Abstract
Simple Summary
The prognosis of osteosarcoma patients with primary metastases affecting multiple organ systems is deemed mostly fatal, with survival rates less than 10%. The aim of this study was to identify potential prognostic factors and to evaluate the impact various therapeutic interventions may have on the outcomes of those patients. The poor prognosis was confirmed in our cohort. Surgical resection at all tumour sites was of the utmost importance for long-term survival, while standard chemotherapy was often insufficiently effective. For unresectable bone metastases, radiotherapy might be considered.
Abstract
Background: To evaluate patient and tumour characteristics, treatment, and their impact on survival in patients with multi-systemic metastases at initial diagnosis of high-grade osteosarcoma. Precedure: Eighty-three consecutive patients who presented with multi-systemic metastases at initial diagnosis of high-grade osteosarcoma were retrospectively reviewed. In cases of curative intent, the Cooperative Osteosarcoma Study Group recommended surgical removal of all detectable metastases in addition to complete resection of the primary tumour and chemotherapy. Results: Eighty-three eligible patients (1.8%) were identified among a total of 4605 individuals with high-grade osteosarcoma. Nine (10.8%) of these achieved complete surgical remission, of whom seven later had recurrences. The median follow-up time was 12 (range, 1–165) months for all patients. Actuarial event-free survival after 1, 2, and 5 years was 9.6 ± 3.2%, 1.4 ± 1.4%, and 1.4 ± 1.4%, and overall survival was 54.0 ± 5.6%, 23.2 ± 4.9%, and 8.7 ± 3.3%. In univariate analyses, elevated alkaline phosphatase before chemotherapy, pleural effusion, distant bones as metastatic sites, and more than one bone metastasis were negative prognostic factors. Among treatment-related factors, the microscopically complete resection of the primary tumour, a good response to first-line chemotherapy, the macroscopically complete resection of all affected tumour sites, and local treatment (surgery ± radiotherapy) of all bone metastases were associated with better outcomes. Tumour progression under first-line treatment significantly correlated with shorter survival times. Conclusion: The outlook for patients with multi-systemic primary metastases from osteosarcoma remains very poor. The utmost importance of surgical resection of all tumour sites was confirmed. For unresectable bone metastases, radiotherapy might be considered. In the patient group studied, standard chemotherapy was often insufficiently effective. In the case of such advanced disease, alternative treatment options are urgently required.
Keywords: osteosarcoma, multi-systemic metastases, combined metastases, primary metastases, survival
1. Introduction
Fewer than one out of four to five patients suffering high-grade osteosarcoma have detectable metastases at initial diagnosis [1,2,3,4,5,6]. If there is detectable metastasis at that time, the lungs are by far the most commonly affected site, followed by bones. Other localisations such as lymph nodes, parenchymal organs, or soft tissue are rarely involved [2,3,4,7,8,9]. Treatment for metastatic osteosarcoma usually includes chemotherapy as well as complete surgical resection at all tumour sites (primary and metastatic) [10,11,12,13].
The prognosis of individuals with primary metastasis is significantly worse than that of individuals with localised disease [2,3]. Primary metastases affecting multiple organ systems are deemed mostly fatal. Previous series have found survival rates of less than 10% for affected patients [7,14,15]. Here, we report on a large series of consecutive patients with such multi-systemic metastases at initial presentation of high-grade osteosarcoma. The aim of this study was to identify potential prognostic factors and to evaluate the impact various therapeutic interventions may have on outcome.
2. Patients and Methods
2.1. Patients
This report includes patients with a primary high-grade osteosarcoma and primary metastases affecting more than one organ system. Patients needed to be registered with the Cooperative Osteosarcoma Study (COSS) between January 1980, and December 2022.
The diagnosis of osteosarcoma needed to have been confirmed histologically. Metastases were required to affect at least two organ systems (lung and bone, lung and other, bone and other, or two distinct organ systems classified as other) and to have occurred within the first four weeks after starting chemotherapy. At least one metastasis of each organ system needed to be strongly suspected radiographically or proven histologically or must have become obvious due to progressive disease. Lesions within the bone of origin of the primary tumour (skip lesions) were not considered metastases.
2.2. Initial Diagnostics and Intended Treatment
The primary tumour site was to be investigated by conventional radiography in all studies, whereas the use of computed tomography and magnetic resonance imaging varied over time and depended on availability. Initial staging included an X-ray and/or a computed tomography scan of the chest for detection of possible pulmonary metastases and a 99Tc-methylene diphosphonate bone scan and/or positron emission tomography for detection of possible bone metastases. Other organ systems were investigated according to symptoms and local practice. During follow-up, X-rays of the chest and of the primary tumour site were mandatory at regular intervals specified in the respective treatment protocols. The intended first-line treatment included polychemotherapy as well as the surgical removal of all tumour sites, whenever feasible.
2.3. Follow-Up and Detection of Recurrence
Regular clinical assessment and X-rays of the former site of the primary tumour as well as the chest were recommended for all patients as part of routine follow-up. Computed tomography of the chest was employed at the treating institutions’ discretion. If a recurrence was suspected, appropriate imaging of the primary tumour site and the chest was recommended, as was a bone scan. The diagnosis of recurrence was based on the assessment of the respective treating institution.
3. Ethics Approval and Patient Consent
All studies and registries within COSS were accepted by the appropriate ethics and/or protocol review committees. Upon enrolment in a study or registry within COSS, informed consent was required from all patients and/or their legal guardians, depending on the patients’ age.
4. Data Collection and Definition of Variables
Data on patient and tumour characteristics at initial diagnosis and first-line treatment were collected and coded as described previously [2]. The following parameters are in need of further explanation:
Patient and tumour characteristics at initial diagnosis (no later than four weeks after start of chemotherapy): pathological fracture—fracture in the area of the primary tumour or other bone metastases; alkaline phosphatase and lactate dehydrogenase—normal vs. elevated levels before the start of chemotherapy (according to the reference values of the respective laboratory); primary tumour site—localisation at an extremity vs. the trunk vs. the head and neck; number of metastatic sites involved—two (lung and bone, lung and other, bone and other, or other and other) vs. three sites; metastatic sites involved—lung and bone, lung and other, bone and other, or other and other (but affecting two distinct organ systems classified as other sites); laterality of pulmonary metastases—uni- vs. bilateral; pleural effusion—evidence of effusion in the pleural space upon imaging; number of bone and other metastases at initial diagnosis—solitary vs. multiple (two or more), other metastases—any metastases neither classified as pulmonary nor bone metastases; complete surgical resection assessed as possible—according to the assessment of the treating institution and, if available, the assessment of one of a panel of COSS reference surgeons.
Treatment-related factors (regarding the time span between initial diagnosis and, if applicable, recurrence or death, whichever occurred first): resection of the primary tumour—none vs. macroscopically complete resection vs. microscopically complete resection (based on the treating institution’s assessment and, if present, surgery and pathology reports); response to first-line chemotherapy—according to Salzer-Kuntschik et al. [16] (good response = tumour viability below 10%); macroscopically complete resection of metastases—by surgery, and based on the treating institution’s assessment and, if present, surgery and pathology reports; radiotherapy—self-explanatory; therapeutic radioactive medication—injection of a radioactive substance with the aim of achieving a therapeutic effect; tumour progression under first-line treatment—based on the treating institution’s assessment and, if present, on a letter of recommendation by COSS; complete local treatment of all bone metastases—no vs. yes to the statement that all bone metastases were treated with local therapy (radiotherapy and/or surgery); type of local treatment of all bone metastases—none vs. surgery vs. radiotherapy plus, if applicable, surgery (provided that all metastases were treated with local therapy); radiotherapy as part of local treatment of all unresected bone metastases—this was answered with a “yes” if all unresected bone metastases were irradiated.
