Abstract
Fifteen cats infected with Chlamydophila felis were monitored for the presence of C. felis DNA on ocular swabs by using real-time PCR and for clinical signs of disease. The cats were assigned to three groups: oral doxycycline at 10 mg/kg of body weight/day for 7 days (six cats), oral doxycycline at 10 mg/kg/day for 14 days (five cats), and an untreated control group (four cats). The untreated cats remained positive for C. felis throughout the trial; clinical signs were most severe on days 14 to 21 postinfection, and then they declined. Treatment with 7 and 14 days of doxycycline decreased C. felis relative copy numbers and clinical signs rapidly. C. felis became undetectable in some of the cats during or after treatment. However, after the cessation of treatment, a recurrence of high relative copy numbers of C. felis and severe clinical signs in all cats was seen. Rescue treatment with 21 days of doxycycline was successful at eliminating infection in eight of the cats; a further 28 days of doxycycline was required to eliminate infection in the remaining three cats. It was concluded that 7, 14, and, in some cases, 21 days of treatment with oral doxycycline will not eliminate C. felis infection. At least 28 days of treatment with doxycycline is required to ensure elimination of the organism. Real-time PCR is a sensitive technique for monitoring C. felis infection and the response to antibiotic treatment.
Chlamydophila felis is now recognized as the most common specific cause of conjunctivitis in cats (9, 16).
Treatment is indicated to alleviate clinical signs of infection and to eliminate carrier status and subsequent shedding of elementary bodies (14). Doxycycline is currently regarded as the treatment of choice for C. felis infection. Previous controlled studies have demonstrated that other systemic and topical treatments are less efficacious at eliminating infection (8, 10, 11). A requirement for prolonged courses (4 to 6 weeks) of doxycycline to eliminate the organism has been advocated, as has the idea that all in-contact cats should be treated (3). However, one study has suggested that shorter courses of doxycycline may also be effective at eliminating infection (12).
In previous treatment studies, C. felis isolation and conventional PCR techniques have been used to monitor the response of infection to treatment (8, 10, 11, 12, 14). PCR has been shown to be more sensitive than isolation techniques (7, 12), and real-time PCR has further advantages as it facilitates quantification of the organism (5).
The aim of this study was to assess the efficacy of 7- and 14-day treatment with doxycycline to eliminate C. felis infection by using quantitative real-time PCR to monitor the course of infection and the response to treatment.
MATERIALS AND METHODS
Fifteen specific-pathogen-free-derived cats were used in the trial. Thirteen cats were unspayed females, and two cats were unneutered males. All of the cats were aged between 4 and 5 years. The cats were randomly assigned to three different groups (A, B, and C), and each group was housed separately.
On day 0, each cat was infected by bilateral inoculation of 3 × 103 infectious units of a field isolate of C. felis onto the conjunctiva, as previously described (8). Treatment was commenced on day 7, by which time all cats had developed significant signs of clinical disease. Group A (four cats) served as untreated controls until day 42 of the trial. Group B (six cats) received 10 mg of doxycycline (Ronaxan, Merial)/kg of body weight orally once daily for 7 days. Group C (five cats) received 10 mg of doxycycline/kg orally for 14 days. If the initial course of treatment was ineffective in eliminating infection (i.e., clinical signs persisted or recurred and the presence of C. felis was demonstrated), a 21-day rescue treatment of 10 mg of doxycycline/kg was to be commenced.
Conjunctival swabs were obtained from the right eye only, by firmly rolling a cotton-tipped swab between the ventral conjunctiva and nictitating membrane. The cats in group A were swabbed on days −7 and 0, and then twice weekly for 2 weeks, and then once weekly until day 42. The cats in groups B and C were swabbed on days −7, 0, 2, 4, 5, 6, 7, and 8, and then twice weekly for 7 weeks, and then once weekly for 5 weeks. The swabs were stored at −70°C until the DNA was extracted.
