg per mouse per injection) or saline. The livers were removed from the mice 120 min after the infection, and liver slices were prepared as described in Materials and Methods. Each slice was incubated for 30 min, and the culture supernatants were collected. The TNF activity in the supernatants was determined by a functional cytotoxicity assay with L929 cells.
DISCUSSION
It has been reported that synthetic rabbit CAP18106–142 and a truncated 32-amino-acid fragment (CAP18106–137) blocked the lethal toxicity of LPS in d-GalN-treated mice and that the activity was highly dependent on structure, because other shorter truncated fragments such as CAP18106–114 and CAP18118–142 were inactive (14, 23). A 32-amino-acid C-terminal fragment of human CAP18 (CAP18104–135) has also been shown to protect mice from LPS lethality when LPS was incubated with CAP18 in vitro before injection (17). In the present study, we demonstrated that injection of synthetic human CAP18109–135 protected d-GalN-sensitized mice from the lethal toxicity of LPS or CAZ-endotoxin (Table 2; Fig. 1). Since CAP18109–135 exerted the protective effect in a dose-dependent fashion, active CAP18109–135 might be consumed by binding to LPS in vivo. Injection of CAP18109–135 also suppressed the levels in serum of TNF generated by injection with LPS or CAZ-endotoxin in d-GalN-sensitized mice in a dose-dependent fashion (Fig. 2). The results, together with those on the lethal toxicity of LPS or CAZ-endotoxin, suggest that CAP18109–135 binds to LPS, neutralizing its ability to induce TNF production by mouse cells, and that suppression of TNF production results in protection of the mice from death by endotoxin shock.
β-Lactam antibiotics, including CAZ, inhibit cell wall synthesis by gram-negative bacteria and cause bacteriolysis and release of endotoxin (19, 36, 37, 44). CAZ induces the release of large amounts of endotoxin and protein (CAZ-endotoxin) from gram-negative bacteria (36). In the present study, the effect of CAP18109–135 on CAZ-endotoxin was shown to be the same as that on LPS (Fig. 1). This indicates that CAP18109–135 can effectively bind to protein-rich CAZ-endotoxin in vivo and neutralize its activity and that the large amount of protein contained in a CAZ-endotoxin preparation does not interfere with the endotoxicity-neutralizing activity of CAP18109–135.
Treatment of bacteremic animals with antibiotics can induce the release of endotoxin from the infecting bacteria and lead to worsening of symptoms such as shock (22). Control of the antibiotic-induced endotoxin release is important to prevent septic shock (22). It has been reported that treatment of d-GalN-sensitized, P. aeruginosa-infected mice with CAZ increased the likelihood of death rather than curing the mice (5, 33, 36). While antibiotic-induced endotoxin release has been extensively documented in experimental studies, evidence of the clinical consequences of endotoxin release in the treatment of infections by gram-negative bacteria in humans remains unproven. Humans seem to be more susceptible to septic shock than are mice, since patients with bacteremia of less than 100 CFU/ml may develop septic shock whereas at least 100 times this number of bacteria is necessary to cause septic shock in mice. The smaller number of bacteria might result in less antibiotic-induced endotoxin release from infecting bacteria in patients, and the released endotoxin might not be present in sufficient amounts to result in manifest shock syndrome.
The present study clearly demonstrated that injection of CAP18109–135 protected d-GalN-sensitized, P. aeruginosa-infected, CAZ-treated mice from death (Fig. 3). Measurement of LPS levels in the plasma of such mice indicated that injection of CAP18109–135 markedly lowered these levels (Fig. 4). Treatment of P. aeruginosa-infected mice with CAZ reduced the number of viable infecting bacteria in the blood to about 1/10 of the number in mice not treated with CAZ, whereas injection of CAP18109–135 in addition to CAZ did not further reduce the numbers of viable infecting bacteria, which remained at the level in mice treated with CAZ alone (Fig. 4). Thus, the present study indicates strongly that the protective effect of CAP18109–135 against death after CAZ treatment of P. aeruginosa-infected mice depends on the LPS-neutralizing activity of CAP18109–135 and not on its antibacterial activity. The secretion of TNF in in vitro culture of liver tissue of CAP18109–135-treated mice that had been infected with P. aeruginosa, treated with CAZ, and sensitized with d-GalN was reduced significantly compared with that in liver tissue of mice not treated with CAP18109–135 (Fig. 6). These results suggest that CAP18109–135 neutralizes the ability of LPS to induce the production of TNF and prevents death from endotoxic shock.