Follow-up information collected prospectively included the date and site of the first disease recurrence as well as of secondary malignancy, should either have occurred; the date the patient was last known to be alive; and, for deceased patients, the date and cause of death. All relevant information included in this report was reviewed by one of the authors (VLM), and the variables stated in Table 1, Table 2 and Table 3 were coded.
Table 1.
Survival estimates by patient- and tumour-related factors of 83 registered osteosarcoma patients with primary multi-systemic metastases.
| Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | % | 1-Year | 2-Year | 5-Year | ||||||
| Rate | SE | Rate | SE | Rate | SE | p * | ||||
| All eligible patients | 83 | 100 | 0.540 | 0.056 | 0.232 | 0.049 | 0.087 | 0.033 | ||
| Age at initial diagnosis, years | ||||||||||
| <14.8 | 41 | 49 | 0.455 | 0.079 | 0.278 | 0.071 | 0.111 | 0.052 | 0.960 | |
| ≥14.8 | 42 | 51 | 0.627 | 0.076 | 0.177 | 0.064 | 0.059 | 0.040 | ||
| Sex | ||||||||||
| Female | 40 | 48 | 0.479 | 0.081 | 0.160 | 0.060 | 0.096 | 0.050 | 0.311 | |
| Male | 43 | 52 | 0.597 | 0.076 | 0.304 | 0.074 | 0.083 | 0.046 | ||
| Duration of pain until initial diagnosis | ||||||||||
| <47 days | 35 | 50 | 0.460 | 0.087 | 0.246 | 0.075 | 0.140 | 0.063 | 0.773 | |
| ≥47 days | 35 | 50 | 0.591 | 0.084 | 0.217 | 0.076 | 0.072 | 0.049 | ||
| No pain/unknown | 13 | |||||||||
| Duration of swelling until initial diagnosis | ||||||||||
| <38 days | 27 | 50 | 0.622 | 0.095 | 0.311 | 0.091 | 0.133 | 0.070 | 0.559 | |
| ≥38 days | 27 | 50 | 0.542 | 0.098 | 0.152 | 0.079 | 0.051 | 0.049 | ||
| No swelling/unknown | 29 | |||||||||
| Pathological fracture at initial diagnosis | ||||||||||
| No | 70 | 89 | 0.537 | 0.060 | 0.248 | 0.053 | 0.099 | 0.038 | 0.402 | |
| Yes | 9 | 11 | 0.292 | 0.173 | 0.146 | 0.135 | 0.000 | 0.000 | ||
| Unknown | 4 | |||||||||
| Alkaline phosphatase before start of chemotherapy | ||||||||||
| Normal | 12 | 18 | 0.750 | 0.125 | 0.536 | 0.156 | 0.214 | 0.133 | 0.019 | |
| Elevated | 56 | 82 | 0.456 | 0.068 | 0.152 | 0.049 | 0.057 | 0.032 | ||
| Unknown | 15 | |||||||||
| Lactate dehydrogenase before start of chemotherapy | ||||||||||
| Normal | 14 | 22 | 0.929 | 0.069 | 0.314 | 0.129 | 0.079 | 0.075 | 0.078 | |
| Elevated | 50 | 78 | 0.388 | 0.071 | 0.186 | 0.058 | 0.093 | 0.044 | ||
| Unknown | 19 | |||||||||
| Primary tumour site | ||||||||||
| Extremity | 72 | 88 | 0.503 | 0.060 | 0.241 | 0.053 | 0.069 | 0.033 | 0.525 | |
| Trunk | 10 | 12 | 0.778 | 0.139 | 0.111 | 0.105 | 0.111 | 0.105 | ||
| Head and neck | 1 | 1.000 | 1.000 | 1.000 | ||||||
| Tumour size at initial diagnosis (limb only) | ||||||||||
| Small (<1/3 of the involved bone’s length) | 23 | 42 | 0.595 | 0.104 | 0.250 | 0.095 | 0.062 | 0.059 | 0.364 | |
| Large (≥1/3 of the involved bone’s length) | 32 | 58 | 0.488 | 0.090 | 0.199 | 0.076 | 0.079 | 0.053 | ||
| Other site/unknown | 28 | |||||||||
| Number of metastatic sites involved at initial diagnosis | ||||||||||
| Two | 57 | 70 | 0.529 | 0.066 | 0.232 | 0.058 | 0.077 | 0.037 | 0.839 | |
| Three | 25 | 30 | 0.438 | 0.103 | 0.246 | 0.093 | 0.123 | 0.077 | ||
| Unknown | 1 | |||||||||
| Metastatic sites involved at initial diagnosis | ||||||||||
| Lung and bone | 42 | 74 | 0.468 | 0.078 | 0.133 | 0.055 | 0.027 | 0.026 | 0.005 | |
| Lung and other | 13 | 23 | 0.923 | 0.074 | 0.508 | 0.144 | 0.169 | 0.109 | ||
| Bone and other | 2 | 4 | 1.000 | 0.500 | 0.354 | 0.500 | 0.354 | |||
| Other and other | 0 | 0 | ||||||||
| Three sites/unknown | 26 | |||||||||
| Pulmonary metastases at initial diagnosis | ||||||||||
| No | 2 | 2 | 1.000 | 0.500 | 0.354 | 0.500 | 0.354 | 0.428 | ||
| Yes | 81 | 98 | 0.529 | 0.056 | 0.225 | 0.049 | 0.075 | 0.032 | ||
| Laterality of pulmonary metastases | ||||||||||
| Unilateral | 4 | 5 | 0.750 | 0.217 | 0.500 | 0.250 | 0.000 | 0.000 | 0.637 | |
| Bilateral | 71 | 95 | 0.519 | 0.061 | 0.215 | 0.052 | 0.083 | 0.035 | ||
| None/unknown | 8 | |||||||||
| Pleural effusion at initial diagnosis | ||||||||||
| No | 35 | 80 | 0.441 | 0.085 | 0.173 | 0.069 | 0.069 | 0.047 | 0.010 | |
| Yes | 9 | 20 | 0.222 | 0.139 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Unknown | 39 | |||||||||
| Bone metastases at initial diagnosis | ||||||||||
| No | 13 | 16 | 0.923 | 0.074 | 0.508 | 0.144 | 0.169 | 0.109 | 0.010 | |
| Yes | 69 | 84 | 0.473 | 0.061 | 0.182 | 0.049 | 0.073 | 0.034 | ||
| Unknown | 1 | |||||||||
| Number of bone metastases at initial diagnosis | ||||||||||
| One | 14 | 21 | 0.701 | 0.126 | 0.390 | 0.136 | 0.156 | 0.101 | 0.028 | |
| At least two | 53 | 79 | 0.393 | 0.068 | 0.133 | 0.051 | 0.053 | 0.035 | ||
| None/unknown | 16 | |||||||||
| Other metastases at initial diagnosis | ||||||||||
| No | 42 | 51 | 0.468 | 0.078 | 0.133 | 0.055 | 0.027 | 0.026 | 0.018 | |
| Yes | 41 | 49 | 0.617 | 0.078 | 0.338 | 0.078 | 0.154 | 0.062 | ||
| Number of other metastases at initial diagnosis | ||||||||||
| One | 17 | 43 | 0.765 | 0.103 | 0.499 | 0.128 | 0.166 | 0.105 | 0.476 | |
| At least two | 23 | 58 | 0.525 | 0.109 | 0.239 | 0.093 | 0.143 | 0.077 | ||
| None/unknown | 43 | |||||||||
| Complete surgical resection assessed as feasible at initial diagnosis | ||||||||||
| No | 45 | 74 | 0.387 | 0.073 | 0.137 | 0.052 | 0.091 | 0.043 | 0.010 | |
| Yes | 16 | 26 | 0.938 | 0.061 | 0.554 | 0.138 | 0.092 | 0.087 | ||
| Unknown | 22 | |||||||||
* p-value, log-rank test.