Each cat was clinically examined at the time of swabbing by a single investigator (R.H.) who was blinded to the cat's treatment group. A clinical score was assigned to each cat based on a modification of a clinical scoring system previously described (14). Twelve clinical parameters were used: depression, sneezing, and nasal discharge; eyelid swelling, blepharospasm, and ocular discharge; conjunctival chemosis, congestion, and follicular development; and nictitating membrane congestion, protrusion, and follicular development. Each individual clinical parameter was awarded a score from 0 (normal) to 3 (very severe), with half units, depending on severity. The scores were then summed to give a total clinical score ranging from 0 to 72 for each cat.
For DNA extraction, each swab was first incubated at 70°C for 10 min with 200 μl of phosphate-buffered saline, 200 μl of buffer AL (DNeasy; QIAGEN, Crawley, United Kingdom), and 20 μl of proteinase K. DNA was then extracted from the resulting solution by using a DNeasy tissue kit (QIAGEN) according to the manufacturer's instructions and eluted into 50 μl of buffer AE. PCR was performed on all samples by using 5 μl of DNA template per 25-μl reaction. Real-time PCR primers and probes for both C. felis and feline 28S rDNA were used (Table 1). The PCR mixture consisted of 12.5 μl of QIAGEN 2× HotStarTaq mix, 100 nM concentrations (each) of the C. felis forward and reverse primers, 200 nM concentrations of the 28S forward and reverse primers, a 100 nM concentration of the C. felis TaqMan probe, a 100 nM concentration of the 28S TaqMan probe, MgCl2 to a final concentration of 4.5 mM, and 5 μl of template DNA made up to a final volume of 25 μl with water.
TABLE 1.
PCR primers and probes used in the multiplex PCR assaya
| Primer or probe | Primer or probe sequence | Region of gene |
|---|---|---|
| Chlam-281F | 5′-GAACTGCAAGCAACACCACTG-3′ | 281-301 |
| Chlam-357R | 5′-CCATTCGGCATCTTGAAGATG-3′ | 337-357 |
| Chlam-303T | 5′-FAM-CGCTGCCGACAGATCAAATTTTGCC-BHQ1-3′ | 303-327 |
| Cat 28S rDNA 521 for | 5′-AGCAGGAGGTGTTGGAAGAG-3′ | 521-540 |
| Cat 28S rDNA 620 rev | 5′-AGGGAGAGCCTAAATCAAAGG-3′ | 600-620 |
| Cat 28S rDNA 557 probe | 5′Texas Red-TGGCTTGTGGCAGCCAAGTGT-BHQ2-3′ | 557-577 |
Fluorescently quenched probes were labeled with either FAM or Texas Red at the 5′ end and an appropriate black hole quencher at the 3′ end. All of the above primers and probes were synthesized by Cruachem, Ltd., Glasgow, Scotland.
An iCycler IQ system (Bio-Rad Laboratories Ltd., Hemel Hempstead, London, United Kingdom) was used to perform the real-time PCR. After an initial incubation at 95°C for 15 min to activate the HotStarTaq polymerase, 45 cycles of 10 seconds at 95°C and 30 seconds at 60°C were performed. At each annealing step, fluorescence was detected at 530 and 620 nm. DNA samples from a known C. felis-infected cat and water were used as positive and negative controls, respectively.
To facilitate comparisons between swabs and calculate relative copy numbers, the C. felis values were normalized to the 28S values. For each swab, the 28S threshold cycle (Ct) was adjusted to 30 through the application of a correction factor (CF), where CF = 30 − 28S Ct. The CF value was then added to the C. felis Ct for each swab to create a normalized C. felis Ct value: normalized C. felis Ct = C. felis Ct + CF. Relative copy number values were generated based upon the notion that a C. felis Ct value of 42 equates to a template copy number of 1. Samples with no measured Ct value were given a relative C. felis copy number of 0. Each positive swab was then given a delta Ct (ΔCt) value, where ΔCt = 42 − corrected C. felis Ct. A relative copy number was assigned to each swab by calculating 1.9ΔCt, as the reaction has been shown to be 92% efficient (4).
The gene sequences used were feline 28S rDNA, accession number AF353617, and C. felis major outer membrane protein gene, accession number AF269258.
