Abstract
This study was performed to determine the factors for predicting the occurrence of acute exacerbation of hepatitis B virus infection in HBeAg-negative patients. Two hundred and sixteen patients with known times of HBeAg seroclearance were recruited. Liver biochemistry and virologic markers were monitored. Precore and core promoter mutations were determined by a line probe assay. The median age at HBeAg seroclearance was 34.5 years. The median follow-up duration was 26.4 months. Fifty-six (27.9%) patients had acute exacerbations. By Cox regression analysis, male gender, older age, and core promoter mutations at the time of HBeAg seroclearance were independently associated with the occurrence of acute exacerbation after HBeAg seroclearance (P = 0.025, 0.018, and 0.001, respectively). Fourteen (7.0%) patients had HBeAg seroreversion within a median follow-up period of 11.6 months after HBeAg seroclearance. By Cox regression analysis, older age at HBeAg seroclearance was independently associated with the chance of HBeAg seroreversion (P = 0.01). We concluded that male patients with core promoter mutations and delayed HBeAg seroclearance had a higher cumulative chance of acute exacerbation in the HBeAg-negative phase. Patients with delayed HBeAg seroclearance had a higher frequency of HBeAg seroreversion.
A majority of Chinese patients with chronic hepatitis B acquired the infection during the neonatal period or at 1 to 2 years of age. They usually have a prolonged hepatitis B e antigen (HBeAg)-positive phase (immune tolerance), followed by the gradual loss of HBeAg (HBeAg seroclearance) with the development of antibody to HBeAg (HBeAg seroconversion) (20). HBeAg seroconversion is usually followed by cessation of active hepatitis B virus (HBV) replication and remission of liver disease (9, 12). However, active HBV replication and a rise in serum alanine aminotransferase (ALT) (a flare-up of hepatitis; acute exacerbation) recur or persist in a proportion of HBeAg-negative patients (8).
Though it has been shown that acute exacerbation of hepatitis B virus infection occurs frequently in HBeAg-positive patients, acute exacerbation in anti-HBe-positive patients is also quite common (22). Multiple episodes of acute exacerbation in HBeAg-negative patients may accelerate the progression of liver disease (1, 6, 15). Acute exacerbation can occur in Chinese anti-HBe-positive patients with low HBV viremia (22). In addition, HBeAg-negative chronic hepatitis B may be more severe (6, 10, 15) and correlated with poor response to interferon therapy (14). It is not known what factors determine the chance of acute exacerbation after HBeAg seroclearance (4, 11). These data may have an impact on the need for antiviral therapy. Recent studies show that HBeAg-negative patients have a response to nucleotide analogues similar to that of HBeAg-positive patients (7, 18). Identifying patients at high risk of acute exacerbation after HBeAg seroconversion may affect the decision as to whether more prolonged treatment is necessary. In fact, patients with a high viral load and active liver inflammation may be considered for treatment irrespective of their HBeAg-anti-HBe status.
This study was performed to determine the predictive value of clinical features and HBV DNA levels and precore and core promoter mutations at the time of HBeAg seroclearance for subsequent acute exacerbation.
MATERIALS AND METHODS
From 1976 to 2001, 216 Chinese patients with chronic hepatitis B with HBeAg seroclearance and subsequent seroconversion to anti-HBe were recruited from the Hepatitis Clinic, Queen Mary Hospital, The University of Hong Kong. All patients were negative for the antibodies to hepatitis C and D viruses. Blood was taken, and serum was stored for each patient at the time of HBeAg seroclearance. All patients were HBsAg and HBeAg positive for at least 6 months prior to HBeAg seroclearance. The patients were followed up every 3 to 6 months, or more frequently when clinically indicated, for liver function, alpha-fetoprotein (AFP), and HBsAg, HBeAg, and anti-HBe.
HBeAg seroclearance was defined as loss of HBeAg on at least two consecutive follow-ups. HBeAg seroconversion was defined as loss of HBeAg with the development of anti-HBe on at least two consecutive follow-ups. HBeAg seroreversion was defined as reappearance of HBeAg on at least two consecutive follow-ups following previous HBeAg seroclearance or seroconversion (12). Acute exacerbation was defined as an increase in the ALT level to >1.5 times the upper limit of normal (ULN) after other common causes of ALT elevation, including other viral hepatitis, drug-induced hepatitis, alcoholic hepatitis (in patients with significant alcohol intake), and steatohepatitis (in patients with fatty infiltration, as demonstrated in ultrasonography) were excluded (22).
