Abstract
Prominent antigens of Treponema denticola have been suggested to be mediators of the cytopathic effects typically seen in periodontal disease. In the present study of the T. denticola major surface protein (Msp) and the surface-expressed chymotrypsinlike protease complex (CTLP), we characterized the ability of these proteins to adhere to and lyse epithelial cells. Msp and CTLP were closely associated in spirochete outer membranes. Purified Msp, both native and recombinant, and CTLP bound to glutaraldehyde-fixed periodontal ligament epithelial cells. Adherence of Msp was partially blocked by specific antibodies. Adherence of CTLP was partially blocked by serine protease inhibitors and was further inhibited by specific antibodies. Both native Msp and CTLP were cytotoxic toward periodontal ligament epithelial cells, and their cytotoxicity was inhibited by the same treatments that inhibited adherence. Msp, but not CTLP, lysed erythrocytes. Msp complex (partially purified outer membranes free of protease activity) was cytotoxic toward a variety of different cell types. Pore-forming activities of recombinant Msp in black lipid model membrane assays and in HeLa cell membranes were similar to those reported for the native protein, supporting the hypothesis that Msp cytotoxicity was due to its pore-forming activity.
Oral spirochetes, most notably Treponema denticola, are associated with severe periodontal disease conditions (54, 61) including acute necrotizing ulcerative gingivitis (41) and early-onset periodontitis (42). While characterization of spirochetes as specific periodontopathogens has been problematic due to difficulties in growing them in vitro, their numerical prevalence in many diseased sites and expression of numerous potential virulence factors suggest that they may play an important role in the progression of the disease (16, 40, 42).
Studies of the role of treponemes in periodontal diseases have concentrated on their in vitro activity in model cell systems. These studies have demonstrated that T. denticola adheres to fibroblasts and epithelial cells (14, 24, 36, 66), as well as to extracellular matrix components present in periodontal tissue (10, 26). T. denticola exhibits hemagglutinating activity (20, 52) hemolysis (8), and hemoxidizing activity (8) toward erythrocytes. In addition, several cytopathic activities of T. denticola against epithelial cells and fibroblasts representative of periodontal tissues have been reported. Carranza et al. (5) observed membrane damage and vacuolization of periodontal tissues associated with oral spirochetes. Epithelial cells exposed in vitro to T. denticola show visible morphological damage, inhibition of proliferation, detachment (58), cytoskeletal rearrangement (11, 64), and loss of volume control (11). T. denticola challenge of fibroblast cultures results in inhibition of proliferation (3), cytoskeletal rearrangement and cell detachment (1), and degradation of plasma membrane fibronectin (15). T. denticola sonicates suppress human lymphocyte proliferative responses to mitogens and antigens without affecting their viability (60).
Molecular characterization of the cytopathic and immunomodulatory effects of T. denticola progressed slowly until quite recently. Several T. denticola components with cytopathic effects have been described, including peptidoglycan (22) and peptides of 62.5, 45, and less than 1 kDa with hemolytic activity (7, 34). While some of these activities were associated with membrane fractions of T. denticola, their cellular locations and roles in periodontal cytopathology remain to be characterized. Of the T. denticola surface-expressed proteins that have been described, only the major surface protein (Msp) (25, 65) and the surface-expressed chymotrypsinlike protease complex, with an Mr of 95,000 (CTLP) (47, 63), have demonstrated adhesin activity (17, 25, 38, 65) or cytopathic effects (49, 64). A recent study showed that a T. denticola outer membrane preparation containing predominantly Msp, with some CTLP activity, triggered the specific release of matrix metalloproteinases by human polymorphonuclear leukocytes (12).
Msp is an adhesin (25) with pore-forming activity both in artificial membranes (13) and in epithelial cell membranes (49). In its oligomeric form, Msp is visible as a hexagonal array in the outer membrane of T. denticola (13, 48). The apparent molecular mass of monomeric Msp and Msp-like proteins varies among strains from approximately 42 to 64 kDa (18). The gene encoding Msp was recently cloned, sequenced and expressed in Escherichia coli, and recombinant Msp and T. denticola adhered similarly to components of the extracellular matrix (ECM) (17).
CTLP (47, 63) has been implicated in T. denticola adherence to epithelial cells (38), as well as in degradation of host cell protease inhibitors (21) and fibronectin degradation and cell detachment in both epithelial cells (64) and fibroblasts (1, 11). These properties may contribute to the observed ability of CTLP to mediate the migration of T. denticola through model basement membranes (23) and to increase the permeability of a multilayer epithelial cell model (64). Recently, the DNA encoding two of the three peptides associated with the CTLP complex was cloned and sequenced, and one of the peptides exhibited homology to subtilisin-type serine proteases (33).
While previous studies have provided indirect evidence for the involvement of Msp and CTLP in cytotoxicity of oral treponemes, the cytotoxicity of the purified proteins has not been reported. Msp is the most abundant protein in the outer membrane of T. denticola (25). CTLP is also highly expressed and is enzymatically active toward a wide range of substrates (47, 63). The present study describes the adherence and cytotoxicity to eukaryotic cells mediated by surface components of this important periodontopathogen. This information will permit further characterization of the cytopathic molecular events resulting from the interaction of T. denticola with periodontal tissues.
MATERIALS AND METHODS
Bacterial strains.
T. denticola ATCC 35405 (American Type Culture Collection, Rockville, Md.) was grown and maintained in NOS broth medium as previously described (26). Cultures were examined for purity by phase-contrast microscopy before use. Four-day-old cultures were harvested by centrifugation at 10,000 × g (10 min at 4°C), washed in phosphate-buffered saline (PBS) (10 mM Na2HPO4, 150 mM NaCl, 2.5 mM KCl, 1.5 mM KH2PO4 [pH 7.2]), and then suspended in PBS to an optical density at 600 nm of 0.2 (5 × 109 cells per ml) for use in assays.
Cell culture.
Porcine periodontal ligament epithelial (PLE) cells (epithelial cell rests of Malassez) were isolated as described previously (4). The Chinese hamster ovary cell line (CHO-K1), the rat osteogenic sarcoma cell line (ROS 17/2.8), and human gingival fibroblasts (HGF) were gifts of F. Tufaro, C. B. Wu, and J. Tonzetich, respectively, of the University of British Columbia. Normal human epidermal keratinocytes (NHEK) were purchased from Clonetics Corp. (San Diego, Calif.). All cell cultures were maintained in a humidified 5% CO2 atmosphere at 37°C. For the experiments, HGF and PLE cells between passages 5 and 10 were used. All the cell cultures except NHEK were grown and maintained in media containing the following antibiotics: 10,000 U of penicillin G per ml, 10 mg of streptomycin per ml, 1.2% (vol/vol) amphotericin B (Life Technologies, Inc., Gaithersburg, Md.). HeLa cells were maintained in modified Eagle’s medium (MEM; Life Technologies, Inc.) containing 10% fetal bovine serum (FBS; Life Technologies, Inc.). CHO-K1 cells were cultured in RPMI 1640 (StemCell Technologies, Vancouver, Canada) containing 10% FBS. ROS 17/2.8 cells were cultured in F-12 medium (Life Technologies, Inc.) containing 5% FBS. PLE cells were cultured in alpha MEM (StemCell Technologies) supplemented with 15% FBS. HGF were grown in Dulbecco’s MEM (StemCell Technologies) supplemented with nonessential amino acids (Life Technologies, Inc.), 50 μg of l-ascorbic acid per ml, 584 μg of l-glutamine per ml, 110 μg of sodium pyruvate per ml, and 10% FBS. NHEK were maintained in KGM medium (Clonetics) supplemented with bovine pituitary extract (30 μg per ml), human recombinant epidermal growth factor (10 ng per ml), insulin (5 μg per ml), hydrocortisone (0.5 μg per ml), gentamicin sulfate (50 μg per ml), and amphotericin B (50 μg per ml).