RESULTS
All swabs were positive for feline 28S DNA throughout the trial. On days −7 and 0, all cats were PCR negative for C. felis on ocular swabs and were clinically normal (clinical score of 0). By day 4, all swabs were positive for C. felis DNA, and cats had developed clinical signs characteristic of C. felis infection (see Fig. 1 to 6). The maximum relative C. felis copy numbers reached during the acute stage of infection in each cat were similar to one another (Fig. 1 to 3).
FIG. 1.
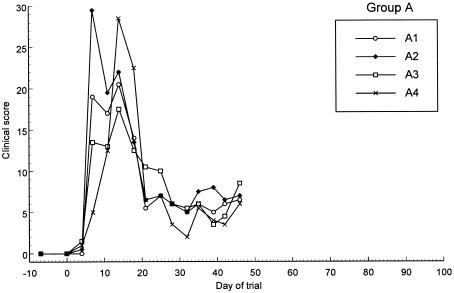
Effect of time on C. felis relative copy numbers of the untreated control cats in group A. Each line represents an individual cat.
FIG. 6.
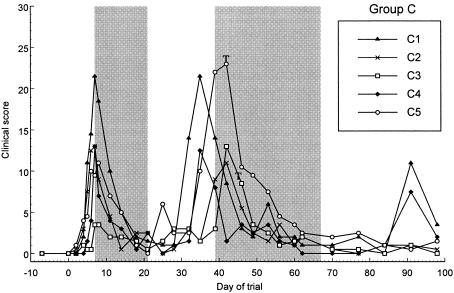
Effect of 14 days of treatment with 10 mg of doxycycline/kg on clinical score for group C. The initial 14-day treatment period is delineated by the first shaded area of the figure, from day 7 to day 21. Rescue treatment with 21 days of 10 mg of doxycycline/kg was initiated in all cats from day 39 to day 46 and is delineated by the second shaded area. Treatment was commenced on day 39 (start of the shaded area) in cats C1, C2, and C4; on day 42 in cat C5 (annotated with a T); and on day 46 in cat C3 (annotated with a T).
FIG. 3.
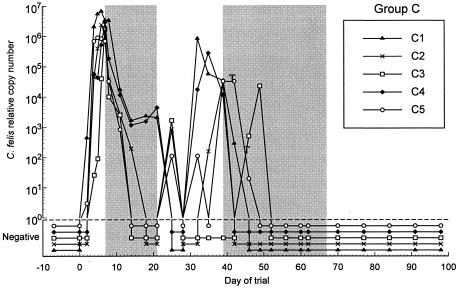
Effect of 14 days of treatment with 10 mg of doxycycline/kg on C. felis relative copy number for group C. The initial 14-day treatment period is delineated by the first shaded area of the figure, from day 7 to day 21. Rescue treatment with 21 days of 10 mg of doxycycline/kg was initiated in all cats from day 39 to day 46 and is delineated by the second shaded area. Treatment was commenced on day 39 (start of the shaded area) for cats C1, C2, and C4; on day 42 for cat C5 (annotated with a T); and on day 46 for cat C3 (annotated with a T).
In group A (untreated group), the C. felis relative copy number peaked in all cats from days 4 to 14 and then gradually diminished approximately 100- to 1,000-fold until day 42, when monitoring ceased (Fig. 1). The clinical signs in these cats increased in severity until day 14 and then diminished until day 21, after which they remained relatively constant (Fig. 4).
FIG. 4.
Effect of time on clinical scores of the untreated control cats in group A. Each line represents an individual cat.