Twenty-two patients had alpha interferon therapy 3.6 to 150 (median, 71.4) months before HBeAg seroclearance. One (4.5%) of these patients had HBeAg seroclearance within 6 months of alpha interferon therapy. None of the patients received lamivudine therapy. None of patients had a history of drug abuse or heavy drinking.
The serum HBV DNA level was measured by the Hybrid Capture II assay (Digene Corp., Gaithersburg, Md.) (lower limit of detection, 1.4 × 105 copies/ml). The serum HBsAg, HBeAg, and anti-HBe were tested by microparticle enzyme immunoassay (Abbott Laboratories, North Chicago, Ill.).
Precore and core promoter mutations were determined using the line probe assay (INNO-LiPA HBV Precore; Innogenetics NV, Ghent, Belgium). The line probe test was performed as described previously (17). The lower detection limit of the LiPA assay is ∼500 copies of mutants/ml in a mixed viral population. The LiPA assay can detect mutants when they are present at >5% among a mixed viral population.
Statistical analysis.
The Statistical Program for Social Sciences (SPSS version 11.0 for Windows; SPSS Inc., Chicago, Ill.) was used for all statistical analyses. Student's t test and the Mann-Whitney U test were used for continuous variables with normal and skewed distributions, respectively. The chi-square test with Yates' correction factor or Fisher's exact test was applied for categorical variables. Kaplan-Meier survival analysis and the log rank test were performed to test the cumulative chance of acute exacerbation(s) and HBeAg seroreversion between different groups. Cox regression with a proportional-hazards model was used to test the associations between different variables and the occurrence of acute exacerbations and HBeAg seroreversion. Results were considered statistically significant if the P value was <0.05.
RESULTS
Demographic data.
The male-to-female ratio was 144/72. The median age at the time of HBeAg seroclearance was 34.5 (range, 15.2 to 80.5) years. The median follow-up period was 26.4 (range, 3.0 to 113.6) months after HBeAg seroclearance. One hundred and ninety-two (88.9%) patients developed anti-HBe at or within 3 months of HBeAg seroclearance; 24 (11.1%) patients developed anti-HBe 3 to 60.5 (median, 6.2) months after HBeAg seroclearance. One patient developed esophageal variceal bleeding, two patients developed ascites, and three patients developed hepatocellular carcinoma during the follow-up.
Ten of 216 (4.6%) patients had HBV DNA levels that were undetectable by the LiPA assay at the time of HBeAg seroclearance. The genotypes of the core promoter region could not be determined in another five patients. Therefore, 201 patients had precore and core promoter genotypes detectable by the LiPA assay at the time of HBeAg seroclearance.
Liver function, HBV DNA level, and precore and core promoter mutations at the time of HBeAg seroclearance.
At the time of HBeAg seroclearance, 106 (52.7%), 69 (34.3%), and 26 (12.9%) patients had pure wild-type precore, mixed wild-type precore and precore mutations, and pure precore mutations, respectively; 60 (29.9%), 55 (27.4%), and 86 (42.8%) patients had pure wild-type core promoter, mixed wild-type core promoter and core promoter mutations, and pure core promoter mutations, respectively. There was no significant difference between the HBV DNA levels in patients with and without precore or core promoter mutations (P = 0.69 for precore mutations and P = 0.99 for core promoter mutations).
Patients with core promoter mutations were older and had higher serum ALT, aspartate aminotransferase (AST), and AFP levels and lower albumin levels than patients without core promoter mutations at the time of HBeAg seroclearance (Table 1) (P = 0.001, 0.012, 0.034, 0.007, and 0.008, respectively). No such differences were observed between patients with and without precore mutations.
TABLE 1.