Table 2.
Survival estimates by treatment-related factors of 83 registered osteosarcoma patients with primary multi-systemic metastases.
| Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | % | 1-Year | 2-Year | 5-Year | ||||||
| Rate | SE | Rate | SE | Rate | SE | p * | ||||
| Resection of the primary tumour | ||||||||||
| None or macroscopically incomplete | 43 | 55 | 0.326 | 0.071 | 0.051 | 0.035 | 0.026 | 0.025 | <0.001 | |
| Macroscopically complete, microscopically incomplete | 3 | 4 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | |||
| Microscopically complete | 32 | 41 | 0.775 | 0.075 | 0.495 | 0.093 | 0.152 | 0.069 | ||
| Unknown | 5 | |||||||||
| Response to first-line chemotherapy † | ||||||||||
| Good (grades 1–3) | 11 | 42 | 1.000 | 0.727 | 0.134 | 0.364 | 0.145 | 0.044 | ||
| Poor (grades 4–6) | 15 | 58 | 0.786 | 0.110 | 0.397 | 0.136 | 0.000 | 0.000 | ||
| Unknown/primary or no tumour resection | 57 | |||||||||
| Macroscopically complete resection of all metastases | ||||||||||
| No | 72 | 89 | 0.481 | 0.059 | 0.135 | 0.042 | 0.068 | 0.032 | 0.001 | |
| Yes | 9 | 11 | 1.000 | 1.000 | 0.250 | 0.153 | ||||
| Unknown | 2 | |||||||||
| Macroscopically complete resection of all pulmonary metastases | ||||||||||
| No | 68 | 87 | 0.450 | 0.061 | 0.111 | 0.041 | 0.056 | 0.030 | <0.001 | |
| Yes | 10 | 13 | 1.000 | 1.000 | 0.222 | 0.139 | ||||
| No pulmonary metastases/unknown | 5 | |||||||||
| Macroscopically complete resection of all bone metastases | ||||||||||
| No | 57 | 86 | 0.424 | 0.065 | 0.129 | 0.046 | 0.064 | 0.035 | 0.062 | |
| Yes | 9 | 14 | 0.778 | 0.139 | 0.519 | 0.176 | 0.130 | 0.121 | ||
| No bone metastases/unknown | 17 | |||||||||
| Macroscopically complete resection of all other metastases | ||||||||||
| No | 25 | 68 | 0.400 | 0.098 | 0.040 | 0.039 | 0.000 | 0.000 | <0.001 | |
| Yes | 12 | 32 | 1.000 | 0.900 | 0.095 | 0.450 | 0.166 | |||
| No other metastases/unknown | 36 | |||||||||
| Radiotherapy | ||||||||||
| No | 46 | 63 | 0.478 | 0.074 | 0.205 | 0.061 | 0.046 | 0.031 | 0.352 | |
| Yes | 27 | 37 | 0.593 | 0.095 | 0.244 | 0.085 | 0.163 | 0.074 | ||
| Unknown | 10 | |||||||||
| Therapeutic radioactive medication | ||||||||||
| No | 63 | 88 | 0.551 | 0.063 | 0.273 | 0.058 | 0.109 | 0.042 | 0.011 | |
| Yes | 9 | 13 | 0.222 | 0.139 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Unknown | 11 | |||||||||
| Duration until start of chemotherapy, days | ||||||||||
| <21 | 57 | 69 | 0.500 | 0.067 | 0.206 | 0.055 | 0.056 | 0.031 | 0.289 | |
| ≥21 | 26 | 31 | 0.636 | 0.097 | 0.300 | 0.099 | 0.180 | 0.089 | ||
| Tumour progression under first-line treatment | ||||||||||
| No | 18 | 27 | 0.941 | 0.057 | 0.627 | 0.121 | 0.314 | 0.116 | <0.001 | |
| Yes | 48 | 73 | 0.396 | 0.071 | 0.090 | 0.043 | 0.000 | 0.000 | ||
| Unknown | 17 | |||||||||
* p-value, log-rank test. † According to Salzer-Kuntschik et al. [16].
Table 3.
Survival estimates by treatment-related factors of 69 registered osteosarcoma patients with primary multi-systemic metastases including bone lesions.
| Overall Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | 1-Year | 2-Year | 5-Year | ||||||
| Rate | SE | Rate | SE | Rate | SE | p * | |||
| All eligible patients with bone metastases | 69 | 0.473 | 0.061 | 0.182 | 0.049 | 0.073 | 0.034 | ||
| Complete local treatment of all bone metastases | |||||||||
| No | 46 | 0.370 | 0.071 | 0.095 | 0.045 | 0.024 | 0.023 | 0.010 | |
| Yes (radiotherapy and/or surgery) | 12 | 0.667 | 0.136 | 0.476 | 0.150 | 0.190 | 0.120 | ||
| Unknown | 11 | ||||||||
| Type of local treatment of all bone metastases | |||||||||
| No | 46 | 0.370 | 0.071 | 0.095 | 0.045 | 0.024 | 0.023 | 0.037 | |
| Surgery only | 9 | 0.778 | 0.139 | 0.519 | 0.176 | 0.130 | 0.121 | ||
| Radiotherapy involved | 3 | 0.333 | 0.272 | 0.333 | 0.272 | 0.333 | 0.272 | ||
| Unknown | 11 | ||||||||
| Type of local treatment of all bone metastases | |||||||||
| Surgery only | 9 | 0.778 | 0.139 | 0.519 | 0.176 | 0.130 | 0.121 | 0.913 | |
| Radiotherapy involved | 3 | 0.333 | 0.272 | 0.333 | 0.272 | 0.333 | 0.272 | ||
| Not all bone metastases treated locally/unknown | 47 | ||||||||
| Macroscopically complete resection of all bone metastases | |||||||||
| No | 57 | 0.424 | 0.065 | 0.129 | 0.046 | 0.064 | 0.035 | 0.062 | |
| Yes | 9 | 0.778 | 0.139 | 0.519 | 0.176 | 0.130 | 0.121 | ||
| Unknown | 3 | ||||||||
| Radiotherapy as part of local treatment of all unresected bone metastases | |||||||||
| No | 55 | 0.436 | 0.067 | 0.163 | 0.052 | 0.041 | 0.028 | 0.250 | |
| Yes, radiotherapy involved | 3 | 0.333 | 0.272 | 0.333 | 0.272 | 0.333 | 0.272 | ||
| Unknown | 11 | ||||||||
* p-value, log-rank test.
5. Statistics
All patients were evaluated retrospectively on an intention-to-treat basis. Median values were given with the range (minimum and maximum), and mean values with the standard deviation. Chi-squared analysis was used to compare unrelated parameters. In survival-time analyses, the date of the diagnostic tumour biopsy was set as the starting point. Event-free survival was calculated until relapse, secondary malignancy, or death, whichever occurred first; overall survival was calculated until death. Patients not achieving surgical remission were assumed to have suffered an event on day 1. Follow-up periods were calculated until the date of last documented information. Survival analyses were performed using the Kaplan-Meier method [17]. The log-rank test was used to compare survival curves [18]. All parameters were investigated by univariate techniques [18]. All p values were two-sided, and a p value of less than 0.05 was considered significant. Statistical analyses were carried out using SPSS (IBM Corp. Released 2022. IBM SPSS Statistics for Windows, Version 29, NY: IBM Corp., New York, NY, USA).
