Abstract
Purpose:
To investigate Cherenkov imaging (Cherenkoscopy) based patient positioning and movement tracking during external beam radiation therapy (EBRT).
Methods:
In a phase 1 clinical trial, including 12 patients undergoing post-lumpectomy whole breast irradiation, Cherenkov emission was imaged with a time-gated ICCD camera synchronized to the LINAC pulse output, during different fractions of the treatment. Patients were positioned with the aid of the AlignRT system in the beginning of each treatment session. Inter-fraction setup variation was studied by rigid image registrations between images acquired at individual treatments to the average image from all imaged treatment fractions. The amplitude of respiratory motion was calculated from the registration of each frame of Cherenkov images to the reference. A Canny edge detection algorithm was utilized to highlight the beam field edges and biological features provided by major blood vessels apparent in the images.
Results:
Real-time Cherenkoscopy can monitor the treatment delivery, patient motion and alignment of the beam edge to the treatment region simultaneously. For all the imaged fractions, the patient positioning errors were within our clinical tolerances (3 mm in shifts and 3 degree in pitch angle rotation), with 4.60% exceeding no more than 1 mm in shifts. The averaged error of repetitive patient positioning was 1.22 mm in linear shift and 0.34 degrees in rotational pitch, consistent with the accuracy reported by the AlignRT system. The edge detection algorithm enhanced features such as field edges and blood vessels. Patient positioning errors and respiratory motion retrieved from rigid image registration were consistent with the edge enhanced images. Besides positioning errors caused by globally inaccurate setups, edge enhanced blood vessels indicate the existence of deformations within the treatment region, especially for large patients.
Conclusions:
Real-time Cherenkoscopy imaging during EBRT is a novel imaging tool that can be used for treatment monitoring, patient positioning and motion tracking.
Introduction:
Radiation therapy is used to treat approximately half of all cancer patients (1), either for palliative or curative intent. In external beam radiation therapy (EBRT) with a linear accelerator (LINAC), doses are prescribed to the clinical target volume (CTV) as simulated in the treatment planning system (TPS) from computed tomography (CT) scans. Fractionated delivery is standard, and to make sure the prescribed dose is delivered to the CTV as planned, accurate positioning of the patient according to the treatment plan is essential, especially for a treatment course including many sessions. Geometric tolerance for small errors in patient positioning are included in the treatment plan by adding adequate margin for error to the planning target volume (PTV). Routine limits of setup accuracy are usually clinically insignificant events; however, if the planned treatment accuracy tolerance is not met, unplanned dose may be delivered to surrounding benign tissue while undertreating the CTV (2). In some specific situations (brain, lung, breast tumor, etc.) where the CTV is fairly close to vital organs (brain stem, lung, heart, etc.), extreme accuracy in patient positioning is required and immobilization techniques such as mask fixation and respiratory gating are important to avoid treatment inaccuracies (3–5). In this study a new method for direct visualization of radiation dose from Cherenkov emission was utilized for real-time monitoring and to assess the ability to estimate positional accuracy.
In general, the positioning of the patient as a rigid body is achieved by matching marks tattooed on the patient to the planned position using room lasers aligned to the treatment isocenter. Depending on stability of the tattooed tissue, routine setup accuracy is limited to approximately 5mm. Setup accuracy may be improved to approximately 1mm – 2 mm by using image-guided radiation therapy (IGRT) (6), techniques such as daily portal imaging or cone beam computed tomography (CBCT) before treatment is delivered (7). Surface detection and matching techniques (8) such as the AlignRT system are used to compare the optically detected patient surface with that derived from the planning CT scan and can achieve setup accuracy up to 3 mm and 3 degrees of rotation (9–12). These techniques assume the patient is a rigid body and challenges exist in several more complex scenarios (13), such as when patients loses significant weight, the tumor changes shape/size, or there is anatomic motion such as bowel displacement or respiration (14–16). Many existing techniques, including portal imaging, respiratory monitoring (RPM) (17), patient monitoring systems such as AlignRT and predictive models (18) can be used to record or monitor these changes at the beginning or during the treatment. Depending on the magnitude of these changes and their effects to the treatment course, adaptive modifications can be made to the original plan during the treatment course (18–20). All of these techniques focus on the patient prior to turning on the radiation beam (validation of patient positioning or motion tracking) and lack the ability to directly monitor the static or dynamic beam actually delivered to the patient during treatment. In order to validate patient positioning, track patient motion and globally monitor how the positioning and motion interactively affect the treatment delivery, both the patient and the radiation field need to be monitored simultaneously. Here, we present a technique based on Cherenkov radiation imaging during EBRT, termed as Cherenkoscopy, which is capable of direct dose imaging and patient position verification in real time.