CAP18-derived peptides exert activity in vitro against various species of gram-positive and gram-negative bacteria (15, 24, 25). In the present study, however, injection of CAP18109–135 into P. aeruginosa-infected mice did not cause a statistically significant decrease in the number of viable infecting bacteria in their blood; furthermore, injection of CAP18109–135 together with CAZ did not decrease the number of viable infecting bacteria that was present after CAZ treatment alone (Fig. 4). These results suggest that CAP18109–135 has no antibacterial activity in vivo. This lack of antibacterial activity in vivo might be explained by the finding that plasma protein interferes strongly with the antibacterial activity of CAP18109–135 (Fig. 5), and the possibility of quick degradation of CAP18111–137 peptide in vivo could not be ruled out. The requirement of larger amounts of CAP18109–135 for neutralization of endotoxic activity in vivo than in vitro (data not shown) may be explained by the same mechanisms.
Several neutrophil cationic peptides have been reported to be toxic to various kinds of eukaryotic cells, such as Giardia lamblia (2), neuronal and glial cells (38), and lymphocytes (40). However, CAP18109–135 does not exhibit any acute toxicity in mice even at doses up to 44 mg/kg of body weight. Some other antibacterial substances in neutrophils, such as indolicidin peptide (8) and defensins (29), are active against bacteria only under hypotonic conditions. However, CAP18 peptides are active under isotonic conditions. New approaches to therapy of septic shock are urgently needed. Several agents, including an IL-1 receptor antagonist (1), a soluble TNF receptor (3), and an LPS antagonist (7), are under clinical investigation. These agents block some of the proinflammatory mediators in the cascade triggered by LPS. Unlike these known agents, CAP18109–135 would block the onset of the cascade of proinflammatory mediators by binding directly to LPS and inactivating its activity. Therefore, CAP18109–135 may provide a new therapeutic procedure for septic shock syndrome.
ACKNOWLEDGMENTS
We thank Kazuhisa Saito, Keio University, Tokyo, Japan, for critical review of the manuscript; Hidekado Tokumoto, Ono Pharmaceutical Co., Osaka, Japan, for many helpful discussions; and Maki Watanabe, Tokyo Research Institute, Seikagaku Corp., Tokyo, for excellent technical assistance with the Limulus test.
This study was supported by grants from the Ministry of Education, Science and Culture, Japan (08457090 to T.K.), from Ono Pharmaceutical Co., and from Seikagaku Corp.
REFERENCES
- 1.Alexander H L, Doherty G M, Buresh C M, Venzon D J, Norton J A. A recombinant human receptor antagonist to interleukin 1 improves survival after lethal endotoxemia. J Exp Med. 1991;173:1029–1036. doi: 10.1084/jem.173.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aley S B, Zimmerman M, Hetsko M, Selsted M E, Gillin F D. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect Immun. 1994;62:5397–5403. doi: 10.1128/iai.62.12.5397-5403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Marsters A S, Capon D J, Chamow S M, Figari I S, Pennica D, Goeddel D V, Palladino M A, Smith D H. Protection against endotoxin shock by a tumor necrosis factor receptor immunoadhesin. Proc Natl Acad Sci USA. 1991;88:10535–10540. doi: 10.1073/pnas.88.23.10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atlas R M. Handbook of microbiological media. Boca Raton, Fla: CRC Press; 1993. [Google Scholar]
- 5.Bucklin S E, Morrison D C. Differences in therapeutic efficacy among cell wall-active antibiotics in a mouse model of gram-negative sepsis. J Infect Dis. 1995;172:1519–1527. doi: 10.1093/infdis/172.6.1519. [DOI] [PubMed] [Google Scholar]
- 6.Cerami A, Beutler B. The role of cachectin/TNF in endotoxic shock and cachexia. Immunol Today. 1988;9:28–31. doi: 10.1016/0167-5699(88)91353-9. [DOI] [PubMed] [Google Scholar]
- 7.Christ W J, Asano O, Robidoux A L C, Perez M, Wang Y, Dubuc G R, Gavin W E, Hawkins L D, McGuinness P D, Mullarkey M A, Lewis M D, Kishi Y, Kawata T, Bristol J R, Rose J R, Rossignol D P, Kobayashi S, Hishumura I, Kimura A, Asakawa N, Katayama K, Yamatsu I. E5531, a pure endotoxin antagonist of high potency. Science. 1995;268:80–83. doi: 10.1126/science.7701344. [DOI] [PubMed] [Google Scholar]