6. Results
6.1. Patient and Tumour Characteristics
For detailed information on patient- and tumour-related characteristics, please see Table 1. Eighty-three patients with multi-systemic metastases of high-grade osteosarcoma evident at initial diagnosis were identified. Their median age at diagnosis was 14.8 years (range, 1.7–62.3 years). Forty patients (48.2%) were female. One patient presented with a background of Rothmund–Thomson syndrome.
The disease presented itself at the time of initial diagnosis as follows: Pain was reported by 79/82 (96.3%) patients with appropriate data, and the median interval until diagnostic biopsy was 47 days (range, 3–412 days). Swelling was reported by 60/75 (80.0%) patients with appropriate information, and the median interval was 38 days (range, 1–227 days). A total of 9/79 (11.4%) patients with appropriate data suffered a pathological fracture. Alkaline phosphatase levels were elevated in 56 (82.4%) of 68 cases with known laboratory parameters, and lactate dehydrogenase levels in 50/64 (78.1%). Elevated AP correlated with the presence of bone metastases (p = 0.016, chi2) but not with their number (p = 0.159, chi2) or the length of the primary tumour (p = 0.720, chi2).
The primary tumour was localised in an extremity in 72/83 (86.7%) patients, in the trunk area in ten (12.0%), and in the cranium in one (1.2%). Small tumours (<1/3 of the involved bone) accounted for 23/55 (41.8%) extremity tumours with appropriate information.
A total of 25/82 (30.5%) patients presented with metastases in three organ systems, while only two sites were involved in 57/82 (69.5%). One further patient had both pulmonary and other metastases, but there was no information about whether bone metastases were also present. Pulmonary metastasis occurred in 81/83 (97.6%) patients (30 histopathologically verified), and pleural effusions in 9/44 (20.5%) patients with relevant data. A total of 69/82 (84.1%) patients with appropriate information presented with bone metastases (20 histopathologically verified), and 41/83 (49.4%) patients presented with metastases outside the lungs or bone (18 histopathologically verified). The latter involved distant lymph nodes in twenty-three (56.1%) patients, soft tissue in nineteen (46.3%) patients, the heart or pericardium in four (9.8%) patients, and the brain in one (2.4%) patient (multiple mentions possible).
At initial diagnosis, complete surgical resection at all tumour sites was deemed possible in 16/61 (26.2%) patients with appropriate data.
6.2. Treatment Strategy at Initial Disease Presentation
Details on treatment strategy are summarized in Table 2.
Macroscopically complete resection of the primary tumour was performed in 35/78 (44.9%) patients with appropriate data, with a good response to neoadjuvant chemotherapy in 11/26 (42.3%) evaluable cases. Macroscopically complete resection of all metastases was achieved in 9/81 (11.1%) patients with appropriate information. A total of 9/83 (10.8%) patients achieved a complete resection of all tumour sites.
Radiotherapy was performed in 27/73 (37.0%) patients with appropriate information, and therapeutic radioactive medication was administered to 9/79 (12.5%) patients with known data.
Local therapy for bone metastases was analysed in more detail and is summarised in Table 3. A total of 12/58 patients received local treatment of all detectable bone metastases. It was performed by surgery in only 9/66 (13.6%) patients with appropriate information and by (additional) radiotherapy in 3/58 (5.2%) patients with available data.
All patients of our cohort received chemotherapy. Twenty-six/83 (31.3%) did so with a delay of more than three weeks. Osteosarcoma was progressive under first-line treatment in 48/66 (72.7%) patients with appropriate information. Tumour progression correlated with response to chemotherapy (p = 0.002, chi2): of 23 patients with appropriate data, progressive disease was observed in 3/10 (30.0%) good responders and in 12/13 (93.2%) poor responders. Information on progressive disease was available for 44/58 (75.9%) patients with unknown response to chemotherapy: Tumour progression was reported in 34 (77.3%) of these patients. There was no correlation between a delay of treatment and progressive disease (p = 0.159, chi2). Second-line chemotherapy was known to have been given in 50/83 (60.2%) patients, of whom eleven had two changes of the systemic therapy regime; six had three; and one each had four, five, and six.
6.3. Prognostic Factors
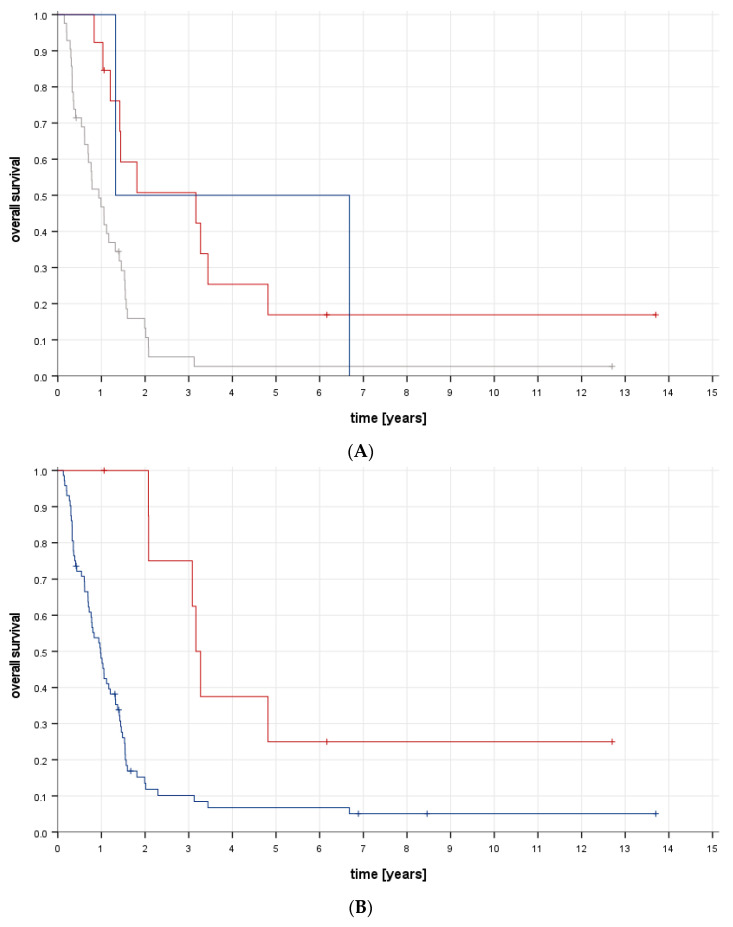
Regarding factors present at diagnosis, elevated alkaline phosphatase (p = 0.019) correlated with poorer survival. There was a trend towards better survival when lactate dehydrogenase levels were normal; however, this was not statistically significant (p = 0.078). Survival was worse in patients with bone and pulmonary metastases (p = 0.005, Figure 1A). There was no difference in survival according to the number of metastatic organ systems involved (two vs. three, p = 0.839). Pleural effusion (p = 0.010) and the appearance (p = 0.010) and number of bone metastases (solitary vs. multiple, p = 0.028) as well as the absence of “other” metastases correlated with poorer survival (p = 0.018). Those patients for whom complete resection at all tumour sites was assessed as possible at initial diagnosis fared significantly better (p < 0.001).