Cherenkov radiation, is optical emission having wavelengths from the ultraviolet to the near-infrared, occurring only in dielectric medium (such as water and biological tissue) when charged particles move with a phase velocity greater than the speed of light in that medium (21, 22). In the scope of radiation therapy, Cherenkov radiation was recently measured from megavoltage external photon and electron beams during EBRT in both water and tissue (23). As shown in Fig. 1(a), high energy particles deposit energy (radiation dose) to the environment through interactions such as soft and hard collisions during their transport. Cherenkov photons are emitted along the path of primary and secondary charged particles (dominated by electrons) and the intensity of Cherenkov radiation emission is proportional to the locally deposited dose (24, 25). Depending on the optical properties of the medium including refractive index, absorption and scattering coefficients, Cherenkov photons will be able to escape the volume to be detected (26–28). For a transparent phantom such as water, photons generated deep within the phantom are detectable while depth of detection is limited to approximately 1 to 6 mm layer underneath the surface for turbid medium such as biological tissue (26, 27). With spectral filtering, the emission can be detected more effectively providing the spectrum of ambient light is significantly different and the sampling depth could also be tuned from sub-millimeter to about 7 mm (26, 27);
Figure. 1:
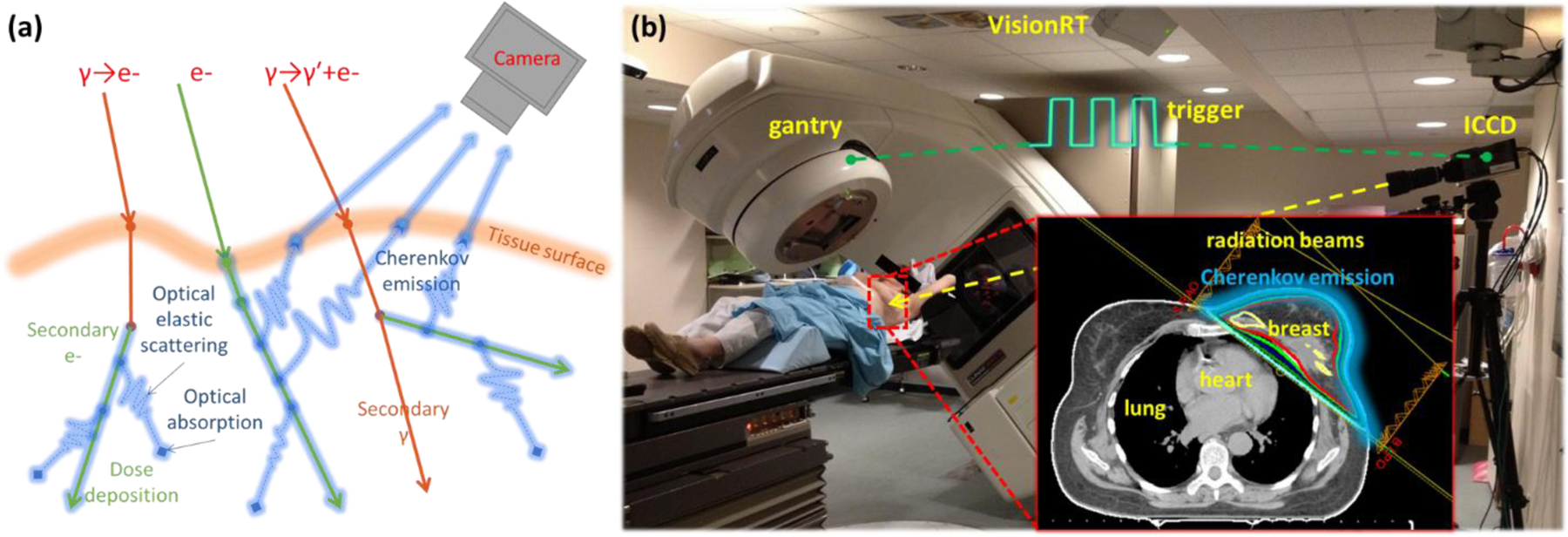
(a) Illustration of the basics of radiation interaction, generating Cherenkov and the optical photon transport in biological tissue. Cherenkov photons emitted by primary or secondary charged particles can be scattered and absorbed in biological tissue, with some escaping surface and being detected by the camera. (b) Setup of the time domain gated imaging system, which is synchronized to the delivered radiation pulses, for a patient undergoing post-lumpectomy whole breast radiation therapy with oppositely directed tangential beams (inset plan).