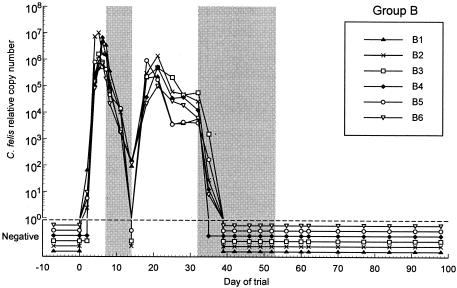
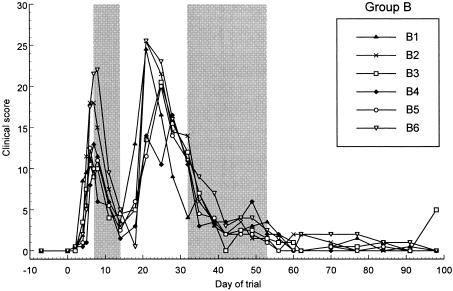
In groups B and C, shortly after initial treatment commenced on day 7, there was a marked decline in C. felis relative copy numbers and clinical signs in all cats (Fig. 2, 3, 5, and 6). In group B, relative copy numbers of C. felis DNA decreased approximately 100,000-fold in three cats and were not detectable in the remaining three cats by the end of the treatment period (day 14). However, on day 18, all swabs were positive again, with high relative copy numbers (Fig. 2). The clinical scores of all six cats decreased markedly during the treatment period; however, severe clinical signs recurred in all cats by day 18 to 21 (Fig. 5).
FIG. 2.
Effect of 7 days of treatment with 10 mg of doxycycline/kg on C. felis relative copy number for group B. The initial 7-day treatment period is delineated by the first shaded area of the figure, from day 7 to day 14. Rescue treatment with 21 days of 10 mg of doxycycline/kg was initiated on day 32 for all cats and is delineated by the second shaded area on the figure.
FIG. 5.
Effect of 7 days of treatment with 10 mg of doxycycline/kg on clinical score for group B. The initial 7-day treatment period is delineated by the first shaded area of the figure, from day 7 to day 14. Rescue treatment with 21 days of 10 mg of doxycycline/kg was initiated on day 32 for all cats and is delineated by the second shaded area on the figure.
In group C, treatment decreased markedly the C. felis relative copy numbers in all cats, and three cats were negative by day 18. The remaining two cats were negative for C. felis DNA by day 24, 3 days after the cessation of treatment. C. felis DNA was intermittently detectable in the swabs of some cats between days 14 and 35 (C2, C3, and C5). C. felis DNA was not detected in swabs from any of the cats on day 28, and Cat C5 remained negative for C. felis DNA for the longest period, from day 28 to day 42 (Fig. 3). C. felis DNA was detected again with high relative copy numbers on swabs from all cats between days 32 and 42. Doxycycline decreased clinical scores by days 11 to 18, and these scores remained low until between days 35 and 42, when they increased once more (Fig. 6).
In most treated cats, the maximum relative copy numbers reached posttreatment were at least 10-fold lower than the pretreatment copy numbers (Fig. 2 and 3). In group B, the relative copy numbers gradually decreased 10- to 100-fold until day 32, when rescue treatment was commenced (Fig. 2). This trend was not observed in group C, as rescue treatment was initiated shortly after those cats became positive for C. felis once more and severe clinical signs recurred. When the clinical signs returned in both groups, the scores were similar or, in some cases, worse than those seen during the initial acute phase of infection (Fig. 5 and 6).
Owing to the recurrence of the clinical signs and positive swab results, rescue treatment (21-day doxycycline, 10 mg/kg) was commenced for the cats in group B on day 32 of the trial (Fig. 2 and 4). In group C, treatment of individual cats was commenced at various time points within a 4-week time period, from day 39 to day 67 (Fig. 3 and 6).
Within 7 days of the commencement of rescue treatment, C. felis was no longer detectable from any of the swabs from the cats in either group (Fig. 2 and 3). The rescue treatment also rapidly decreased the clinical score of all cats in both groups (Fig. 4 and 6).
Between days 91 and 98, three cats (B3, C1, and C4) had an increase in total clinical score due to a recurrence of clinical signs in the left eye (Fig. 5 and 6). The routine swab taken from the right eye remained PCR negative, but swabs from the left eye proved to be positive for C. felis DNA. These three cats received a further 28 days of treatment with doxycycline, which resolved the clinical signs once more. None of the cats in groups B or C showed evidence of a recurrence of clinical signs over the following 6 months.