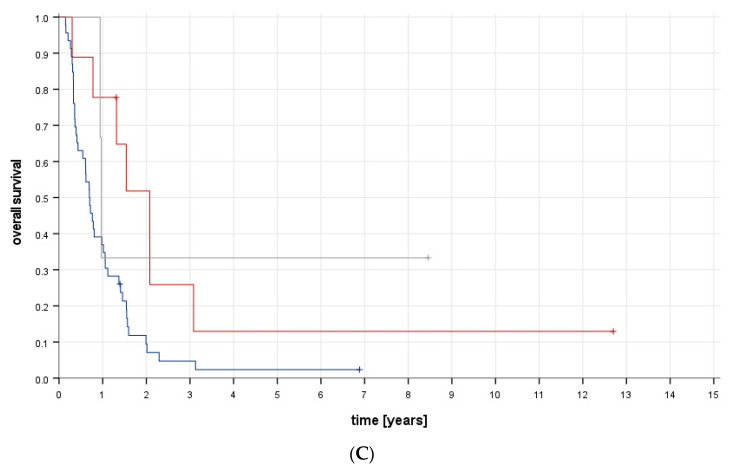
Figure 1.
(A). Overall survival according to metastatic sites involved at initial diagnosis: lung and other (red; n = 13), lung and bone (grey; n = 42), bone and other (blue; n = 2); p = 0.005; log-rank test. (B). Overall survival according to achievement of complete resection of all metastases: macroscopically complete (red; n = 9), macroscopically incomplete (blue; n = 72); p = 0.001; log-rank test. (C). Patients with bone metastases as part of dissemination: overall survival according to type of local treatment of all bone metastases; surgery only (red; n = 9), radiotherapy ± surgery (grey; n = 3), no remission (blue; n = 46); p = 0.037; log-rank test.
Treatment-related factors associated with better outcomes were microscopically complete resection of the primary tumour (p < 0.001); a good response to first-line chemotherapy (p = 0.044); (macroscopically) complete resection of all metastases (p = 0.001, Figure 1B), as well as of all pulmonary and other metastases on an individual basis (p < 0.001); and complete local therapy (surgical resection and/or radiotherapy) of all bone metastases (p = 0.010). Overall survival according to type of local treatment of all bone metastases is shown in Figure 1C). There was no difference in survival for patients receiving radiotherapy as part of local treatment for all unresected bone metastases (p = 0.250); there was a trend toward better survival when all bone metastases were resected, but this was not statistically significant (p = 0.062). Patients receiving therapeutic radioactive medication fared worse (p = 0.011). Tumour progression under first-line treatment correlated with poorer survival (p < 0.001).
In summary, elevated alkaline phosphatase before chemotherapy, pleural effusion, distant bones as metastatic sites, and the presence of more than one bone metastasis were negative prognostic factors. Among treatment-related factors, microscopically complete resection of the primary tumour, a good response to first-line chemotherapy, and macroscopically complete resection of all affected tumour sites as well as local treatment (surgery ± radiotherapy) of all bone metastases were associated with better outcomes. Tumour progression under first-line treatment significantly correlated with shorter survival times.
6.4. Survival and Follow-Up
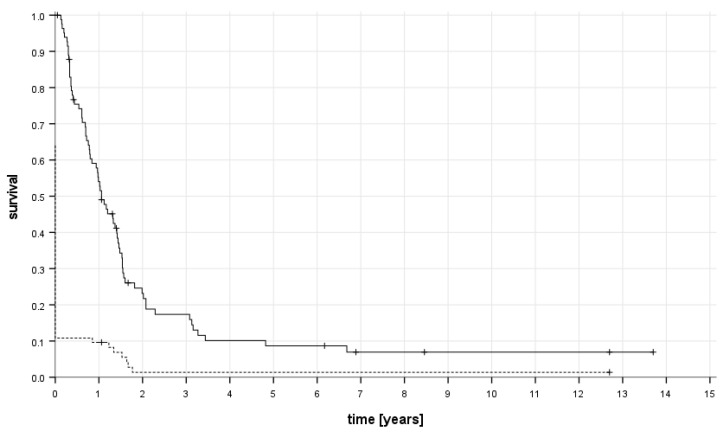
The median follow-up was 12 (range, 1–164) months for all patients and 18 (range, 1–164) months for patients still alive at last contact. The event-free survival rates for all patients at one, two, and five years after initial diagnosis were 9.6 ± 3.2%, 1.4 ± 1.4%, and 1.4 ± 1.4%, and the corresponding overall survival rates were 54.0 ± 5.6%, 23.2 ± 4.9%, and 8.7 ± 3.3% (Figure 2).
Figure 2.
Overall (solid line) and event-free survival (dashed line) of all 83 patients.
At last follow-up, nine (10.8%) patients out of the total sample of eighty-three had achieved a first surgical remission as defined. Two of these remained in their first remission (1.1 and 12.7 years after diagnosis), four died following their first recurrence, two died following their second recurrence, and one was in a third complete remission (6.2 years after diagnosis). Of seventy-four (89.2%) patients never achieving a first complete remission, sixty-three died of osteosarcoma, one due to unknown causes, and one due to septic shock in neutropenia. Of twelve survivors, the follow-up was longer than five years in five patients: In two of those patients (alive for 6.9 and 8.5 years after diagnosis), all remaining bone metastases had been irradiated. One patient (alive at 13.7 years) was under continuous therapy with interferon-alpha. One survivor was in a first remission at 12.7 years and one in a third remission at 6.2 years after initial disease.
7. Discussion
This study, the first to report on such a large patient cohort, confirms the exceedingly poor prognosis of patients in whom several organ systems are affected by primary osteosarcoma metastases [7,14,15]. Only one in ten patients in our cohort had achieved a first surgical remission, and one in fourteen patients survived for more than five years after osteosarcoma diagnosis.
A poor prognosis for patients with extra-pulmonary osteosarcoma metastases, including those to the bones and other sites, has already been reported, both by our study group and by others [7,8,9,14,15,19]. Based on the patients examined in this study, it can further be deducted that patients with bony metastases in addition to pulmonary metastases fare worse than those with lesions at other sites in addition to pulmonary lesions.
A comparatively high number of our patients presented with elevated AP values at initial diagnosis. This was to be expected, as it has been reported that elevated AP values correlate with the occurrence of metastases [20]. The predictive value of elevated AP levels seems to be maintained even within the group of patients with metastatic disease—possibly as an indicator of a higher tumour burden [14,20,21]. Bone metastases in particular are reported to be associated with elevated AP values [22]. This observation was confirmed in our cohort. Ultimately, one could conclude that an increase in AP values could possibly be a consequence of the presence of prognostically poor bone metastases.
It was not surprising that the presence of pleural effusion was accompanied by a fatal prognosis: in the presence of malignant pleural effusion, it can be assumed that tumour cells have already spread within the pleural space. Complete surgical remission is then hardly conceivable or even impossible. In concordance, Saoud et al. reported a poor prognosis for patients with malignant pleural effusion in the presence of sarcoma. Both of their patients with pleural effusion accompanying osteosarcoma survived only a few months [23].
Regarding bony involvement, solitary metastases were found to be associated with a better prognosis than more extensive disease. As multiple metastases pose a greater challenge in terms of local treatment, this is not unexpected. A higher number of pulmonary metastases or metastases in general has repeatedly been associated with poorer survival [3,7,15,24,25,26]. However, we could not find a survival benefit for patients with either solitary metastasis to other sites or unilateral lung involvement. For the former, the possibility of a lower detection rate of “other” metastases (due to the lack of standard diagnostics in this regard) may have led to an incorrect classification. For the latter, the small number of cases of unilateral pulmonary metastases might be an explanation.