Comparison of clinical data at the time of HBeAg seroclearance between patients with and without precore and core promoter mutations
| Parameter | Value for groupa
|
|||||
|---|---|---|---|---|---|---|
| Core promoter
|
P value | Precore
|
P value | |||
| MT | WT | MT | WT | |||
| Age (yr) | 36.6 (15.3-80.5) | 30.3 (15.2-54.3) | 0.001 | 33.1 (15.2-69.8) | 36.1 (15.3-80.5) | 0.095 |
| Albumin (g/liter) | 46 (22-60) | 48 (36-53) | 0.008 | 47 (32-53) | 46 (22-60) | 0.50 |
| ALT (U/liter) | 38 (13-313) | 24 (6-393) | 0.012 | 29.0 (6-393) | 39 (13-313) | 0.086 |
| AST (U/liter) | 29 (11-218) | 25 (15-181) | 0.034 | 26 (15-218) | 29 (11-164) | 0.17 |
| Bilirubin (μmol/liter) | 10 (4-187) | 11 (4-59) | 0.87 | 11 (4-73) | 11 (4-187) | 0.79 |
| AFP (ng/liter) | 5 (1-1700) | 3 (1-32) | 0.007 | 4 (1-284) | 4 (1-1700) | 0.32 |
| HBV DNA levels by Digene assay (×106 copies/ml) | <0.14 (<0.14-442.14) | <0.14 (<0.14-279.89) | 0.99 | <0.14 (<0.14-329.87) | <0.14 (<0.14-442.14) | 0.69 |
Values are expressed as median (range). MT, mutant; WT, wild type.
No significant difference was found in age; ALT, bilirubin, AFP, and HBV DNA levels; or prevalence of precore and core promoter mutations at the time of HBeAg seroclearance between patients who had previous alpha interferon therapy and those who did not receive the therapy (all P > 0.05).
Thirty-four (16.9%) patients had ALT levels of >1.5 times the ULN at the time of HBeAg seroclearance. ALT levels returned to normal within 0.9 to 36.4 (median, 4.6) months after HBeAg seroclearance in 30 (14.9%) patients. Four (2.0%) patients had persistently elevated ALT levels after HBeAg seroclearance (median follow-up period, 17.4 months; range, 4.2 to 37.3 months).
Frequency of acute exacerbation of chronic hepatitis B after HBeAg seroclearance.
Fifty-six of 201 (27.9%) patients had acute exacerbation of chronic hepatitis B after HBeAg seroclearance. Thirty-nine, eight, six, and three patients had one, two, three, and four episodes, respectively, of acute exacerbation within a median follow-up period of 45.2 (range, 4.9 to 119.3) months after HBeAg seroclearance.
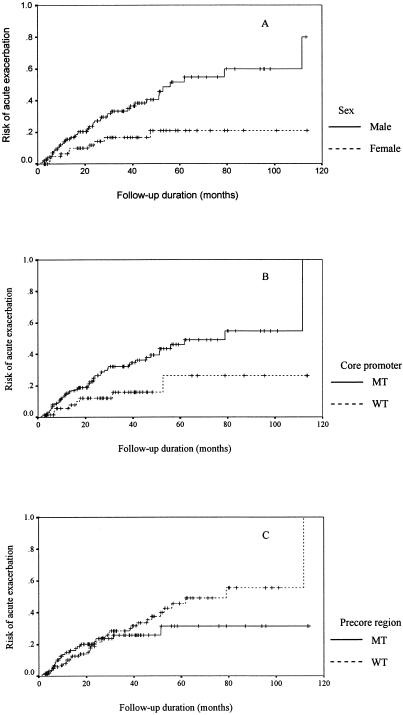
In the first acute exacerbation after HBeAg seroclearance, 38 (67.9%) patients had peak ALT levels of >2.0 times the ULN, and 18 (32.1%) patients had peak ALT levels within 1.5 to 2.0 times the ULN. Male patients had a higher cumulative chance of acute exacerbation than female patients (Fig. 1A) (46 of 134 [34.3%] versus 10 of 67 [14.9%]; P = 0.0054).
FIG. 1.
Risk of acute exacerbation after HBeAg seroclearance in (A) male and female patients, (B) patients with and without core promoter mutations, and (C) patients with and without precore mutations. MT, mutant; WT, wild type.
Compared with patients without acute exacerbation, patients with acute exacerbation after HBeAg seroclearance were older (median [range], 38.0 [15.2 to 60.0] years versus 33.1 [15.3 to 80.5] years; P = 0.019). The occurrence of acute exacerbation in patients within different age groups at HBeAg seroclearance is shown in Table 2.