In this work, video imaging of Cherenkov emission was captured by a time domain gated system for a clinical trial of patients undergoing post-lumpectomy whole breast irradiation. Images from different treatment sessions were registered to the average image to calculate errors in patient positioning and then compared to the tolerance value preset in the AlignRT system. A Canny edge detection algorithm was applied to Cherenkov images to highlight beam edges and blood vessels, which shows the potential of vessels as internal biological markers. Respiratory motion tracking was investigated by calculating the positioning difference for every frame of Cherenkov images taken within one fraction of treatment. The entire study was conducted in realistic clinical environment without affecting the normal process of planned radiation therapy. Initial results indicate that Cherenkoscopy is a robust technique for simultaneous patient and radiation field monitoring.
Materials and methods:
Post-lumpectomy Whole Breast Radiation Therapy:
This clinical trial was conducted at the radiation oncology department of Norris-Cotton Cancer Center. The external beam irradiator was a Varian Clinic 2100CD linear accelerator (LINAC, Varian Medical Systems, Palo Alto, CA USA). As illustrated in Fig. 1b, in post-lumpectomy whole breast radiation therapy, tangential beams were designed to irradiate the whole breast and spare the heart and lung (Fig. 1b). For the 12 patients investigated in this study (IRB approved), the radiation treatment planning was performed in the Varian Eclipse planning system (Varian Medical Systems, Palo Alto, CA USA). In general, a normal treatment course will be followed by a boost treatment course for each patient; however, this study will only focus on the first treatment course. The first treatment course was delivered with either standard (28 whole breast fractions) or hypo-fractionation (16 whole breast fractions) with each fraction delivering a dose of 180 or 266 cGy. Depending on the specific clinical considerations for each patient, radiation beams with different configurations (energies, monitor units (MU), field sizes, incident angles, etc.) were delivered for each fraction. Information about the treatment course related to this study for each patient is summarized in Table 1. At the beginning of each treatment fraction, the patient was set up to the planned position with the aid of the AlignRT system. The tolerance values set for the AlignRT system were 3 mm in linear shift and 1 degree in pitch rotation.
Table. 1:
Treatment information and acquisition procedures for each patient.