The initial assessment regarding the possibility of achieving complete remission proved to be significant in terms of survival. However, on closer inspection, it can be deduced that this correlated with short-term survival only. Five-year outcomes, in contrast, were almost the same regardless of whether remission was considered feasible. The reason for this might not be so much that the initial assessment of resectability was incorrect, but rather that some individual patients became long-term survivors despite incomplete surgical resections.
Nevertheless, the high significance of complete surgical resection for further survival was confirmed in our cohort—both resection of the primary tumour and resection of all metastases [2,7,8,10,11,12,13,14,24,27].
In the case of pulmonary metastases, an open thoracotomy is usually advisable, as not all lung metastases can be reliably detected by imaging [28,29,30,31,32]. In addition to the removal of all conspicuous foci, lesions that elude imaging can then be detected by manual exploration [33]. In patients with multiple metastases, however, complete resection of all metastatic lesions is naturally often difficult if not impossible. Alternative therapeutic approaches are particularly important for these patients.
In the particular case of bony metastases, only a trend towards a better prognosis with complete surgical resection of these metastases could be demonstrated. This may be due to the rather limited cohort size—we can only surmise that statistical significance could be achieved with a higher number of cases. There was no advantage at all for patients who received radiotherapy for all non-resected bone metastases. It should be noted here that such a constellation was extremely rare in our cohort (n = 3)—it is possible that a higher number of cases could also reveal a significant advantage for patients receiving such therapy. However, if any local treatment was performed for all bone metastases, regardless of whether it was a surgical or radiotherapeutic procedure, the survival rate was significantly higher than for metastases that were not or only partially treated. Furthermore, two of our patients with some bone metastases only treated by radiotherapy were among the few long-term survivors (6.9 and 8.5 years). This also suggests a possible benefit of adequate radiotherapy. Various studies suggest that radiotherapy with appropriate doses, achieved particularly by heavy ions and/or protons, might achieve long-term local osteosarcoma control [34,35,36,37,38,39]. This report indicates that radiotherapy might be a therapeutic alternative for those bony metastases that are not suitable for surgical removal.
It must, however, be noted that no benefit of radiotherapy in general could be detected in our total cohort. The reason for this observation is probably the predominant use of this treatment modality in clearly palliative situations. Therefore, a selection bias must be assumed. The administration of radioactive medications was even associated with worse survival. It seems that patients receiving such therapy were highly selected in the same manner as described above. Altogether, long-term survival was not observed in any patient who received internal radiotherapy. This finding is in line with past reports on internal radiotherapy [40,41].
All of our patients received chemotherapy. As expected, tumour progression under first-line therapy led to a very poor prognosis, with no survivors beyond two years after initial diagnosis. It must be noted that progressive disease did not necessarily occur during actual chemotherapy itself but might also have been caused by delays in the onset of treatment or by prolonged chemotherapy interruptions. However, no clear correlation between chemotherapy delay and tumour progression could be established.
Histological response to upfront chemotherapy is a well-known predictive factor for reduced overall survival [2,7,8]. In our cohort, it was poor in nearly 60% of the evaluable cases. Compared to a poor response in just over 40% of patients with osteosarcoma in general, this seems rather high [2]. In those patients with response data and progressive disease, we even observed an almost uniformly poor tumour response. Since, for patients without information on tumour response, tumour progression under therapy was reported in the majority of cases, in total, an even higher proportion of poor responders than indicated can be assumed. All in all, the overwhelmingly high rate of poor response impressively illustrates that administered systemic therapy appeared to be of very limitedly value in the examined subgroup of patients. Ultimately, the question arises as to whether chemotherapy is justified in this subgroup, as it shows little effect overall. Still, in some patients, disease stabilization or even a good response to systemic treatment was observed. Therefore, in the absence of superior alternatives, first-line chemotherapy should not be abandoned, at least initially. If the tumour is progressive and there are no curative treatment options, a palliative approach might then also be discussed instead of intensive chemotherapy—for example, a therapy attempt with a tyrosine kinase inhibitor might be considered. In phase II trials, cabozantinib, regorafenib, and sorafenib were able to extend the progression-free interval in treatment-refractory or relapsed osteosarcoma patients [42,43,44,45,46,47].
Over the last three decades, no significant improvement in survival has been achieved in primary or metastasised osteosarcoma [12,47,48,49]. The survival rate of patients examined in various clinical trials evaluating new therapies such as immunotherapy, tyrosine kinase inhibitors, and other drug therapies has increased only slightly at best [47,49,50,51]. However, for patients with multisystemic osteosarcoma, new therapeutic approaches are urgently needed, as standard therapy is clearly not sufficient.
8. Conclusions
In conclusion, this report confirms the extremely poor prognosis for patients with multi-systemic primary metastases of osteosarcoma. Surgical resection of all tumour sites is of the utmost importance for long-term survival. For unresectable bone metastases, radiotherapy might be considered. In the patient group studied, standard chemotherapy was often insufficiently effective. More effective treatment options for affected patients are direly needed.
Acknowledgments
We thank all patients and their families as well as the staff of the participating institutions.
Author Contributions
Conceptualization, V.L.M.; methodology, V.L.M.; validation, V.L.M.; formal analysis, V.L.M.; investigation, V.L.M., C.B., G.F., S.H., T.v.K., L.K., T.K., M.N., M.W., S.S.B. and S.H.-N.; data curation, V.L.M., M.K. and B.S.; writing—original draft, V.L.M.; writing—review and editing, C.B., G.F., S.H., T.v.K., L.K., T.K., M.N., M.W., S.S.B. and S.H.-N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. All studies within COSS were accepted by the appropriate ethics and/or protocol review committees. Use of patient data was approved by the ethics committees of Hamburg (protocol codes 500 and 1147), of Munster (protocol code 182/98 Biel2), of Ärztekammer Westfalen-Lippe (protocol codes 4I, 4IV Bie4, and 5V Bielack, 30.06.05), and of Landesärztekammer Baden-Württemberg (protocol code F-2021-007, 01.04.22).
Informed Consent Statement
Informed consent was obtained from all patients and/or, depending on the patients’ age, their legal guardians.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
S. Bielack reports personal fees for participation in advisory boards from Hoffmann-La Roche, MAP-Biopharma, Y-mAbs, and SERB SAS and personal fees for expert testimony from Zschimmer & Schwarz Mohsdorf GmbH & Co. KG, all outside the submitted work. S. Hecker-Nolting reports payment from EISAI to her institution for her role as deputy PI and grants from Förderkreis krebskranke Kinder Stuttgart e.V. and Deutsche Kinderkrebsstiftung, all outside the submitted work. V. Mettmann, C. Blattmann, G. Friedel, S. Harrabi, L. Kager, T. von Kalle, M. Kevric, T. Kühne, M. Nathrath, B. Sorg, and M. Werner have nothing to disclose.