Preparation of antisera.
Polyclonal rabbit antisera to T. denticola ATCC 35405 whole cells and to purified components (CTLP, native Msp, and recombinant Msp [rMsp]) were prepared as described previously (17, 23, 25, 26). Polyclonal mouse antiserum to Msp was prepared by injecting native Msp subcutaneously into the abdominal area of shaved BALB/c mice. After 4 days, blood samples were removed from the tail vein. Immunoglobulins (Igs) were purified from immune serum by using protein A-Sepharose (30).
Electron microscopy.
Preparation of T. denticola cells for transmission electron microscopy and immunogold labeling of surface proteins were done as described previously (13, 25). Primary antibodies consisted of a mixture of mouse antiserum raised against Msp and rabbit antiserum raised against CTLP. Secondary antibodies were a cocktail of goat anti-mouse IgG conjugated to 10-nm gold beads and goat anti-rabbit IgG conjugated to 5-nm gold beads (Ted Pella, Inc.). Negative staining was carried out as described previously (13). Specimens were examined with a Philips 300 electron microscope operating at 60 or 80 kV.
Gel electrophoresis and immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western immunoblotting were done as described previously (17). Proteins in gels were detected by silver staining or Coomassie brilliant blue staining. Proteins blotted to nitrocellulose membranes were probed with rabbit polyclonal primary antibodies and alkaline phosphatase-conjugated anti-rabbit secondary antibodies, and the membranes were developed with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (Sigma, St. Louis, Mo.).
Preparation of Msp complex.
Extraction of Msp complex from T. denticola with 1% n-octyl-polyoxyethylene (Bachem, King of Prussia, Penn.) was performed as described previously (49). The soluble fraction of the n-octyl-polyoxyethylene extract was then incubated for 24 to 48 h at 37°C to allow autoproteolysis, after which CTLP could no longer be detected with anti-CTLP IgG or chromogenic substrates for trypsinlike and chymotrypsinlike proteinases (63). The resulting material, Msp complex, was concentrated and prepared for use in assays as described previously (49).
Triton X-114 extraction and phase partitioning.
Extraction and phase partitioning of treponemal outer membrane proteins were performed as described for T. pallidum (9) with slight modifications. Treponemes were harvested by centrifugation at 10,000 × g (10 min at 4°C), washed in Tris-buffered saline, suspended in 1/40 volume of 20 mM Tris-HCl (pH 7.5)–2 mM EDTA–1 mM dithiothreitol containing 1% Triton X-114, and stirred gently overnight at 4°C. Detergent-extracted cells were centrifuged at 17,000 × g for 10 min at 4°C. The supernatant, enriched for outer membrane components, was partitioned into aqueous and detergent phases, and each phase was reextracted (9).
Preparative electrophoresis of Msp and CTLP.
Msp and CTLP were purified from the aqueous and detergent phases, respectively, of Triton X-114 extracts of 3-liter batch cultures of T. denticola by preparative SDS-PAGE with a model 491 Prep Cell (Bio-Rad Laboratories, Richmond, Calif.). Each sample was concentrated to approximately 5 ml in a CentriPrep 30 ultrafiltration unit (Amicon Inc., Beverly, Mass.), mixed with an appropriate volume of standard sample buffer containing reducing agent, and layered (without preheating) on the stacking-gel matrix. Samples were electrophoresed at 60 mA and 4°C through the 4% acrylamide stacking gel and the 7.5% acrylamide gel. The running and elution buffers consisted of 25 mM Tris (pH 8.3), 192 mM glycine, and 0.1% SDS. The eluate was collected in fractions of 2.5 ml at a flow rate of 1 ml per min. Fractions containing proteins of interest were concentrated by ultrafiltration, precipitated in acetone to remove detergent (27), and stored in aliquots at −70°C. For use in assays, Msp and CTLP were resuspended in alpha MEM lacking FBS and phenol red, PBS, or saline (150 mM NaCl). The protein concentration was determined as described previously (17). The chymotrypsinlike activity of CTLP samples was monitored periodically during storage and during assays by using the chromogenic substrate succinyl-l-alanyl-l-alanyl-l-prolyl-l-phenylalanine-p-nitroanilide (SAAPNA) as described previously (23).
Preparation of rMsp.
rMsp was purified by immunoaffinity chromatography as described previously (17), and then samples were washed repeatedly through a desalting column (EconoPac 10DG; Bio-Rad) in PBS containing 0.03% Triton X-100, concentrated by ultrafiltration, and stored at −70°C until use. For use in assays, aliquots of rMsp were thawed, precipitated in acetone to remove all traces of detergent, and resuspended in PBS, saline, or serum-free alpha-MEM as described above.
Adherence assay.
Glutaraldehyde-fixed PLE cells (FPLE cells) in 96-well plates were used as a substrate for assays of adherence of T. denticola proteins. Confluent monolayers of PLE cells were washed in PBS containing 0.5 mM CaCl2 and 0.5 mM MgCl2 and fixed in 0.25% glutaraldehyde by the method of Mintz and Fives-Taylor (53). Confluence of FPLE cells after fixation was confirmed by visual inspection in an inverted microscope. Cell or protein preparations in 0.1 ml of PBS were incubated with FPLE cells at room temperature or 4°C for the times indicated, after which the FPLE cells were washed twice with PBS. T. denticola cells and purified proteins adhering to FPLE cells were detected by enzyme-linked immunosorbent assay (ELISA) as described previously (17) with antibodies raised against T. denticola cells, Msp, or CTLP as appropriate. For inhibition studies, samples were pretreated with saturating levels of one or more of the following for 1 h before addition to FPLE cells: phenylmethylsulfonyl fluoride (PMSF; 100 μM), N-tosyl-l-phenylalanine chloromethyl ketone (TPCK; 284 μM), sodium p-tosyl-l-lysine chloromethyl ketone (TLCK; 135 μM), anti-rMsp IgG (20 μg per ml), anti-CTLP IgG (20 μg per ml), or normal rabbit IgG (20 μg per ml). Protease inhibitors were removed before adherence assays by washing T. denticola cells with PBS and by washing treated protein samples with PBS in a CentriPrep 30 ultrafiltration unit. Parallel untreated samples were subjected to identical incubation and wash conditions. The SAAPNA activities of CTLP samples were monitored before and after treatment and after the adherence assay. Neither primary nor secondary antibodies adhered to bovine serum albumin (BSA)-blocked ELISA plate wells or to FPLE cell monolayers.
Cytotoxicity assays.
The cytotoxicity of T. denticola components toward cultured cells was quantified by measuring the release of the cytosolic enzyme lactate dehydrogenase (LDH) in culture supernatants (37) with the CytoTox 96 kit (Promega, Madison, Wis.). Confluent cell cultures in 96-well plates (Falcon) were washed in growth medium lacking FBS and antibiotics; bathed in growth medium lacking FBS, antibiotics, and phenol red; and then challenged with T. denticola preparations in tissue culture medium or PBS. After challenge, 50 μl of the supernatant was transferred to a fresh 96-well plate, and the LDH activity was detected as specified by the manufacturer. The absorbance of each well at 490 nm was determined with a Bio-Rad model 3550 microplate reader and compared to that of a positive lysis control (supplied by the manufacturer) and a negative lysis control (saline or alpha MEM). Cultures were also inspected microscopically before and after challenge. The ability of various compounds and antibodies to inhibit cytotoxic effects of T. denticola proteins was tested as described above for adherence assays.
Alternatively, the cytotoxicity of T. denticola components was measured by a viability assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) as described by Mosmann (55). The cells were grown and washed as described above and then challenged with 100 μl of T. denticola preparation in culture medium as described above. MTT (10 μl per well, 5 mg per ml in PBS) was added to the wells, and the mixtures were incubated at 37°C for 2 to 4 h. To dissolve any formazan crystals, 150 μl of 20% SDS–50% dimethyl formamide (pH 4.7) was added to each well and mixed thoroughly before the plate was read. The absorbance of each well at 595 nm was determined, and the viability of the cells after challenge was computed as described for the LDH assay.