DISCUSSION
The model of induced C. felis infection used in this study produces consistent and predictable clinical disease. The time course of infection, clinical signs, and response to treatment in this study were similar to those seen in previous studies (8, 10, 11, 14). The C. felis isolate used in this study was the same as that used in a 2003 study by Owen et al. (8).
This is the first study in which real-time PCR was used to longitudinally monitor experimentally induced C. felis infection and the response to treatment. The real-time PCR used in this study is highly reproducible and sensitive and facilitates quantification of the organism (5), thereby allowing any changes in bacterial load over time and in response to treatment to be accurately assessed. The clinical scoring system used in this study has been shown to be reproducible, and the results were consistent with those from previous studies (8, 10, 11, 14). In the untreated control group, the PCR results mirrored the clinical signs during the acute stage of infection. However, as the infection became more chronic and the clinical signs became milder, the relative copy numbers of C. felis stayed relatively high and there was less correlation between clinical scores and bacterial load. In the treated groups, the clinical scores and PCR results showed very similar patterns throughout the study. However, when the disease recurred after the initial treatment period, the marked increase in relative copy numbers of C. felis preceded the increases in clinical scores.
After the initial treatment with doxycycline was begun, there was a rapid decline in the relative copy numbers of C. felis recovered from the conjunctiva. This decline was accompanied by a concurrent marked decline in the severity of the clinical signs, a result which is consistent with those of previous studies (8, 10, 11). The results of this study demonstrate that treatment with 7 and 14 days of doxycycline is effective at reducing the numbers of organisms present and at alleviating clinical signs of infection. In some cats, C. felis DNA became undetectable in swabs following or during the initial course of treatment. However, shortly after treatment ceased, ocular swabs became PCR positive for C. felis and clinical signs returned in all cats. These findings suggest that treatment with doxycycline for 7 and 14 days is ineffective at eliminating C. felis infection. Treatment for 14 days resulted in lower relative copy numbers and reduced the clinical signs for a longer period than the 7-day course. However, there was little difference in the relative copy numbers or the severity of the clinical signs between the two groups when the disease returned.
One study by Sykes et al. (12) suggested that short courses of doxycycline may be sufficient at eliminating infection. In that study, all of the cats were negative by conventional PCR by day 6 of a 21-day treatment period and remained negative for 14 days after treatment, at which point monitoring was ceased. It was therefore suggested that 1 to 2 weeks of treatment may be sufficient to successfully eliminate infection. The present study demonstrates that if treatment is stopped after 7 or 14 days, infection is not eliminated, despite negative PCR results. The cats in the present study received 21 days of doxycycline rescue treatment and rapidly became negative for C. felis on PCR. However, 25 to 34 days following cessation of this treatment, signs of disease returned and C. felis was subsequently detected in three cats (Fig. 5 and 6). These findings suggest that it may be advisable to continue swabbing for at least 35 days following the cessation of treatment to ensure that cats have eliminated C. felis infection.
As all of the cats in the present study had previously been treated with shorter courses of doxycycline, it is impossible to assess the efficacy of an initial course of 21 or 28 days of doxycycline in eliminating C. felis infection. Other treatment studies have been successful in eliminating C. felis infection using 19-day (11) and 25-day (8) courses of doxycycline treatment; however, these studies used isolation techniques, which are less sensitive than real-time PCR (7, 12). Strain difference may also account for some variation between studies; future studies using other isolates of C. felis would be necessary to investigate this possibility. For all of the cats, even the three cats for which a third course of treatment was required, rescue therapy with longer courses of doxycycline was subsequently effective at eliminating C. felis infection. This finding suggests that this strain of C. felis did not develop resistance to doxycycline therapy during the initial shorter courses of treatment.