- 8.Del Sal G, Storici P, Schnerider C, Romeo D, Zanetti M. cDNA cloning of the netrophil bactericidal peptide indolicin. Biochem Biophys Res Commun. 1992;187:467–472. doi: 10.1016/s0006-291x(05)81517-7. [DOI] [PubMed] [Google Scholar]
- 9.Elsbach P, Weiss J, Franson R C, Beckerdite-Quagliata A, Schneider A, Harris L. Separation and purification of a potent bactericidal/permeability increasing protein and a closely related phospholipase A2 from PMNs: observation on their relationship. J Biol Chem. 1979;254:11000–11008. [PubMed] [Google Scholar]
- 10.Flegel W A, Baumstark M W, Weinstock C, Berg A, Northoff H. Prevention of endotoxin-induced monokine release by human low- and high-density lipoproteins and by apolipoprotein A-I. Infect Immun. 1993;61:5140–5146. doi: 10.1128/iai.61.12.5140-5146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galanos C, Freudenberg M A, Reuter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;75:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazzano-Santoro H, Parent J B, Grinna L, Horwitz A, Persons T, Elsbach P, Weiss J, Conlon P J. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun. 1992;60:4754–4761. doi: 10.1128/iai.60.11.4754-4761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirata M, Shimomura Y, Yoshida M, Morgan J G, Palings I, Wilson D, Yen M H, Wright S C, Larrick J W. Characterization of a rabbit cationic protein (CAP18) with lipopolysaccharide-inhibitory activity. Infect Immun. 1994;62:1421–1426. doi: 10.1128/iai.62.4.1421-1426.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirata M, Shimonura Y, Yoshida M, Wright S C, Larrick J W. Endotoxin-binding synthetic peptides with endotoxin-neutralizing, antibacterial and anticoagulant activities. In: Levin J, van Deventer S J H, van der Poll T, Sturk A, editors. Bacterial endotoxin: basic science to anti-sepsis strategies. New York, N.Y: Wiley-Liss, Inc.; 1994. pp. 147–159. [PubMed] [Google Scholar]
- 15.Hirata M, Wright S C, Larrick J W. Endotoxin-neutralizing proteins for sepsis and endotoxin shock. In: Okada K, Ogata H, editors. Shock. Amsterdam: Elsevier Science, B.V.; 1996. pp. 109–115. [Google Scholar]
- 16.Hirata M, Yoshida M, Inada K, Kirikae T. Investigation of endotoxin binding cationic proteins from granulocyte. Agglutination of erythrocytes sensitized with Re-LPS. Adv Exp Med Biol. 1990;256:287–299. doi: 10.1007/978-1-4757-5140-6_25. [DOI] [PubMed] [Google Scholar]
- 17.Hirata M, Zhong J, Wright S C, Larrick J W. Structure and functions of endotoxin-binding peptides derived from CAP18. In: Levin J, Alving C R, Munford R S, Redl H, editors. Bacterial endotoxins: lipopolysaccharides from genes to therapy. New York, N.Y: Wiley-Liss, Inc.; 1995. pp. 317–326. [PubMed] [Google Scholar]
- 18.Hitchcock P J, Leive L, Makela P H, Rietschel E T, Stritimatter W, Morrison D C. Lipopolysaccharide nomenclature—past, present, and future. J Bacteriol. 1986;166:699–705. doi: 10.1128/jb.166.3.699-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson J J, Kropp H. Differences in mode of action of β-lactam antibiotics influence morphology, LPS release and in vivo antibiotic efficacy. J Endotoxin Res. 1996;3:201–218. [Google Scholar]
- 20.Kayama F, Yoshida T, Elwell M R, Luster M I. Role of tumor necrosis factor-α in cadmium-induced hepatotoxicity. Toxicol Appl Pharmacol. 1995;131:224–234. doi: 10.1006/taap.1995.1065. [DOI] [PubMed] [Google Scholar]
- 21.Kirikae F, Kirikae T, Qureshi N, Takayama K, Morrison D C, Nakano M. CD14 is not involved in Rhodobacter sphaeroides diphosphoryl lipid A inhibition of tumor necrosis factor alpha and nitric oxide induction by taxol in murine macrophages. Infect Immun. 1995;63:486–497. doi: 10.1128/iai.63.2.486-497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirikae T, Nakano M, Morrison D C. Antibiotic-induced endotoxin release from bacteria and its clinical significance. Microbiol Immunol. 1997;41:285–294. doi: 10.1111/j.1348-0421.1997.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 23.Larrick J W, Hirata M, Balint R F, Huang T-H, Chen C, Zhong J, Wright S. CAP18: a novel LPS-binding/antimicrobial protein. In: Morrison D C, Ryan J L, editors. Novel therapeutic stratiagies in the treatment of sepsis. New York, N.Y: Marcel Dekker, Inc.; 1996. pp. 71–95. [Google Scholar]