Hemolysis assay.
Hemolytic activities of T. denticola and outer membrane components were assayed in triplicate samples by the method described by Grenier (20). Human erythrocytes (2% [vol/vol] in 0.1 ml of saline) were prepared in 96-well plates. Washed T. denticola (approximately 2.5 × 108 cells), Msp (0.25 μg), rMsp (0.25 μg), or CTLP (up to 2.0 μg) in 0.1 ml of saline was added to erythrocytes and incubated at room temperature for 2 h. The positive hemolysis control was 10 μl of 10% SDS in 0.1 ml of saline. Hemolysis was observed visually and microscopically and was quantitated by measuring the absorbance at 550 nm with a model 3550 microplate reader. The ability of various compounds and antibodies to inhibit hemolysis was tested as described above for the adherence assays.
Black lipid bilayer analysis.
Lipid membranes formed from a 1.5% solution of oxidized cholesterol in n-decane were bathed in 1 M KCl (pH 7.0) (2). Single-channel conductance experiments at an applied voltage of 10 mV, in which T. denticola proteins were added to the aqueous phase, were conducted as described previously (13).
Patch clamp recordings from HeLa cells.
Cell-attached patch clamp recordings from HeLa cells were made as described previously (29). For cell-attached recordings, both the bath and pipette contained 10 mM HEPES, 135 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM glucose. The patch electrode solution also contained 0.03% Triton X-100. Monomeric rMsp or PBS containing 0.03% Triton X-100 was added directly to the patch electrode solution. Channel conductance was estimated from the single-channel current-voltage relation (29).
RESULTS
Visualization of Msp and CTLP in T. denticola outer membrane.
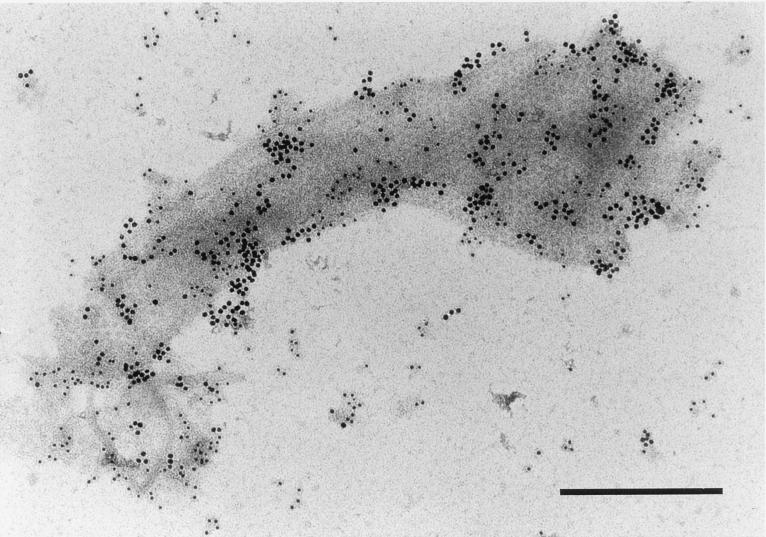
T. denticola subjected to short periods of sonication releases outer membrane material that contains CTLP (23) and Msp (25). Msp has been proposed to comprise the hexagonal array ultrastructure typical of T. denticola outer membranes (13, 18, 32, 48). The immunogold electron micrograph in Fig. 1 shows the alignment of Msp (10-nm gold beads) with the centers of the subunits of the hexagonal array. CTLP (5-nm gold beads) appears to be similarly abundant in the outer membrane.
FIG. 1.
Transmission electron micrograph of T. denticola ATCC 35405 outer membrane material released by mild sonication and probed with antibodies directed against Msp (10-nm gold beads) and CTLP (5-nm gold beads). Bar, 0.3 μm.
Purification of Msp and CTLP.
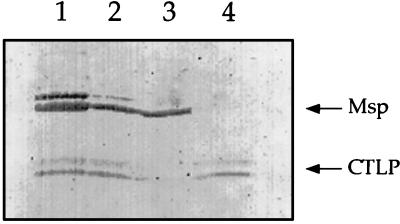
To further characterize the activities of outer membrane components, T. denticola outer membrane material was extracted with Triton X-114. Msp and CTLP segregated into the aqueous and detergent phases, respectively, of the detergent-extracted material. Msp was present in all samples except the detergent phase of the Triton X-114 extract (Fig. 2, lane 4), while CTLP was visible in all samples except the aqueous phase (lane 3). Msp and CTLP were further purified by preparative electrophoresis from the aqueous and detergent phases of the Triton X-114 extract. The fractions containing high-molecular-weight oligomers of Msp resolved as a mixture of Msp oligomers and 53-kDa monomers when unheated samples were subjected to a final round of analytical SDS-PAGE (Fig. 3, lanes 2 and 3). Fractions containing purified Msp had no detectable protease activity (data not shown). Fractions containing unheated CTLP were visible as a typical doublet at 95 kDa (lane 5) which, when heated, migrated as peptides of approximately 72, 43, and 38 kDa (data not shown).
FIG. 2.
Segregation of Msp and CTLP in Triton X-114 extracts, as shown by Western immunoblot analysis. Samples were not heated before electrophoresis. The blots were probed with a mixture of antisera raised against Msp and CTLP. Lanes: 1, crude Triton X-114 extract; 2, extract following high-speed centrifugation; 3, aqueous phase of Triton X-114 extract; 4, detergent phase of Triton X-114 extract. Arrows: Msp oligomers with an apparent molecular mass of approximately 150 kDa; CTLP doublet at approximately 95 kDa.
FIG. 3.
SDS-PAGE of Triton X-114 extract and preparative electrophoresis fractions. Lanes: 1, aqueous phase of Triton X-114 extract prior to preparative electrophoresis fractions. 2, fraction containing Msp, unheated; 3, fraction containing Msp, heated; 4, detergent phase of Triton X-114 extract prior to preparative electrophoresis; 5, fraction containing CTLP, unheated. Molecular mass standards are shown to the left of the panel.
Adherence of T. denticola and its outer membrane components to FPLE cells.
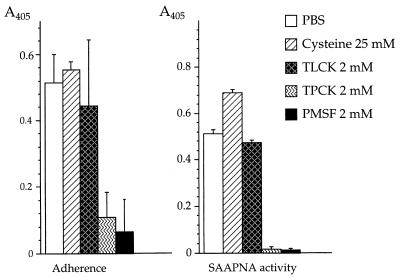
Both Msp and CTLP have been proposed to mediate adherence of treponemes to cells and ECM components associated with the periodontium. To further characterize these activities, the adherence of T. denticola cells and outer membrane components to FPLE cell monolayers was measured. As shown in Table 1, antibodies directed against Msp and CTLP partially blocked the binding of T. denticola to FPLE. Inhibition of adherence was not significantly greater when a mixture of anti-rMsp and anti-CTLP IgGs was used. Pretreatment of T. denticola cells with PMSF or TPCK (protease inhibitors that block the chymotrypsinlike activity of CTLP [63]) inhibited the attachment of T. denticola to FPLE cells, while TLCK, which does not inhibit CTLP enzymatic activity (63), had no significant effect on binding (Fig. 4). These results suggested that both Msp and CTLP are important adherence determinants of T. denticola.
TABLE 1.
Inhibition of T. denticola adherence by specific antibodies
| Pretreatmenta | % Adherenceb |
|---|---|
| PBS | 100 ± 4.0 |
| Anti-T. denticola IgG | 62 ± 6.0 |
| Anti-Msp IgG | 62 ± 3.3 |
| Anti-rMsp IgG | 71 ± 9.8 |
| Anti-CTLP IgG | 50 ± 0.6 |
| Anti-rMsp IgG + anti-CTLP IgG | 45.7 ± 5.2 |
| Rabbit IgG | 100 ± 6.9 |
T. denticola cells were incubated in specific IgG for 1 h and then washed in PBS before adherence assay.
Adherence of T. denticola to glutaraldehyde-fixed PLE cells is expressed as a percentage of the adherence of PBS-treated control. All reported values represent the means ± standard deviations for at least three experiments using samples in triplicate.