In this study, some of the treated cats demonstrated negative C. felis PCR results and improvement of clinical signs following treatment but later showed recurrence of clinical disease and C. felis infection. This result implies either that the C. felis infection had not been eliminated despite the negative PCR result (false negative) or that the cats eliminated the infection and then became reinfected. A false-negative result could be due to the presence of low numbers of the organism at the conjunctiva or the persistence of the organism elsewhere. The assay used in this study is highly sensitive and under optimal conditions can detect C. felis when fewer than 10 genomic copies are present (5). However, the dilution effect of extraction and the small volume of extracted material used in each reaction could lead to false-negative results. Notably, cats C2, C3, and C5 fluctuated between negative PCR results and very low relative copy numbers from day 14 to day 28. This finding would be supportive of the hypothesis that low levels of C. felis persisted in the conjunctiva but were undetected at some time points. However, swabs from cat C3 remained negative on five consecutive occasions (day 28 to 42) (Fig. 3), making false negatives for this cat less likely.
Alternatively, the negative PCR result could imply that the organism is no longer in the conjunctival cells of the eye being swabbed but is persistent elsewhere in the cat and that when treatment ceased there was reexcretion of the organism at the conjunctiva. In this study, it has been shown that in some situations in cats with established infection it is possible to detect the organism in one eye but not the other. Latent infection and carrier status of C. felis in cats has also been postulated (14). C. felis has been isolated from the gastrointestinal tract, genital tract, lung, tonsil, liver, spleen, kidney, and blood (4, 6, 15). It has been shown that C. felis recovered from gastric mucosa of cats can induce ocular infection (1). The significance of the presence of the organism at these sites and the importance in transmission and persistence of the infection is still unknown (1, 15). These findings may indicate that in order to maximize the chance of detecting C. felis infection, both eyes (and possibly other sites) should be swabbed if there is clinical suspicion of C. felis infection.
The possibility of reinfection between cats as a contributory factor to the recurrence of disease cannot be excluded as the cats in each group were housed together. Due to ethical considerations and space, management, and financial constraints, it was not possible to keep the cats in isolation. In group B, reinfection is possible, as not all cats had negative PCR results at the same time. However, in group C, C. felis DNA was not detected from any of the cats on day 28 but the cats became PCR positive again 4 to 18 days later. Transmission via fomites in the cats' environment may also be a possible method of reinfection (13).
In the untreated group (group A), the relative copy numbers for all of the cats began to decrease from day 21. This decrease was mirrored by a decrease in the clinical scores, which has also been observed in previous studies (8, 10, 11). The cats in group B showed a similar decline in relative copy and clinical scores between days 21 and 32 (before rescue treatment commenced). This finding may indicate that the cats were mounting an immune response which was effective at reducing bacterial load and replication and the severity of the disease but was clearly not effective at eliminating infection. Following the recurrence of infection after the initial treatment period, the relative copy numbers remained 100-fold lower than at the time of initial infection in both groups (Fig. 2 and 3). This finding may be due to the previously administered treatment or, again, this may indicate the development of an immune response associated with a reduction of the amount of organism present.
Interestingly, when the clinical signs returned following the initial treatment, they were of equal or, in some cases, increased severity compared to the signs seen during the primary acute phase of infection (Fig. 5 and 6), despite a apparently lower bacterial load. The clinical scores of the cats in groups B and C posttreatment were, in fact, worse than those of the untreated controls at this time point of the trial (Fig. 4, 5, and 6). This finding may indicate that the immune response or chronic carriage of C. felis infection may contribute to the severity of the clinical signs seen, similar to the severe sequelae of repeated ocular infection with C. trachomatis in humans (2).
In conclusion, the results of this study show that 7 and 14 days of systemic doxycycline treatment do not eliminate C. felis infection, despite a reduction in clinical signs and bacterial load. Furthermore, in some situations, 21-day treatment may also be inadequate, and the current recommendation to treat all cats for a minimum of 4 weeks is supported by the results of this study. In addition, this study demonstrates that real-time PCR is a useful, sensitive technique for quantifying the progression of C. felis infection and its response to antibiotic treatment.
Acknowledgments
We thank Fort Dodge Animal Health, United Kingdom, for providing the grant to support Rachel Dean, and Séverine Tasker and the animal technicians at the University of Bristol for help in medicating and caring for the cats.