Funding Statement
The studies from which these patients originate were supported by Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, Fördergemeinschaft Kinderkrebs Zentrum Hamburg, and Förderkreis krebskranke Kinder Stuttgart. Michaela Nathrath was supportet by the Helga und Heinrich Holzhauer Stiftung.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Marko T.A., Diessner B.J., Spector L.G. Prevalence of Metastasis at Diagnosis of Osteosarcoma: An International Comparison. Pediatr. Blood Cancer. 2016;63:1006–1011. doi: 10.1002/pbc.25963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bielack S.S., Kempf-Bielack B., Delling G., Exner G.U., Flege S., Helmke K., Kotz R., Salzer-Kuntschik M., Werner M., Winkelmann W., et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 3.Kaste S.C., Pratt C.B., Cain A.M., Jones-Wallace D.J., Rao B.N. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: Imaging features. Cancer. 1999;86:1602–1608. doi: 10.1002/(SICI)1097-0142(19991015)86:8<1602::AID-CNCR31>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G., Ferrari S., Longhi A., Forni C., Zavatta M., Versari M., Smith K. High-grade osteosarcoma of the extremity: Differences between localized and metastatic tumors at presentation. J. Pediatr. Hematol. Oncol. 2002;24:27–30. doi: 10.1097/00043426-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Miller B.J., Cram P., Lynch C.F., Buckwalter J.A. Risk factors for metastatic disease at presentation with osteosarcoma: An analysis of the SEER database. J. Bone Joint Surg. Am. 2013;95:e89. doi: 10.2106/JBJS.L.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duchman K.R., Gao Y., Miller B.J. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol. 2015;39:593–599. doi: 10.1016/j.canep.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Kager L., Zoubek A., Potschger U., Kastner U., Flege S., Kempf-Bielack B., Branscheid D., Kotz R., Salzer-Kuntschik M., Winkelmann W., et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J. Clin. Oncol. 2003;21:2011–2018. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 8.Meyers P.A., Heller G., Healey J.H., Huvos A., Applewhite A., Sun M., LaQuaglia M. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J. Clin. Oncol. 1993;11:449–453. doi: 10.1200/JCO.1993.11.3.449. [DOI] [PubMed] [Google Scholar]
- 9.Harris M.B., Gieser P., Goorin A.M., Ayala A., Shochat S.J., Ferguson W.S., Holbrook T., Link M.P. Treatment of metastatic osteosarcoma at diagnosis: A Pediatric Oncology Group Study. J. Clin. Oncol. 1998;16:3641–3648. doi: 10.1200/JCO.1998.16.11.3641. [DOI] [PubMed] [Google Scholar]
- 10.Tsukamoto S., Errani C., Angelini A., Mavrogenis A.F. Current Treatment Considerations for Osteosarcoma Metastatic at Presentation. Orthopedics. 2020;43:e345–e358. doi: 10.3928/01477447-20200721-05. [DOI] [PubMed] [Google Scholar]
- 11.Strauss S.J., Frezza A.M., Abecassis N., Bajpai J., Bauer S., Biagini R., Bielack S., Blay J.Y., Bolle S., Bonvalot S., et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2021;32:1520–1536. doi: 10.1016/j.annonc.2021.08.1995. [DOI] [PubMed] [Google Scholar]
- 12.Eaton B.R., Schwarz R., Vatner R., Yeh B., Claude L., Indelicato D.J., Laack N. Osteosarcoma. Pediatr. Blood Cancer. 2021;68((Suppl. S2)):e28352. doi: 10.1002/pbc.28352. [DOI] [PubMed] [Google Scholar]
- 13.Ritter J., Bielack S.S. Osteosarcoma. Ann. Oncol. 2010;21((Suppl. S7)):vii320–vii325. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 14.Mialou V., Philip T., Kalifa C., Perol D., Gentet J.C., Marec-Berard P., Pacquement H., Chastagner P., Defaschelles A.S., Hartmann O. Metastatic osteosarcoma at diagnosis: Prognostic factors and long-term outcome--the French pediatric experience. Cancer. 2005;104:1100–1109. doi: 10.1002/cncr.21263. [DOI] [PubMed] [Google Scholar]
- 15.Slade A.D., Warneke C.L., Hughes D.P., Lally P.A., Lally K.P., Hayes-Jordan A.A., Austin M.T. Effect of concurrent metastatic disease on survival in children and adolescents undergoing lung resection for metastatic osteosarcoma. J. Pediatr. Surg. 2015;50:157–160; discussion 160. doi: 10.1016/j.jpedsurg.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 16.Salzer-Kuntschik M., Brand G., Delling G. Determination of the degree of morphological regression following chemotherapy in malignant bone tumors. Pathologe. 1983;4:135–141. [PubMed] [Google Scholar]
- 17.Kaplan E.L., Meier P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 18.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 19.Bacci G., Fabbri N., Balladelli A., Forni C., Palmerini E., Picci P. Treatment and prognosis for synchronous multifocal osteosarcoma in 42 patients. J. Bone Joint Surg. Br. 2006;88:1071–1075. doi: 10.1302/0301-620X.88B8.17809. [DOI] [PubMed] [Google Scholar]
- 20.Bacci G., Dallari D., Battistini A., Orlandi M., Ferrari S., Avella M., Picci P., Casadei R., Ruggieri P. The prognostic value of serum alkaline phosphatase in osteosarcoma of the limbs. Chir. Organi Mov. 1992;77:171–180. [PubMed] [Google Scholar]
- 21.Basoli S., Cosentino M., Traversari M., Manfrini M., Tsukamoto S., Mavrogenis A.F., Bordini B., Donati D.M., Errani C. The Prognostic Value of Serum Biomarkers for Survival of Children with Osteosarcoma of the Extremities. Curr. Oncol. 2023;30:7043–7054. doi: 10.3390/curroncol30070511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marais L.C., Bertie J., Rodseth R., Sartorius B., Ferreira N. Pre-treatment serum lactate dehydrogenase and alkaline phosphatase as predictors of metastases in extremity osteosarcoma. J. Bone Oncol. 2015;4:80–84. doi: 10.1016/j.jbo.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saoud C., Ali S.Z. Metastatic sarcomas to pleural effusion: A 10-year large tertiary care center experience with emphasis on clinical features and cytomorphologic characteristics. J. Am. Soc. Cytopathol. 2023;12:216–228. doi: 10.1016/j.jasc.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Buddingh E.P., Anninga J.K., Versteegh M.I., Taminiau A.H., Egeler R.M., van Rijswijk C.S., Hogendoorn P.C., Lankester A.C., Gelderblom H. Prognostic factors in pulmonary metastasized high-grade osteosarcoma. Pediatr. Blood Cancer. 2010;54:216–221. doi: 10.1002/pbc.22293. [DOI] [PubMed] [Google Scholar]
- 25.Chen F., Miyahara R., Bando T., Okubo K., Watanabe K., Nakayama T., Toguchida J., Date H. Prognostic factors of pulmonary metastasectomy for osteosarcomas of the extremities. Eur. J. Cardiothorac. Surg. 2008;34:1235–1239. doi: 10.1016/j.ejcts.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchiya H., Kanazawa Y., Abdel-Wanis M.E., Asada N., Abe S., Isu K., Sugita T., Tomita K. Effect of timing of pulmonary metastases identification on prognosis of patients with osteosarcoma: The Japanese Musculoskeletal Oncology Group study. J. Clin. Oncol. 2002;20:3470–3477. doi: 10.1200/JCO.2002.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L., Akiyama T., Fukushima T., Iwata S., Takeshita K., Kawai A., Tanaka S., Kobayashi H. Surgical resection of the primary lesion for osteosarcoma patients with metastasis at initial diagnosis. Jpn. J. Clin. Oncol. 2021;51:416–423. doi: 10.1093/jjco/hyaa204. [DOI] [PubMed] [Google Scholar]