FIG. 4.
Effects of protease inhibitors on the adherence of T. denticola. Adherence of T. denticola to glutaraldehyde-fixed junctional epithelium cell monolayers (FPLE cells) was measured by ELISA with anti-T. denticola serum. CTLP activity was measured by ELISA of SAAPNA hydrolysis. A405, absorbance at 405 nm.
The T. denticola outer membrane components Msp (both native and recombinant) and CTLP adhered to FPLE cells. Adherence was, in most instances, inhibitable by the same treatments that inhibited the adherence of whole cells. Heating Msp and CTLP to 90°C completely inhibited adherence (data not shown). Pretreatment of Msp or rMsp with anti-rMsp IgG significantly inhibited adherence (Table 2). As shown in Table 3, adherence of CTLP was partially blocked by inhibitors of serine proteases. Pretreatment with anti-CTLP IgG alone had no adherence-blocking activity but significantly inhibited SAAPNA activity. Adherence of PMSF-treated CTLP to FPLE cells was inhibited to a much greater degree by anti-CTLP IgG than was adherence of untreated CTLP by either PMSF or anti-CTLP IgG alone. CTLP adhered rapidly to FPLE cells, reaching maximum levels within 15 min (data not shown). Adherence of Msp and rMsp to FPLE was similar at room temperature and at 4°C, while adherence of CTLP was significantly higher at room temperature than at 4°C (data not shown).
TABLE 2.
Adherence and cytotoxicity of Msp to epithelial cells
| Pretreatment | % Adherencea
|
% Cytotoxicityb
|
||
|---|---|---|---|---|
| Mspc | rMspc | Msp | rMsp | |
| PBS | 100 ± 0.5 | 100 ± 1.2 | 100 ± 1.2 | 100 ± 0.5 |
| Anti-rMsp IgG | 43 ± 2.7 | 64 ± 3.8 | 54 ± 4.3 | 61 ± 9.8 |
| Rabbit IgG | 100 ± 12.4 | 100 ± 6.0 | 97 ± 6.7 | 92 ± 3.1 |
Adherence to glutaraldehyde-fixed PLE cells is expressed as a percentage of that for the untreated control after a 1-h challenge.
Cytotoxicity to PLE cells as measured by release of cytoplasmic LDH is expressed as a percentage of that for the positive-lysis control after a 1-h challenge.
Native Msp was used at 1.75 μg per ml. rMsp was used at 2.5 μg per ml. Assays were conducted at room temperature.
TABLE 3.
Adherence and cytotoxicity of CTLPa to epithelial cells
| Pretreatmentb | % Adherencec | SAAPNAd | % Cytotoxicitye |
|---|---|---|---|
| PBS | 100 ± 9.6 | 100 ± 2.0 | 100 ± 1.6 |
| Anti-CTLP IgG | 92 ± 11.2 | 38 ± 3.9 | 87 ± 6.3 |
| Rabbit IgG | 98 ± 14.1 | 100 ± 0.3 | 97 ± 5.1 |
| PMSF | 73 ± 1.3 | 6.2 ± 1.5 | 71 ± 3.2 |
| TLCK | 100 ± 3.2 | 88 ± 11.6 | 96 ± 0.4 |
| TPCK | 85 ± 1.6 | 12.8 ± 3.3 | 83 ± 5.0 |
| PMSF + anti-CTLP | 46 ± 7.3 | 8.4 ± 1.0 | 42 ± 4.0 |
CTLP was used at 0.15 μg per ml. Assays were conducted at room temperature.
Samples were pretreated by incubation for 1 h and washed by ultrafiltration.
Adherence to glutaraldehyde-fixed PLE cells after a 1-h challenge, detected by anti-CTLP IgG, is expressed as a percentage of that for the PBS-treated control.
Chymotrypsin-like activity of pretreated CTLP before the PLE challenge, as measured by cleavage of the chromogenic substrate.
Cytotoxicity to PLE cells after a 1-h challenge, as measured by release of cytoplasmic LDH, is expressed as a percentage of that for the positive-lysis control.
Cytotoxicity of purified T. denticola outer membrane proteins toward PLE cells.
Both native and rMsp, as well as CTLP, induced high levels of LDH release in PLE cell cultures, indicating severe cytotoxic effects. Cytotoxicity data shown in Tables 2 and 3 represent experiments with the lowest concentrations of proteins which caused complete lysis of PLE cells within 1 h. Msp and rMsp were cytotoxic at 40 to 50 nM, while CTLP was cytotoxic at approximately 1.5 nM. To confirm that cytotoxicity was not due to detergent contamination, the effects of BSA and a 70-kDa T. denticola protein present in the detergent phase of Triton X-114 extracts were tested. Following preparative SDS-PAGE and acetone precipitation, the control proteins did not induce release of LDH (data not shown). Inhibition of cytotoxicity followed the same pattern as inhibition of adherence for Msp, rMsp, and CTLP, suggesting that cytotoxic effects resulted from adherence of the treponemal protein to the epithelial cell surface. Anti-rMsp IgG inhibited the cytotoxic effects of Msp and rMsp by 40 to 50%. Partial inhibition of cytotoxic effects (15 to 30%) was attained by pretreatment of CTLP with PMSF. Anti-CTLP IgG alone had no significant effect on the cytotoxicity of CTLP, but when CTLP was pretreated with PMSF, anti-CTLP IgG inhibited the cytotoxicity by more than 50%. The effects of heat denaturation, assay time, and assay temperature on cytotoxicity were similar to those observed in adherence assays (data not shown).
Hemolytic activity of T. denticola outer membrane proteins.
The ability of the purified outer membrane components of T. denticola to lyse human erythrocytes was tested and compared with hemolytic activity of intact T. denticola. As shown in Table 4, hemolytic activity was clearly evident with T. denticola, Msp, or rMsp and was inhibitable by anti-rMsp IgG. Msp and rMsp were hemolytic at concentrations similar to those cytotoxic to PLE cells. CTLP at concentrations up to 50× the concentration cytotoxic to PLE cells did not lyse erythrocytes, even though SAAPNA activity was still high at the end point of the assay (data not shown).
TABLE 4.
T. denticola hemolytic activity mediated by Msp
| Challengea | % Hemolytic activityb after pretreatment with:
|
||
|---|---|---|---|
| Saline | Anti-rMsp IgG | Rabbit IgG | |
| Saline | 12 ± 6.0 | NDc | ND |
| SDS | 100 ± 5.7 | ND | ND |
| T. denticola | 92 ± 5.4 | 31 ± 8.6 | 98 ± 4.6 |
| Msp | 95 ± 3.2 | 45 ± 9.0 | 91 ± 11.1 |
| rMsp | 96 ± 5.0 | 42 ± 2.6 | 97 ± 5.3 |
Erythrocytes were incubated with the listed materials in saline for 2 h at room temperature.
Hemolytic activity is expressed as a percentage of the positive-lysis control (SDS), as measured by absorbance at 550 nm.
ND, not done.
Cytotoxicity of the Msp complex.
Msp partially purified by mild detergent extraction of T. denticola and extended incubation at 37°C (Msp complex) was electrophoretically similar to Msp purified by fast protein liquid chromatography and contained neither proteolytic activity nor detectable CTLP (49). The effect of Msp complex on PLE cells was assayed by LDH release and MTT reduction assays, and the results are shown in Fig. 5A. The two assays gave similar results for Msp complex at final concentrations between 6 and 50 μg per ml. Cell lysis as measured by LDH release from PLE cells was not detected at concentrations of Msp complex below 6 μg per ml, while culture viability as measured by reduction of MTT was reduced by approximately 25% at Msp complex concentrations between 2 and 6 μg per ml, suggesting that there were intracellular effects of the Msp complex on PLE cells that preceded cytolysis.