- 24.Larrick J W, Hirata M, Balint R F, Lee J, Zhong J, Wright S C. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larrick J W, Hirata M, Shimomura Y, Yoshida M, Zheng H, Zhong J, Wright S C. Antimicrobial activity of rabbit CAP18-derived peptides. Antimicrob Agents Chemother. 1993;37:2534–2539. doi: 10.1128/aac.37.12.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larrick J W, Hirata M, Zheng H, Zhong J, Bolin D, Cavaillon J M, Warren H S, Wright S C. A novel granulocyte-derived peptide with lipopolysaccharide-neutralizing activity. J Immunol. 1994;152:231–240. [PubMed] [Google Scholar]
- 27.Larrick J W, Morgan J G, Palings I, Hirata M, Yen M H. Complementary DNA sequence of rabbit CAP18. A unique lipopolysaccharide binding protein. Biochem Biophys Res Commun. 1991;179:170–175. doi: 10.1016/0006-291x(91)91350-l. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann V, Freudenberg M A, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J Exp Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehrer R I, Lichtenstein A K, Ganz T. Defensins: antimicrobial and cytotoxic peptide of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 30.Marra M N, Wilde C G, Collins M S, Snabel J L, Thronton M B, Scott R W. The role of BPI as a natural inhibitor of bacterial endotoxin. J Immunol. 1992;148:532–540. [PubMed] [Google Scholar]
- 31.Morrison D C. 4th International Congress on the Immune Consequences of Trauma, Shock, and Sepsis. Whitehouse Station, N.J: Merck & Co., Inc.; 1997. Antibiotics, endotoxin, and the pathogenesis of gram-negative sepsis; pp. 1–4. [Google Scholar]
- 32.Morrison D C. Endotoxin/antibiotics and gram-negative sepsis. J Endotoxin Res. 1996;3:171. [Google Scholar]
- 33.Morrison D C, Bucklin S E, Leeson M C, Norimatsu M. Contribution of soluble endotoxin released from Gram-negative bacteria by antibiotics to the pathogenesis of experimental sepsis in mice. J Endotoxin Res. 1996;3:237–243. [Google Scholar]
- 34.Morrison D C, Danner R L, Dinarello C A, Munford R S, Natanson C, Pollack M, Spitzer J J, Ulevitch R J, Vogel S N, McSweegan E. Bacterial endotoxins and pathogenesis of Gram-negative infections: current status and future direction. J Endotoxin Res. 1994;1:71–83. [Google Scholar]
- 35.Morrison D C, Ryan J L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 36.Nakano M, Kirikae T. Biological characterization of Pseudomonas aeruginosa endotoxin released by antibiotic treatment in vitro. J Endotoxin Res. 1996;3:195–200. [Google Scholar]
- 37.Neu H C. Relation of structural properties of β-lactam antibiotics to antibacterial activity. Am J Med. 1985;79:2–13. doi: 10.1016/0002-9343(85)90254-2. [DOI] [PubMed] [Google Scholar]
- 38.Radermacher S W, Schoop V M, Schluesener H J. Bactenecin, a leukocytic antimicrobial peptide, is cytotoxic to neuronal and glial cells. J Neurosci Res. 1993;36:657–662. doi: 10.1002/jnr.490360606. [DOI] [PubMed] [Google Scholar]
- 39.Rietschel E T, Brade H, Holst O, Brade L, Müller-Loennies S, Mamat U, Zähringer U, Beckmann F, Seydel U, Brandenburg K, Ulmer A J, Mattern T, Heine H, Schletter J, Loppnow H, Schönbeck U, Flad H-D, Hauschildt S. Bacterial endotoxin: chemical constitution, biological recognition, host response, and immunological detoxification. Curr Top Microbiol Immunol. 1996;216:39–81. doi: 10.1007/978-3-642-80186-0_3. [DOI] [PubMed] [Google Scholar]
- 40.Schluesener H J, Radermacher S, Melms A, Jung S. Leucocytic antimicrobial peptides kill autoimmune T cells. J Neuroimmunol. 1993;47:119–202. doi: 10.1016/0165-5728(93)90030-3. [DOI] [PubMed] [Google Scholar]
- 41.Schumann R R, Leong S R, Flaggs G W, Gray P W, Wright S D, Mathison J C, Tobias P S, Ulevitch R J. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 42.Shafer W M, Martin L E, Spitznage J K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphates. Infect Immun. 1984;45:29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka S, Iwanaga S. Limulus test for detecting bacterial endotoxins. Methods Enzymol. 1993;223:358–364. doi: 10.1016/0076-6879(93)23057-t. [DOI] [PubMed] [Google Scholar]
- 44.Tuomanen E, Gilbert K, Tomasz A. Modulation of bacteriolysis by cooperative effects of penicillin-binding proteins 1a and 3 in Escherichia coli. Antimicrob Agents Chemother. 1986;30:659–663. doi: 10.1128/aac.30.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.VanderMerr T J, Menconi M J, Zhuang J, Wang H, Murtaugh R, Bouza C, Stevens P, Fink M P. Protective effects of a novel 32-amino acid C-terminal fragment of CAP18 in endotoxemic pigs. Surgery. 1995;117:656–662. doi: 10.1016/s0039-6060(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 46.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]