- 28.Kayton M.L., Huvos A.G., Casher J., Abramson S.J., Rosen N.S., Wexler L.H., Meyers P., LaQuaglia M.P. Computed tomographic scan of the chest underestimates the number of metastatic lesions in osteosarcoma. J. Pediatr. Surg. 2006;41:200–206; discussion 200–206. doi: 10.1016/j.jpedsurg.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Ciccarese F., Bazzocchi A., Ciminari R., Righi A., Rocca M., Rimondi E., Picci P., Bacchi Reggiani M.L., Albisinni U., Zompatori M., et al. The many faces of pulmonary metastases of osteosarcoma: Retrospective study on 283 lesions submitted to surgery. Eur. J. Radiol. 2015;84:2679–2685. doi: 10.1016/j.ejrad.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Gao E., Li Y., Zhao W., Zhao T., Guo X., He W., Wu W., Zhao Y., Yang Y. Necessity of thoracotomy in pulmonary metastasis of osteosarcoma. J. Thorac. Dis. 2019;11:3578–3583. doi: 10.21037/jtd.2019.07.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su W.T., Chewning J., Abramson S., Rosen N., Gholizadeh M., Healey J., Meyers P., La Quaglia M.P. Surgical management and outcome of osteosarcoma patients with unilateral pulmonary metastases. J. Pediatr. Surg. 2004;39:418–423; discussion 418–423. doi: 10.1016/j.jpedsurg.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 32.Heaton T.E., Hammond W.J., Farber B.A., Pallos V., Meyers P.A., Chou A.J., Price A.P., LaQuaglia M.P. A 20-year retrospective analysis of CT-based pre-operative identification of pulmonary metastases in patients with osteosarcoma: A single-center review. J. Pediatr. Surg. 2017;52:115–119. doi: 10.1016/j.jpedsurg.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrle D., Bielack S. Osteosarcoma lung metastases detection and principles of multimodal therapy. Cancer Treat. Res. 2009;152:165–184. doi: 10.1007/978-1-4419-0284-9_8. [DOI] [PubMed] [Google Scholar]
- 34.Ciernik I.F., Niemierko A., Harmon D.C., Kobayashi W., Chen Y.L., Yock T.I., Ebb D.H., Choy E., Raskin K.A., Liebsch N., et al. Proton-based radiotherapy for unresectable or incompletely resected osteosarcoma. Cancer. 2011;117:4522–4530. doi: 10.1002/cncr.26037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamada T., Tsujii H., Tsuji H., Yanagi T., Mizoe J.E., Miyamoto T., Kato H., Yamada S., Morita S., Yoshikawa K., et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J. Clin. Oncol. 2002;20:4466–4471. doi: 10.1200/JCO.2002.10.050. [DOI] [PubMed] [Google Scholar]
- 36.Seidensaal K., Mattke M., Haufe S., Rathke H., Haberkorn U., Bougatf N., Kudak A., Blattmann C., Oertel S., Kirchner M., et al. The role of combined ion-beam radiotherapy (CIBRT) with protons and carbon ions in a multimodal treatment strategy of inoperable osteosarcoma. Radiother. Oncol. 2021;159:8–16. doi: 10.1016/j.radonc.2021.01.029. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz R., Bruland O., Cassoni A., Schomberg P., Bielack S. The role of radiotherapy in oseosarcoma. Pediatr. Adolesc. Osteosarcoma. 2009;152:147–164. doi: 10.1007/978-1-4419-0284-9_7. [DOI] [PubMed] [Google Scholar]
- 38.Matsunobu A., Imai R., Kamada T., Imaizumi T., Tsuji H., Tsujii H., Shioyama Y., Honda H., Tatezaki S., Working Group for Bone et al. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer. 2012;118:4555–4563. doi: 10.1002/cncr.27451. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W., Tanaka M., Sugimoto Y., Takigawa T., Ozaki T. Carbon-ion radiotherapy of spinal osteosarcoma with long-term follow. Eur. Spine J. 2016;25((Suppl. S1)):113–117. doi: 10.1007/s00586-015-4202-9. [DOI] [PubMed] [Google Scholar]
- 40.Anderson P.M., Subbiah V., Rohren E. Bone-seeking radiopharmaceuticals as targeted agents of osteosarcoma: Samarium-153-EDTMP and radium-223. Adv. Exp. Med. Biol. 2014;804:291–304. doi: 10.1007/978-3-319-04843-7_16. [DOI] [PubMed] [Google Scholar]
- 41.Berger M., Grignani G., Giostra A., Ferrari S., Ferraresi V., Tamburini A., Cefalo G., Carnevale-Schianca F., Vassallo E., Picci P., et al. 153Samarium-EDTMP administration followed by hematopoietic stem cell support for bone metastases in osteosarcoma patients. Ann. Oncol. 2012;23:1899–1905. doi: 10.1093/annonc/mdr542. [DOI] [PubMed] [Google Scholar]
- 42.Italiano A., Mir O., Mathoulin-Pelissier S., Penel N., Piperno-Neumann S., Bompas E., Chevreau C., Duffaud F., Entz-Werle N., Saada E., et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:446–455. doi: 10.1016/S1470-2045(19)30825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis L.E., Bolejack V., Ryan C.W., Ganjoo K.N., Loggers E.T., Chawla S., Agulnik M., Livingston M.B., Reed D., Keedy V., et al. Randomized Double-Blind Phase II Study of Regorafenib in Patients With Metastatic Osteosarcoma. J. Clin. Oncol. 2019;37:1424–1431. doi: 10.1200/JCO.18.02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duffaud F., Mir O., Boudou-Rouquette P., Piperno-Neumann S., Penel N., Bompas E., Delcambre C., Kalbacher E., Italiano A., Collard O., et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: A non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019;20:120–133. doi: 10.1016/S1470-2045(18)30742-3. [DOI] [PubMed] [Google Scholar]
- 45.Grignani G., Palmerini E., Dileo P., Asaftei S.D., D’Ambrosio L., Pignochino Y., Mercuri M., Picci P., Fagioli F., Casali P.G., et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: An Italian Sarcoma Group study. Ann. Oncol. 2012;23:508–516. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]
- 46.Grignani G., Palmerini E., Ferraresi V., D’Ambrosio L., Bertulli R., Asaftei S.D., Tamburini A., Pignochino Y., Sangiolo D., Marchesi E., et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: A non-randomised phase 2 clinical trial. Lancet Oncol. 2015;16:98–107. doi: 10.1016/S1470-2045(14)71136-2. [DOI] [PubMed] [Google Scholar]
- 47.Beird H.C., Bielack S.S., Flanagan A.M., Gill J., Heymann D., Janeway K.A., Livingston J.A., Roberts R.D., Strauss S.J., Gorlick R. Osteosarcoma. Nat. Rev. Dis. Primers. 2022;8:77. doi: 10.1038/s41572-022-00409-y. [DOI] [PubMed] [Google Scholar]
- 48.Rothzerg E., Pfaff A.L., Koks S. Innovative approaches for treatment of osteosarcoma. Exp. Biol. Med. 2022;247:310–316. doi: 10.1177/15353702211067718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris M.A., Hawkins C.J. Recent and Ongoing Research into Metastatic Osteosarcoma Treatments. Int. J. Mol. Sci. 2022;23:3817. doi: 10.3390/ijms23073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pilavaki P., Gahanbani Ardakani A., Gikas P., Constantinidou A. Osteosarcoma: Current Concepts and Evolutions in Management Principles. J. Clin. Med. 2023;12:2785. doi: 10.3390/jcm12082785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gazouli I., Kyriazoglou A., Kotsantis I., Anastasiou M., Pantazopoulos A., Prevezanou M., Chatzidakis I., Kavourakis G., Economopoulou P., Kontogeorgakos V., et al. Systematic Review of Recurrent Osteosarcoma Systemic Therapy. Cancers. 2021;13:1757. doi: 10.3390/cancers13081757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.