FIG. 5.
Reduction of viability of cultured cells due to challenge by Msp complex. (A) Effects of Msp complex on PLE cells as detected by LDH release (% PLE cytotoxicity) and MTT reduction (% PLE viability). (B) Effects of the Msp complex on cultured eukaryotic cells, as detected by MTT reduction. Cell cultures are as described in Materials and Methods.
The Msp complex had similar cytotoxic effects on other cell types, as measured by the MTT reduction assay (Fig. 5B). Msp complex was cytotoxic to all cell types tested, including several epithelial cells, fibroblasts, keratinocytes, and osteoblast-like cells. In this assay, NHEK were found to be most sensitive to Msp complex, while ROS 17/2.8, HeLa, PLE, CHO-K1, and HGF cell cultures were progressively more tolerant. All the cells were killed within 24 h by Msp complex at 15 μg per ml.
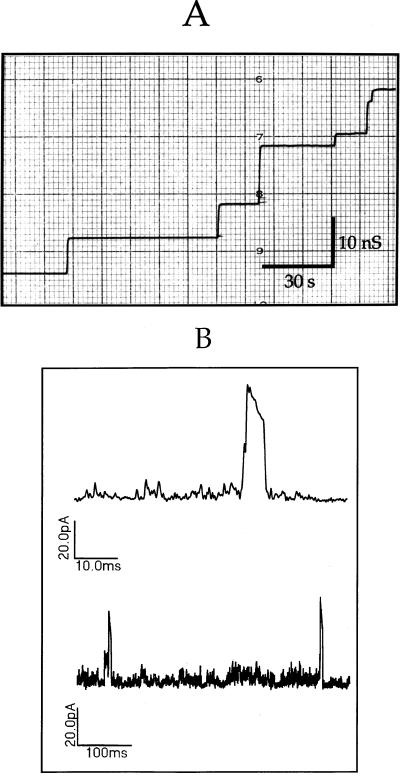
Channel-forming activity of rMsp.
Previous studies showed that native oligomeric Msp had porin activity in a black lipid bilayer system (13) and that the Msp complex depolarized epithelial cells and induced extremely large ion channels in their membranes (49). To determine whether the recombinant molecule had similar pore-forming activities, rMsp was tested under the same assay conditions. Addition of rMsp to the salt solution bathing a model lipid bilayer led to stepwise increases in membrane conductance consistent with the incorporation of pore-forming units into the membrane (Fig. 6A). In a series of 124 incorporation events, the mean single channel conductance of rMsp was 10.3 nS, which was similar to that of native Msp under the same assay conditions (reference 13 and data not shown). To determine whether rMsp had channel-forming activity in epithelial cell membranes, patch clamp recordings were made of HeLa cell membranes with rMsp in the cell-attached electrode. Monomeric rMsp at 1.5 μM caused transient conductance events in HeLa cell membranes consistent with the formation of very large, nonselective pores (Fig. 6B). The conductance of these channels was in the nanosiemen range, far higher than that of any native HeLa cell ion channel under these conditions. These observations are in agreement with the previous reports of channel-forming activity of Msp (13, 49).
FIG. 6.
Pore-forming activity of recombinant Msp in model membranes and epithelial cell membranes. (A) Single-channel conductance events due to incorporation of rMsp into black lipid bilayer model membranes. The scale shows conductance in nanosiemens on the vertical axis and time in seconds on the horizontal axis. The mean single-channel conductance at an applied voltage of 10 mV was calculated to be 10.3 nS. (B) Current recordings consistent with the gating of large conductance channels were obtained from a cell-attached patch of HeLa cell membrane with a patch electrode containing 1.5 μM rMsp. The scale associated with each recording shows the current in picoamperes on the vertical axis and time in milliseconds on the horizontal axis.
DISCUSSION
Outer membrane-associated or secreted proteins of T. denticola have been assumed to mediate the cytopathic effects of oral spirochetes. Molecular characterization of these effects has proven difficult due to several factors, including the heterogeneity of Msp proteins among different T. denticola strains (18), the association of a number of distinct proteolytic activities with oral treponemes (43, 45, 46, 51, 59), and the variety of cell models used by different researchers. The objective of the present study was to quantify and compare the adherence activity and cytotoxic effects of two important outer membrane proteins of the T. denticola type strain ATCC 35405: the pore-forming adhesin Msp, and the chymotrypsinlike protease CTLP. Prior to this study, the specific roles of each of these proteins in adherence and cytotoxicity had not been thoroughly examined.
It has been our experience that quantitative studies of adherence of T. denticola cells and purified outer membrane components to epithelial cells were difficult to replicate (24, 38). We suspected that the excessive variability observed between assays might be due to cell lysis or substrate cell detachment. The most reliable quantitative studies of T. denticola adherence have used cultured fibroblasts (reviewed in reference 14). Interestingly, we found that HGF were considerably more resistant to the cytotoxic effects of Msp complex than were any of the other cell types tested. For these reasons, we used glutaraldehyde-fixed epithelial cell cultures in adherence assays and viable epithelial cell cultures to study the cytotoxic effects of T. denticola components. The FPLE cell model gave consistent results that were in accord with previous studies of adherence of T. denticola to epithelial cells.
In previous studies of T. denticola outer membrane proteins, the ability to distinguish between the effects of Msp and CTLP has been problematical, since they tend to copurify in most detergent extracts of treponemal surface components (12, 49). The use of selective Triton X-114 partitioning to separate Msp from CTLP was useful in differentiating between the effects of Msp and CTLP. This technique has proven useful in studies of hydrophobic and amphipathic outer membrane proteins of other pathogenic spirochetes (9). In the present study, we have been able to obtain detergent-free proteins in milligram or higher quantities as necessary for use in functional assays.
Demonstration of the pore-forming activity of rMsp supported the hypothesis that Msp acts as a pore-forming cytotoxin. The porin activity of rMsp was essentially identical to that of native Msp in the black lipid bilayer system. When tested in HeLa cells, the high conductance events associated with rMsp incorporation into the cell membrane were of shorter duration than those reported previously for native Msp (56). We have not detected rMsp in the oligomeric form typical of native Msp (17). The minor differences in duration of incorporation events might be due to differences in quaternary structure between the oligomeric native Msp and monomeric rMsp, resulting in decreased stability of the monomeric molecule in the cell membrane.
Higher concentrations of rMsp were required for activity in patch clamp studies conducted on HeLa cells compared to those in cytotoxicity assays with PLE cells. There are several possible reasons for this. HeLa cells appeared to be somewhat more resistant than PLE cells to cytotoxic concentrations of Msp complex (Fig. 5B). In our previous study (49), approximately four times as much Msp complex was required to show pore-forming activity in the patch clamp assay as was required in the present study for cytotoxicity to HeLa cells (Fig. 5B), suggesting that differences in the assays themselves were partially responsible. In addition, BSA was not included in the electrode buffer when patch recordings with rMsp were made, thus allowing significant amounts of rMsp to adhere to the glass electrode.
Cytopathic effects on eukaryotic cells due to translocation of bacterial porin-like molecules to the cell membrane have been reported for Neisseria gonorrhoeae (28), Salmonella typhimurium (19), Porphyromonas gingivalis (56), and Eikenella corrodens (62). A recent study implicated bacterial porins in bone resorption (50), presumably through modulation of proinflammatory cytokines (31). The mechanism of Msp-induced cytotoxicity has not yet been identified, but data presented here suggest that Msp pore-forming activity is involved. The three Msp preparations used in this study had similar effects on PLE cultures. Msp was cytotoxic at monomer concentrations of under 50 nM. This is approximately the same concentration as reported for porin-induced bone resorption by Meghi et al., taking into account that porins were calculated as trimeric molecules in that study (50). The inability to completely block the biological activity of Msp with antibodies raised against denatured Msp may be due to exposure of unrecognized epitopes on properly folded molecules. Compared with the purified native and recombinant molecules, higher concentrations of the Msp complex were required for cytotoxicity. This apparently modulated effect could be due to one or more factors, including the formation of inactive Msp aggregates or some other artifact of the lengthy incubation protocol used during preparation of the Msp complex (56).