TABLE 2.
Occurrence of acute exacerbation and HBeAg seroreversion after HBeAg seroclearance in different age groups
| Age at HBeAg seroclearance (yr) | No. of patients | No. (%) with:
|
|
|---|---|---|---|
| Acute exacerbation | HBeAg seroreversion | ||
| ≤20 | 12 | 1 (8.3) | 1 (8.3) |
| 20-30 | 60 | 13 (21.7) | 3 (5.0) |
| 30-40 | 71 | 20 (28.2) | 11 (15.5) |
| 40-50 | 45 | 16 (35.6) | 7 (15.6) |
| >50 | 13 | 6 (46.2) | 2 (15.4) |
Patients with acute exacerbation after HBeAg seroclearance had higher ALT, AST, bilirubin, and AFP levels at the time of HBeAg seroclearance than patients without acute exacerbation (Table 3) (P = 0.002, <0.001, 0.003, and 0.003, respectively). However, the HBV DNA levels in the two groups at the time of HBeAg seroclearance were comparable (P = 0.16).
TABLE 3.
Comparison of clinical data at the time of HBeAg seroclearance between patients with and without exacerbations after HBeAg seroclearance
| Parameter | Value in patientsa:
|
P value | |
|---|---|---|---|
| With acute exacerbation | Without acute exacerbation | ||
| Albumin (g/liter) | 47 (36-60) | 46 (22-53) | 0.17 |
| ALT (U/liter) | 44 (14-191) | 29 (6-393) | 0.002 |
| AST (U/liter) | 32.5 (20-101) | 27 (11-218) | <0.001 |
| Bilirubin (μmol/liter) | 12.5 (4-59) | 10 (4-187) | 0.003 |
| AFP (ng/liter) | 6.5 (1-178) | 4 (1-1700) | 0.003 |
| HBV DNA by Digene assay (106 copies/ml) | <0.14 (<0.14-442.14) | <0.14 (<0.14-329.87) | 0.16 |
| Precore mutations | 21/56 (37.5%) | 74/145 (51.0%) | 0.12 |
| Core promoter mutations | 48/56 (85.7%) | 93/145 (64.1%) | 0.003 |
Values are expressed as median (range).
Patients with core promoter mutations at the time of HBeAg seroclearance had a significantly higher cumulative chance of acute exacerbation than patients without core promoter mutations (Fig. 1B) (48 of 141 [34.0%] versus 8 of 60 [13.3%]; P = 0.016). Patients with and without precore mutations at the time of HBeAg seroclearance had comparable cumulative chances of acute exacerbation (Fig. 1C) (21 of 95 [22.1%] versus 35 of 106 [33.0%]; P = 0.38).
Patients with and without a history of interferon therapy had comparable cumulative risks of acute exacerbation after HBeAg seroclearance (5 of 22 [22.7%] versus 51 of 179 [28.5%]; P = 0.21).
Cox regression analysis for the development of acute exacerbation after HBeAg seroclearance.
Factors at the time of HBeAg seroclearance that were identified by univariate analysis to be associated with a higher frequency of acute exacerbation after HBeAg seroclearance were male gender; older age; higher serum ALT, AST, bilirubin, and AFP levels; and the presence of core promoter mutations at the time of HBeAg seroclearance. Using Cox regression with a proportional-hazards model, the male gender (P = 0.025), the presence of core promoter mutations (P = 0.018), and old age (P = 0.001) at the time of HBeAg seroclearance were found to be independent factors associated with higher cumulative risk for the occurrence of acute exacerbation after HBeAg seroclearance.
Frequency of HBeAg seroreversion after HBeAg seroclearance.
Fourteen (7.0%) patients had HBeAg seroreversion within a median follow-up period of 11.6 (range, 3.9 to 35.8) months of HBeAg seroclearance and 11.3 (3.9 to 35.1) months after HBeAg seroconversion. Of these 14 patients, 8 (57.1%) had a flare-up of ALT accompanying the seroreversion to HBeAg.