REFERENCES
- 1.Gaillard, E. T., A. M. Hargis, D. J. Prieur, J. F. Evermann, and A. S. Dhillon. 1984. Pathogenesis of feline gastric chlamydial infection. Am. J. Vet. Res. 45:2314-2321. [PubMed] [Google Scholar]
- 2.Grayston, J. T., S. P. Wang, L. J. Yeh, and C. C. Kuo. 1985. Importance of reinfection in the pathogenesis of trachoma. Rev. Infect. Dis. 7:717-725. [DOI] [PubMed] [Google Scholar]
- 3.Greene, C. E. 1998. Chlamydial infections. In C. E. Greene (ed.), Infectious diseases of the dog and cat, 2nd ed. W. B. Saunders, Philadelphia, Pa.
- 4.Hargis, A. M., D. J. Prieur, and E. T. Gaillard. 1983. Chlamydial infection of the gastric mucosal in twelve cats. Vet. Pathol. 20:170-178. [DOI] [PubMed] [Google Scholar]
- 5.Helps, C., N. Reeves, K. Egan, P. Howard, and D. Harbour. 2003. Detection of Chlamydophila felis and feline herpesvirus by multiplex real-time PCR analysis. J. Clin. Microbiol. 41:2734-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masubuchi, K., H. Nosaka, K. Iwamoto, T. Kokubu, M. Yamanaka, and Y. Shimizu. 2002. Experimental infection of cats with Chlamydophila felis. J. Vet. Med. Sci. 64:1165-1168. [DOI] [PubMed] [Google Scholar]
- 7.McDonald, M., B. J. Willett, O. Jarrett, and D. D. Addie. 1998. A comparison of DNA amplification, isolation and serology for the detection of Chlamydia psittaci infection in cats. Vet. Rec. 143:97-101. [DOI] [PubMed] [Google Scholar]
- 8.Owen, W. M. A., C. P. Sturgess, D. A. Harbour, K. Egan, and T. Gruffydd-Jones. 2003. Efficacy of azithromycin for the treatment of feline chlamydophilosis. J. Feline Med. Surg. 5:305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shewen, P. E., R. C. Povey, and M. R. Wilson. 1978. Feline chlamydial infection. Can. Vet. J. 19:289-292. [PMC free article] [PubMed] [Google Scholar]
- 10.Sparkes, A. H., S. M. A. Caney, C. P. Sturgess, and T. Gruffydd-Jones. 1999. The clinical efficacy of topical and systemic therapy for the treatment of feline ocular chlamydiosis. J. Feline Med. Surg. 1:31-35. [DOI] [PubMed] [Google Scholar]
- 11.Sturgess, C. P., T. Gruffydd-Jones, D. A. Harbour, and R. L. Jones. 2001. Controlled study of the efficacy of clavulanic acid-potentiated amoxicillin in the treatment of Chlamydia psittaci in cats. Vet. Rec. 149:73-76. [DOI] [PubMed] [Google Scholar]
- 12.Sykes, J. E., V. P. Studdert, and G. F. Browning. 1999. Comparison of the polymerase chain reaction and culture for the detection of Chlamydia psittaci in untreated and doxycycline-treated experimentally infected cats. J. Vet. Intern. Med. 13:146-152. [DOI] [PubMed] [Google Scholar]
- 13.Sykes, J. E. 2004. Chlamydial infections. In E. A. Chandler, C. J. Gaskell, and R. M. Gaskell (ed.), Feline medicine and therapeutics, 3rd ed. Blackwell Publishing, Oxford, United Kingdom.
- 14.Wills, J. M. 1986. Chlamydial infection in cats. Ph.D. thesis. University of Bristol, Bristol, United Kingdom.
- 15.Wills, J. M., T. J. Gruffydd-Jones, S. J. Richmond, R. M. Gaskell, and F. J. Bourne. 1987. Effect of vaccination on feline Chlamydia psittaci infection. Infect. Immun. 55:2653-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wills, J. M., P. E. Howard, T. J. Gruffydd-Jones, and C. M. Wathes. 1988. Prevalence of Chlamydia psittaci in different cat populations in Britain. J. Small Anim. Practice 29:327-339. [Google Scholar]