The data presented here complement and extend the results of recent studies of adherence and cytopathic effects mediated by CTLP. In studies of the interaction of T. denticola with HGF, both cell detachment and degradation of endogenous fibronectin on attached cells were inhibited by pretreatment of the bacteria with serine protease inhibitors (1, 15). Leung et al. (38) suggested that CTLP might mediate adherence of T. denticola to epithelial cells, since PMSF and anti-CTLP IgG inhibited the attachment of T. denticola to PLE cells. We obtained similar results when using FPLE monolayers challenged with whole T. denticola cells. The absence of increased inhibition of adherence when a mixture of anti-Msp and anti-CTLP IgGs was used could suggest a close spatial arrangement between Msp and CTLP. Adherence of T. denticola to FPLE cells was not inhibited by IgGs that bind to a 70-kDa protein on T. denticola cells (data not shown) or by normal rabbit IgG. This supports the conclusion that anti-Msp and anti-CTLP IgGs blocked adherence by binding to specific adhesins. Adherence blocking by inhibitors of CTLP enzymatic activity further supports the hypothesis that CTLP is involved in the initial interaction of the spirochete with host cells. While proteolytic enzymes other than CTLP appear to be secreted by T. denticola, none have been implicated in adherence or shown to be localized on the cell surface (44). The role of specific proteases in microbe-host cell interactions will become clearer as specific genetic mutants become available.
Under the conditions of the present study, the inability of anti-CTLP IgG to effectively block the adherence of CTLP was not surprising. Igs are substrates for CTLP protease activity (63) and could have been degraded by the highly active purified enzyme, thus preventing effective antibody-antigen recognition. When the antibody inhibition assay was done with PMSF-treated CTLP, inhibition of binding was significantly greater than that due to protease inhibitor alone. At present, nothing is known about the orientation of the CTLP catalytic site or adhesin epitope on the T. denticola cell surface, nor has the three-dimensional structure of the purified active enzyme (which consists of three peptides) been predicted. With the recent cloning and recombinant expression of the peptide that apparently contains the catalytic domain of CTLP (33), some of these issues are now potentially addressable.
Several investigators have noted cytoskeletal changes in cells exposed to T. denticola, but none have directly linked CTLP activity and cytoskeletal rearrangement (1, 11, 64). A recent study by Uitto et al. (64) described visible cytopathic effects of T. denticola including membrane blebbing, cytoplasmic vacuolization, and cytoskeletal rearrangement. The authors also reported fibronectin degradation and intercellular permeability changes attributable to CTLP chymotrypsinlike activity. In that study, assays required the use of CTLP preparations at a protein concentration greater than 2 log units higher than that used in the present study, emphasizing the need for careful storage and quantitation of enzyme activity when working with proteolytic enzymes.
It is apparent from the present study that the effects of Msp and CTLP occur rapidly and are the results of distinct mechanisms. Both molecules adhere to ECM components, while CTLP can also degrade them. When we used patch clamp techniques to study the electrophysiological responses of epithelial cells to challenge by CTLP, we were unable to obtain stable membrane patches (data not shown), suggesting that CTLP seriously disrupted cell surfaces with which it was in contact. Both Msp and CTLP were cytotoxic toward epithelial cells, but only Msp had hemolytic activity. This is in agreement with a report that protease inhibitors did not reduce the hemolytic activity of T. denticola cells (20) and suggests that CTLP might interact with a specific cell surface receptor absent on human erythrocytes. Earlier studies showing that lymphocyte proliferation was inhibited by a T. denticola factor of approximately 100 kDa (60) while fibroblast proliferation was inhibited by a 50-kDa factor (3) are intriguing in this context and may have been the first reports of the distinct cellular tropisms seen in the cytopathic activities of Msp and CTLP. The cytotoxicity of Msp was not affected by the inclusion of serum in the cytotoxicity assay mixture, while 1% FBS partially modulated the cytotoxicity of CTLP toward PLE cells (data not shown). Further studies are in progress to identify potential receptors for CTLP and to further characterize its cytotoxic effects at the molecular level.
A recent study with an epithelioid oral carcinoma cell model suggested that T. denticola induced the detachment of a specific subpopulation of epithelial cells (11), and earlier studies reported enhanced T. denticola attachment to a subpopulation of cultured epithelial cells and speculated on the apparent affinity of the spirochetes for actively dividing cells (35, 57). This implies that T. denticola may bind to epithelial cell receptors expressed at specific stages of the cell cycle. We recently identified a 65-kDa epithelial cell receptor for Msp (49). Further studies with synchronized cell cultures are needed to characterize the Msp receptor and determine whether its expression is cell cycle dependent.
Studies are in progress to accurately model the membrane topology of Msp, based on comparison of Msp molecules of different Treponema strains (18), and to identify functional domains of Msp. In light of our previous studies showing pore-forming activity by Msp and induction of large conductance ion channels in HeLa cells by Msp complex (13, 49), we hypothesized that Msp acts similarly to pore-forming cytotoxins (67). Alternatively, the binding of Msp complex to a HeLa cell surface receptor (49) might suggest the triggering of a cascade of cellular events leading to cell membrane permeabilization and cell death. The potential role of Msp in T. denticola-induced cytoskeletal changes remains to be determined. Studies are in progress to characterize the mechanism by which Msp associates with and disrupts cell membranes.
The Msp peptide, encoded by a single conserved genetic locus in T. denticola, is antigenically distinct among strains of T. denticola and other oral spirochetes (18). Recently, Msp was reported to be homologous to predicted products of a number of repetitive sequences present in the T. pallidum genome (Treponema pallidum Server, 16 June 1997 [http://utmmg .med.uth.tmc.edu/treponema/tpall.html]). It is intriguing to speculate on the significance of these homologies and on the possible role of Msp-like proteins in chronic infectious diseases other than periodontal disease. There have been no reports of antigenic variability of outer membrane proteins in T. denticola strains, such as is well known in Borrelia species (68). However, the differences in Msp peptide sequences between some strains of T. denticola are confined to the predicted surface-exposed regions (18), suggesting, at the very least, strong selection pressures for interstrain variation in this prevalent outer membrane protein. Destructive periodontal disease lesions often contain multiple strains and species of cultivable and uncultivable spirochetes (6). Even without a genetic mechanism of antigenic variation, the wide variety of Msp proteins present in this heterogeneous population could ensure a continuing source of “new” (or at least newly predominant) strains following immune responses to successive strains.
The molecular pathways involved in cytopathic effects of T. denticola are just beginning to be characterized. The availability of treponemal proteins in recombinant form will facilitate these efforts. Similarly, the recent development of methods of genetic transformation of T. denticola (39) will for the first time permit the construction of defined mutants whose interaction with host cells can be studied. Future studies will concentrate on further characterization of the cytopathological effects described here and on the molecular characterization of Msp and CTLP.
ACKNOWLEDGMENTS
We acknowledge the assistance of Andre Wong in electron microscopy and David Mathers in patch clamp experiments. We thank Robert Hancock for helpful discussions.
This study was supported by the Medical Research Council of Canada.