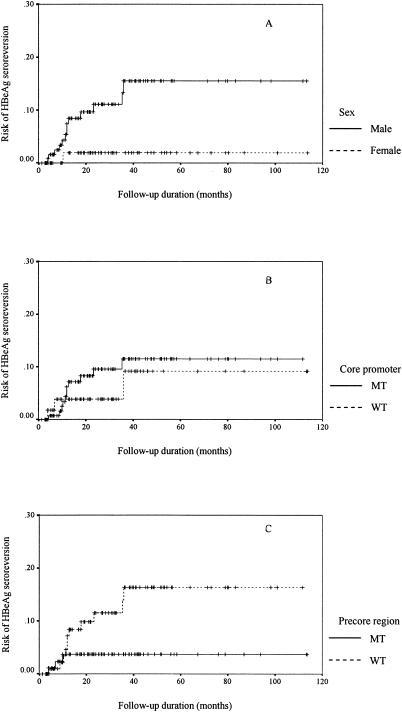
Male patients had a significantly higher cumulative chance of HBeAg seroreversion than female patients (Fig. 2A) (13 of 134 [9.7%] versus 1 of 67 [1.49%]; P = 0.026). Patients with HBeAg seroreversion were older and had higher ALT, AST, bilirubin, and AFP levels at the time of initial HBeAg seroclearance than patients without HBeAg seroreversion (Table 4) (P = 0.013, 0.005, 0.004, 0.025, and 0.02, respectively).
FIG. 2.
Cumulative risk of HBeAg seroreversion after HBeAg seroclearance in (A) male and female patients, (B) patients with and without core promoter mutations, and (C) patients with and without precore mutations. MT, mutant; WT, wild type.
TABLE 4.
Comparison of clinical data at the time of HBeAg seroclearance between patients with and without HBeAg seroreversion
| Parameter | Value for patientsa
|
P value | |
|---|---|---|---|
| With HBeAg seroreversion | Without HBeAg seroreversion | ||
| Age (yr) | 40.2 (28.6-55.7) | 34.2 (15.2-80.5) | 0.013 |
| Albumin (g/liter) | 47 (36-53) | 47 (22-60) | 0.47 |
| ALT (U/liter) | 57 (14-155) | 33 (6-393) | 0.005 |
| AST (U/liter) | 42.5 (19-94) | 28 (11-218) | 0.004 |
| Bilirubin (μmol/liter) | 13.7 (6-45) | 10 (4-187) | 0.025 |
| AFP (ng/liter) | 9 (2-154) | 4 (1-1700) | 0.02 |
| HBV DNA by Digene assay (106 copies/ml) | 0.16 (<0.14-5.52) | <0.14 (<0.14-442.1) | 0.15 |
| Precore mutations | 3/14 (21.4%) | 92/187 (49.2%) | 0.054 |
| Core promoter mutations | 11/14 (78.6%) | 130/187 (69.5%) | 0.56 |
Continuous values are expressed as median (range).
Patients with precore mutations tended to have a lower cumulative risk of HBeAg seroreversion than patients without precore mutations (Fig. 2C) (3 of 95 [3.2%] versus 11 of 106 [10.4%]; P = 0.058). Patients with and without core promoter mutations had comparable cumulative chances of HBeAg seroreversion (Fig. 2B) (11 of 141 [7.8%] versus 3 of 60 [5.0%]; P = 0.54).
Using Cox regression with a proportional-hazards model, only increased age at HBeAg seroclearance was independently associated with the occurrence of HBeAg seroreversion (P = 0.01).
DISCUSSION
In this study of male patients, increased age and presence of core promoter mutations at the time of HBeAg seroclearance were found to be independently associated with a higher chance for the occurrence of acute exacerbation after HBeAg seroclearance.
Chinese patients usually acquire HBV infection perinatally or early in life. The duration of the immune tolerance phase was proposed to be inversely correlated with the age at acquiring HBV infection (8). In this study, it was further found that patients with later HBeAg seroclearance had higher frequencies of HBeAg seroreversion and acute exacerbation of chronic hepatitis B after HBeAg seroclearance. Consistent with this finding, the frequency of acute exacerbation after HBeAg seroclearance was reported to be low in previous studies of anti-HBe-positive children (5 to 20%) (5, 16). Whether the frequent exacerbation after HBeAg seroclearance in the older age groups leads to the development of cirrhosis-related complications cannot be determined in the present study because of the relatively few complications that developed during the follow-up period.