REFERENCES
- 1.Baehni P C, Song M, McCulloch C A, Ellen R P. Treponema denticola induces actin rearrangement and detachment of human gingival fibroblasts. Infect Immun. 1992;60:3360–3368. doi: 10.1128/iai.60.8.3360-3368.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benz R, Schmid A, Hancock R E. Ion selectivity of gram-negative bacterial porins. J Bacteriol. 1985;162:722–727. doi: 10.1128/jb.162.2.722-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehringer H, Taichman N S, Shenker B J. Suppression of fibroblast proliferation by oral spirochetes. Infect Immun. 1984;45:155–159. doi: 10.1128/iai.45.1.155-159.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunette D M, Melcher A H, Moe H K. Culture and origin of epithelium-like and fibroblast-like cells from porcine periodontal ligament explants and cell suspensions. Arch Oral Biol. 1976;21:393–400. doi: 10.1016/0003-9969(76)90001-7. [DOI] [PubMed] [Google Scholar]
- 5.Carranza F A, Jr, Saglie R, Newman M G, Valentin P L. Scanning and transmission electron microscopic study of tissue-invading microorganisms in localized juvenile periodontitis. J Periodontol. 1983;54:598–617. doi: 10.1902/jop.1983.54.10.598. [DOI] [PubMed] [Google Scholar]
- 6.Choi B K, Paster B J, Dewhirst F E, Gobel U B. Diversity of cultivable and uncultivable oral spirochetes from a patient with severe destructive periodontitis. Infect Immun. 1994;62:1889–1895. doi: 10.1128/iai.62.5.1889-1895.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu L, Holt S C. Purification and characterization of a 45 kDa hemolysin from Treponema denticola ATCC 35404. Microb Pathog. 1994;16:197–212. doi: 10.1006/mpat.1994.1020. [DOI] [PubMed] [Google Scholar]
- 8.Chu L, Kennell W, Holt S C. Characterization of hemolysis and hemoxidation activities by Treponema denticola. Microb Pathog. 1994;16:183–195. doi: 10.1006/mpat.1994.1019. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham T M, Walker E M, Miller J N, Lovett M A. Selective release of the Treponema pallidum outer membrane and associated polypeptides with Triton X-114. J Bacteriol. 1988;170:5789–5796. doi: 10.1128/jb.170.12.5789-5796.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson J R, Ellen R P. Tip-oriented adherence of Treponema denticola to fibronectin. Infect Immun. 1990;58:3924–3928. doi: 10.1128/iai.58.12.3924-3928.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Filippo A B, Ellen R P, McCulloch C A. Induction of cytoskeletal rearrangements and loss of volume regulation in epithelial cells by Treponema denticola. Arch Oral Biol. 1995;40:199–207. doi: 10.1016/0003-9969(95)98809-d. [DOI] [PubMed] [Google Scholar]
- 12.Ding Y, Uitto V-J, Haapasalo M, Lounatmaa K, Konttinen Y T, Salo T, Grenier D, Sorsa T. Membrane components of Treponema denticola trigger proteinase release from human polymorphonuclear leukocytes. J Dent Res. 1996;75:1986–1993. doi: 10.1177/00220345960750121101. [DOI] [PubMed] [Google Scholar]
- 13.Egli C, Leung W K, Müller K H, Hancock R E, McBride B C. Pore-forming properties of the major 53-kilodalton surface antigen from the outer sheath of Treponema denticola. Infect Immun. 1993;61:1694–1699. doi: 10.1128/iai.61.5.1694-1699.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellen R P, Dawson J R, Yang P F. Treponema denticola as a model for polar adhesion and cytopathogenicity of spirochetes. Trends Microbiol. 1994;2:114–119. doi: 10.1016/0966-842x(94)90597-5. [DOI] [PubMed] [Google Scholar]
- 15.Ellen R P, Song M, McCulloch C A. Degradation of endogenous plasma membrane fibronectin concomitant with Treponema denticola 35405 adhesion to gingival fibroblasts. Infect Immun. 1994;62:3033–3037. doi: 10.1128/iai.62.7.3033-3037.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenno, J. C., and B. C. McBride. Virulence factors of oral treponemes. Anaerobe, in press. [DOI] [PubMed]
- 17.Fenno J C, Müller K-H, McBride B C. Sequence analysis, expression and binding activity of recombinant major outer sheath protein (Msp) of Treponema denticola. J Bacteriol. 1996;178:2489–2496. doi: 10.1128/jb.178.9.2489-2497.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenno J C, Wong G W K, Hannam P M, Müller K-H, Leung W K, McBride B C. Conservation of msp, the gene encoding the major outer membrane protein of oral Treponema spp. J Bacteriol. 1997;179:1082–1089. doi: 10.1128/jb.179.4.1082-1089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galdiero F, Cippollaro de L’Ero G, Benedetto N, Galdiero M, Tufano M A. Release of cytokines induced by Salmonella typhimurium porins. Infect Immun. 1993;61:155–161. doi: 10.1128/iai.61.1.155-161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grenier D. Characteristics of hemolytic and hemagglutinating activities of Treponema denticola. Oral Microbiol Immunol. 1991;6:246–249. doi: 10.1111/j.1399-302x.1991.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 21.Grenier D. Degradation of host protease inhibitors and activation of plasminogen by proteolytic enzymes from Porphyromonas gingivalis and Treponema denticola. Microbiology. 1996;142:955–961. doi: 10.1099/00221287-142-4-955. [DOI] [PubMed] [Google Scholar]
- 22.Grenier D, Uitto V J. Cytotoxic effect of peptidoglycan from Treponema denticola. Microb Pathog. 1993;15:389–397. doi: 10.1006/mpat.1993.1088. [DOI] [PubMed] [Google Scholar]
- 23.Grenier D, Uitto V J, McBride B C. Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infect Immun. 1990;58:347–351. doi: 10.1128/iai.58.2.347-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haapasalo M, Hannam P, McBride B C, Uitto V J. Hyaluronan, a possible ligand mediating Treponema denticola binding to periodontal tissue. Oral Microbiol Immunol. 1996;11:156–160. doi: 10.1111/j.1399-302x.1996.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 25.Haapasalo M, Müller K H, Uitto V J, Leung W K, McBride B C. Characterization, cloning, and binding properties of the major 53-kilodalton Treponema denticola surface antigen. Infect Immun. 1992;60:2058–2065. doi: 10.1128/iai.60.5.2058-2065.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haapasalo M, Singh U, McBride B C, Uitto V J. Sulfhydryl-dependent attachment of Treponema denticola to laminin and other proteins. Infect Immun. 1991;59:4230–4237. doi: 10.1128/iai.59.11.4230-4237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hager D A, Burgess R R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980;109:76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- 28.Haines K A, Reibman J, Tang X Y, Blake M, Weissmann G. Effects of protein I of Neisseria gonorrhoeae on neutrophil activation: generation of diacylglycerol from phosphatidylcholine via a specific phospholipase C is associated with exocytosis. J Cell Biol. 1991;114:433–442. doi: 10.1083/jcb.114.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch Eur J Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 30.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 31.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holt S C, Bramanti T E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 33.Ishihara K, Miura T, Kuramitsu H K, Okuda K. Characterization of the Treponema denticola prtP gene encoding a prolyl-phenylalanine-specific protease (dentilisin) Infect Immun. 1996;64:5178–5186. doi: 10.1128/iai.64.12.5178-5186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karunakaran T, Holt S C. Cloning and expression of hemolysin genes from Treponema denticola strains ATCC 35404 (TD-4) and human clinical isolate GM-1 in Escherichia coli. Microb Pathog. 1994;16:337–348. doi: 10.1006/mpat.1994.1034. [DOI] [PubMed] [Google Scholar]
- 35.Keulers R A, Maltha J C, Mikx F H, Wolters-Lutgerhorst J M. Attachment of T. denticola strains ATCC 33520, ATCC 35405, B11 and Ny541 to a morphologically distinct population of rat palatal epithelial cells. J Periodontal Res. 1993;28:274–280. doi: 10.1111/j.1600-0765.1993.tb02094.x. [DOI] [PubMed] [Google Scholar]
- 36.Keulers R A, Maltha J C, Mikx F H, Wolters-Lutgerhorst J M. Attachment of Treponema denticola strains to monolayers of epithelial cells of different origin. Oral Microbiol Immunol. 1993;8:84–88. doi: 10.1111/j.1399-302x.1993.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 37.Korzeniewski C, Callewaert D M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 38.Leung W K, Haapasalo M, Uitto V-J, Hannam P M, McBride B C. The surface proteinase of Treponema denticola may mediate attachment of the bacteria to epithelial cells. Anaerobe. 1996;2:39–46. [Google Scholar]
- 39.Li H, Ruby J, Charon N, Kuramitsu H. Gene inactivation in the oral spirochete Treponema denticola: construction of an flgE mutant. J Bacteriol. 1996;178:3664–3667. doi: 10.1128/jb.178.12.3664-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Listgarten M A, Levin S. Positive correlation between the proportions of subgingival spirochetes and motile bacteria and susceptibility of human subjects to periodontal deterioration. J Clin Periodontol. 1981;8:122–138. doi: 10.1111/j.1600-051x.1981.tb02352.x. [DOI] [PubMed] [Google Scholar]
- 41.Loesche W J, Syed S A, Laughon B E, Stoll J. The bacteriology of acute necrotizing ulcerative gingivitis. J Periodontol. 1982;53:223–230. doi: 10.1902/jop.1982.53.4.223. [DOI] [PubMed] [Google Scholar]
- 42.Loesche W J, Syed S A, Schmidt E, Morrison E C. Bacterial profiles of subgingival plaques in periodontitis. J Periodontol. 1985;56:447–456. doi: 10.1902/jop.1985.56.8.447. [DOI] [PubMed] [Google Scholar]
- 43.Mäkinen K K, Chen C Y, Mäkinen P L. Proline iminopeptidase from the outer cell envelope of the human oral spirochete Treponema denticola ATCC 35405. Infect Immun. 1996;64:702–708. doi: 10.1128/iai.64.3.702-708.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mäkinen K K, Mäkinen P L. The peptidolytic capacity of the spirochete system. Medical Microbiol Immunol. 1996;185:1–10. doi: 10.1007/s004300050008. [DOI] [PubMed] [Google Scholar]
- 45.Mäkinen K K, Mäkinen P L, Loesche W J, Syed S A. Purification and general properties of an oligopeptidase from Treponema denticola ATCC 35405—a human oral spirochete. Arch Biochem Biophys. 1995;316:689–698. doi: 10.1006/abbi.1995.1092. [DOI] [PubMed] [Google Scholar]
- 46.Mäkinen K K, Mäkinen P L, Syed S A. Purification and substrate specificity of an endopeptidase from the human oral spirochete Treponema denticola ATCC 35405, active on furylacryloyl-Leu-Gly-Pro-Ala and bradykinin. J Biol Chem. 1992;267:14285–14293. [PubMed] [Google Scholar]
- 47.Mäkinen P L, Mäkinen K K, Syed S A. Role of the chymotrypsin-like membrane-associated proteinase from Treponema denticola ATCC 35405 in inactivation of bioactive peptides. Infect Immun. 1995;63:3567–3575. doi: 10.1128/iai.63.9.3567-3575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masuda K, Kawata T. Isolation, properties, and reassembly of outer sheath carrying a polygonal array from an oral treponeme. J Bacteriol. 1982;150:1405–1413. doi: 10.1128/jb.150.3.1405-1413.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathers D A, Leung W K, Fenno J C, Hong Y, McBride B C. Major surface protein complex of Treponema denticola depolarizes and induces ion channels in HeLa cell membranes. Infect Immun. 1996;64:2904–2910. doi: 10.1128/iai.64.8.2904-2910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meghi S, Henderson B, Nair S P, Tufano M A. Bacterial porins stimulate bone resorption. Infect Immun. 1997;65:1313–1316. doi: 10.1128/iai.65.4.1313-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mikx F H. Comparison of peptidase, glycosidase and esterase activities of oral and non-oral Treponema species. J Gen Microbiol. 1991;137:63–68. doi: 10.1099/00221287-137-1-63. [DOI] [PubMed] [Google Scholar]
- 52.Mikx F H, Keulers R A. Hemagglutination activity of Treponema denticola grown in serum-free medium in continuous culture. Infect Immun. 1992;60:1761–1766. doi: 10.1128/iai.60.5.1761-1766.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mintz K P, Fives-Taylor P M. Adhesion of Actinobacillus actinomycetemcomitans to a human oral cell line. Infect Immun. 1994;62:3672–3678. doi: 10.1128/iai.62.9.3672-3678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore W E, Moore L H, Ranney R R, Smibert R M, Burmeister J A, Schenkein H A. The microflora of periodontal sites showing active destructive progression. J Clin Periodontol. 1991;18:729–739. doi: 10.1111/j.1600-051x.1991.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 55.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 56.Novak M J, Cohen H J. Depolarization of polymorphonuclear leukocytes by Porphyromonas (Bacteroides) gingivalis 381 in the absence of respiratory burst activation. Infect Immun. 1991;59:3134–3142. doi: 10.1128/iai.59.9.3134-3142.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olsen I. Attachment of Treponema denticola to cultured human epithelial cells. Scand J Dent Res. 1984;92:55–63. doi: 10.1111/j.1600-0722.1984.tb00860.x. [DOI] [PubMed] [Google Scholar]
- 58.Reijntjens F M, Mikx F H, Wolters-Lutgerhorst J M, Maltha J C. Adherence of oral treponemes and their effect on morphological damage and detachment of epithelial cells in vitro. Infect Immun. 1986;51:642–647. doi: 10.1128/iai.51.2.642-647.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosen G, Naor R, Kutner S, Sela M N. Characterization of fibrinolytic activities of Treponema denticola. Infect Immun. 1994;62:1749–1754. doi: 10.1128/iai.62.5.1749-1754.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shenker B J, Listgarten M A, Taichman N S. Suppression of human lymphocyte responses by oral spirochetes: a monocyte-dependent phenomenon. J Immunol. 1984;132:2039–2045. [PubMed] [Google Scholar]
- 61.Socransky S S, Haffajee A D. Microbial mechanisms in the pathogenesis of destructive periodontal diseases: a critical assessment. J Periodontal Res. 1991;26:195–212. doi: 10.1111/j.1600-0765.1991.tb01646.x. [DOI] [PubMed] [Google Scholar]
- 62.Tufano M A, Sommese L, Galdiero F. Some biological activities of Eikenella corrodens major outer membrane proteins. Eur J Epidemiol. 1986;2:305–311. doi: 10.1007/BF00419495. [DOI] [PubMed] [Google Scholar]
- 63.Uitto V J, Grenier D, Chan E C, McBride B C. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988;56:2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uitto V J, Pan Y M, Leung W K, Larjava H, Ellen R P, Finlay B B, McBride B C. Cytopathic effects of Treponema denticola chymotrypsin-like proteinase on migrating and stratified epithelial cells. Infect Immun. 1995;63:3401–3410. doi: 10.1128/iai.63.9.3401-3410.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinberg A, Holt S C. Chemical and biological activities of a 64-kilodalton outer sheath protein from Treponema denticola strains. J Bacteriol. 1991;173:6935–6947. doi: 10.1128/jb.173.21.6935-6947.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weinberg A, Holt S C. Interaction of Treponema denticola TD-4, GM-1, and MS25 with human gingival fibroblasts. Infect Immun. 1990;58:1720–1729. doi: 10.1128/iai.58.6.1720-1729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Welch R A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 68.Wilske B, Barbour A G, Bergstrom S, Burman N, Restrepo B I, Rosa P A, Schwan T, Soutschek E, Wallich R. Antigenic variation and strain heterogeneity in Borrelia spp. Res Microbiol. 1992;143:583–596. doi: 10.1016/0923-2508(92)90116-6. [DOI] [PubMed] [Google Scholar]