In vitro studies have demonstrated that HBV strains with core promoter mutations have higher replication capacities (2, 3). However, there are conflicting reports about the effect of core promoter mutations on the serum HBV DNA levels in anti-HBe-positive patients (13, 21). In this study, patients with and without core promoter mutations had comparable low HBV DNA levels at the time of HBeAg seroclearance (<0.14 × 106 copies/ml). However, patients with core promoter mutations had higher frequencies of acute exacerbation after HBeAg seroclearance. This result suggests that after HBeAg seroclearance, patients with core promoter mutations were more likely to mount a significant immune response and suffer from acute exacerbation of chronic hepatitis B.
Patients with and without interferon therapy had comparable chances of acute exacerbation and comparable prevalences of precore and core promoter mutations. Most of these patients received interferon therapy ∼6 years before HBeAg seroclearance. These results support our previous study showing that the benefit of interferon therapy in Chinese patients is short term (19). It has no significance for the selection of precore and core promoter mutations and also could not reduce the risk of acute exacerbation.
In conclusion, patients with delayed HBeAg seroclearance had a higher frequency of HBeAg seroreversion. Male patients with delayed HBeAg seroclearance and the presence of core promoter mutations at the time of HBeAg seroclearance had a higher frequency of acute exacerbation after HBeAg seroclearance. Precore mutations play no role in causing acute exacerbation after HBeAg seroclearance. Over 46.2% of patients who had HBeAg seroclearance after the age of 50 had subsequent acute exacerbation. This implies that patients who have delayed HBeAg seroconversion should be followed up more carefully for possible exacerbation.
REFERENCES
- 1.Brunetto, M. R., F. Oliveri, B. Coco, G. Leandro, P. Colombatto, J. M. Gorin, and F. Bonino. 2002. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J. Hepatol. 36:263-270. [DOI] [PubMed] [Google Scholar]
- 2.Buckwold, V. E., Z. Xu, M. Chen, T. S. Yen, and J. H. Ou. 1996. Effects of a naturally occurring mutation in the hepatitis B virus basal core promoter on precore gene expression and viral replication. J. Virol. 70:5845-5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckwold, V. E., Z. Xu, T. S. Yen, and J. H. Ou. 1997. Effects of a frequent double-nucleotide basal core promoter mutation and its putative single-nucleotide precursor mutations on hepatitis B virus gene expression and replication. J. Gen. Virol. 78:2055-2065. [DOI] [PubMed] [Google Scholar]
- 4.Chan, H. L., Y. Hui, N. W. Leung, J. Y. Ching, F. K. Chan, and J. J. Sung. 2000. Risk factors for active liver disease in HBeAg-negative chronic hepatitis B virus-infected patients. Am. J. Gastroenterol. 95:3547-3551. [DOI] [PubMed] [Google Scholar]
- 5.Chang, M. H., H. Y. Hsu, H. C. Hsu, Y. H. Ni, J. S. Chen, and D. S. Chen. 1995. The significance of spontaneous hepatitis B e antigen seroconversion in childhood with special emphasis on the clearance of hepatitis B e antigen before 3 years of age. Hepatology 22:1387-1392. [PubMed] [Google Scholar]
- 6.Fattovich, G. 2003. Natural history and prognosis of hepatitis B. Semin. Liver Dis. 23:47-58. [DOI] [PubMed] [Google Scholar]
- 7.Hadziyannis, S. J., N. C. Tassopoulos, E. J. Heathcote, T. T. Chang, G. Kitis, M. Rizzetto, P. Marcellin, S. G. Lim, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, and C. L. Brosgart. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N. Engl. J. Med. 348:800-807. [DOI] [PubMed] [Google Scholar]
- 8.Hadziyannis, S. J., and D. Vassilopoulos. 2001. Immunopathogenesis of hepatitis B e antigen negative chronic hepatitis B infection. Antiviral Res. 52:91-98. [DOI] [PubMed] [Google Scholar]
- 9.Hoofnagle, J. H., D. A. Shafritz, and H. Popper. 1987. Chronic type B hepatitis and the “healthy” HBsAg carrier state. Hepatology 7:758-763. [DOI] [PubMed] [Google Scholar]
- 10.Liaw, Y. F., D. I. Tai, C. M. Chu, C. C. Pao, and T. J. Chen. 1987. Acute exacerbation in chronic type B hepatitis: comparison between HBeAg and antibody-positive patients. Hepatology 7:20-23. [DOI] [PubMed] [Google Scholar]
- 11.Lok, A. S., and C. L. Lai. 1990. Acute exacerbations in Chinese patients with chronic hepatitis B virus (HBV) infection. Incidence, predisposing factors and etiology. J. Hepatol. 10:29-34. [DOI] [PubMed] [Google Scholar]
- 12.Lok, A. S., and B. J. McMahon. 2001. Chronic hepatitis B. Hepatology 34:1225-1241. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto, H., F. Tsuda, Y. Akahane, Y. Sugai, M. Yoshiba, K. Moriyama, T. Tanaka, Y. Miyakawa, and M. Mayumi. 1994. Hepatitis B virus with mutations in the core promoter for an e antigen-negative phenotype in carriers with antibody to e antigen. J. Virol. 68:8102-8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papatheodoridis, G. V., and S. J. Hadziyannis. 2001. Diagnosis and management of pre-core mutant chronic hepatitis B. J. Viral Hepat. 8:311-321. [DOI] [PubMed] [Google Scholar]
- 15.Perrillo, R. P. 2001. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology 120:1009-1022. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Moreno, M., M. Otero, A. Millan, I. Castillo, M. Cabrerizo, F. J. Jimenez, H. Oliva, S. Cajal, and V. Carreno. 1999. Clinical and histological outcome after hepatitis B e antigen to antibody seroconversion in children with chronic hepatitis B. Hepatology 29:572-575. [DOI] [PubMed] [Google Scholar]
- 17.Stuyver, L., S. De Gendt, J. F. Cadranel, C. Van Geyt, G. Van Reybroeck, R. Dorent, I. Gandjbachkh, M. Rosenheim, F. Charlotte, P. Opolon, J. M. Huraux, and F. Lunel. 1999. Three cases of severe subfulminant hepatitis in heart-transplanted patients after nosocomial transmission of a mutant hepatitis B virus. Hepatology 29:1876-1883. [DOI] [PubMed] [Google Scholar]
- 18.Tassopoulos, N. C., R. Volpes, G. Pastore, J. Heathcote, M. Buti, R. D. Goldin, S. Hawley, J. Barber, L. Condreay, D. F. Gray, et al. 1999. Efficacy of lamivudine in patients with hepatitis B e antigen-negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis B. Hepatology 29:889-896. [DOI] [PubMed] [Google Scholar]
- 19.Yuen, M. F., C. K. Hui, C. C. Cheng, C. H. Wu, Y. P. Lai, and C. L. Lai. 2001. Long-term follow-up of interferon alfa treatment in Chinese patients with chronic hepatitis B infection: the effect on hepatitis B e antigen seroconversion and the development of cirrhosis-related complications. Hepatology 34:139-145. [DOI] [PubMed] [Google Scholar]
- 20.Yuen, M. F., and C. L. Lai. 2000. Natural history of chronic hepatitis B virus infection. J. Gastroenterol. Hepatol. 15(Suppl.):E20-E24. [DOI] [PubMed] [Google Scholar]
- 21.Yuen, M. F., E. Sablon, H. J. Yuan, C. K. Hui, D. K. Wong, J. Doutreloigne, B. C. Wong, A. O. Chan, and C. L. Lai. 2002. Relationship between the development of precore and core promoter mutations and hepatitis B e antigen seroconversion in patients with chronic hepatitis B virus. J. Infect. Dis. 186:1335-1338. [DOI] [PubMed] [Google Scholar]
- 22.Yuen, M. F., H. J. Yuan, C. K. Hui, D. K. Wong, W. M. Wong, A. O. Chan, B. C. Wong, and C. L. Lai. 2003. A large population study of spontaneous HBeAg seroconversion and acute exacerbation of chronic hepatitis B infection: implications for antiviral therapy. Gut 52:416-419. [DOI] [PMC free article] [PubMed] [Google Scholar]