| Patient # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment side | Left | Right | Left | Right | Left | Right | Right | Right | Right | Right | Right | Left |
| Imaged fractions/total fractions | 7/16 | 6/16 | 8/28 | 10/28 | 7/28 | 10/28 | 7/28 | 6/28 | 9/16 | 7/16 | 10/25 | 7/16 |
| Dose(cGy)/monitor units(MU) per fraction | 266/315 | 266/306 | 180/215 | 180/205 | 180/205 | 180/218 | 180/213 | 180/212 | 266/317 | 266/319 | 180/211 | 266/289 |
| Beam configurations | RAO 6MV-83MU; RAO 10MV-76MU; LPO 6MV-82MU; LPO 10MV-74MU | LAO 6MV-136MU; LAO 10MV-28MU; RPO 6MV-112MU; RPO 10MV-30MU | RAO 6MV-110MU; LPO 6MV-105MU | LAO 6MV-107MU; RPO 6MV-98MU | RAO 6MV-78MU; RAO 10MV-29MU; LPO 6MV-68MU; LPO 10MV-30MU | LAO 6MV-136MU; LAO 10MV-28MU; RPO 6MV-112MU; RPO 10MV-30MU | LAO 6MV-108MU; RPO 6MV-105MU(SCV tx LAO 10MV-141MU and RPO 10MV-50MU) | LAO 6MV-94MU; LAO 10MV-13MU; RPO 6MV-59MU; RPO 10MV-46MU | LAO 6MV-167MU; RPO 6MV-150MU | LAO 6MV-160MU; RPO 6MV-159MU | LAO 6MV-110MU; RPO 6MV-101MU | RAO 6MV-147MU; LPO 6MV-152MU |
| Camera | PI-MAX3(ICCD) | PI-MAX3(ICCD) | PI-MAX3(ICCD) | PI-MAX3(ICCD) | PI-MAX4(emICCD) | PI-MAX3(ICCD) | PI-MAX3(ICCD) | PI-MAX3(ICCD) | PI-MAX3(ICCD) | PI-MAX3(ICCD) | PI-MAX3(ICCD) | PI-MAX3(ICCD) |
| Lens(focal length (mm)/f number) | 135/2.0 | 135/2.0 | 135/2.0 | 135/2.0 | 135/2.0 | 100/2.0 | 100/2.0 | 100/2.0 | 100/2.0 | 100/2.0 | 100/2.0 | 135/2.0 |
| Intensifier | ×100 | ×100 | ×100 | ×100 | ×10000 | ×100 | ×100 | ×100 | ×100 | ×100 | ×100 | ×100 |
| Accumulated pulses per frame | 100 | 50 | 100 | 50 | 1 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Frame rate (fps) | 2.8 | 4.7 | 2.8 | 4.7 | 30 | 4.7 | 4.7 | 4.7 | 4.7 | 4.7 | 4.7 | 4.7 |
Time gated Cherenkov imaging (Cherenkoscopy):
Our imaging system, as described in previous studies (29–31) and shown in Fig. 1b, consisted of an intensified charge-coupled device (ICCD) camera (ICCD, PI-MAX3 (×100, 1024 × 1024), electron multiplying charge-coupled device (emICCD), PI-MAX4 (×10000, 512 × 512), a commercial lens (Canon EF 135mm or 100mm, f/2L USM) and a tripod, was mounted approximately 3 meters from the treatment couch in order to provide full access to the patient and permit gantry rotation. Depending on the treatment side, the camera was focused on the left or right side of the patient and remained in this position (except for patient 5) over the entire normal treatment course. The room light was dimmed during the treatment to a level which balanced patients’ comfort, ability of the treating therapist to monitor the patient, and the acquisition of the weak Cherenkov signal. To detect the Cherenkov emission effectively and reject most of the ambient light, the camera was synchronized to the LINAC (by the trigger signal shown in Fig. 1b), which delivered radiation in pulses of 3.25 μs temporal length, at a repetition frequency of approximately 200 Hz. For each patient, 10 fractions during the whole treatment course (first course and boost treatment) were imaged. This study was only based on the data acquired from the first course, whole breast treatments.
Cherenkov images were acquired continuously (frame rates of 2.8, 4.7 or 30 fps) during irradiation with each frame an accumulation of certain number (100, 50 or 1) of radiation pulses. The acquisition procedures, including the camera, lens, intensifier, accumulation settings and the corresponding frame rate, are summarized in Table 1 for each patient. Background images were acquired before the treatment for each planned gantry rotation with the same imaging procedure but radiation off and then subtracted from the acquired Cherenkov images. White light images of the treatment region, to which the Cherenkov images could be overlaid for post processing, were also captured before the radiation delivery. As summarized in Table 1, similar acquisition procedures were applied to the patients with changes made to realize specific experimental goals. For example, due to limits in the treatment schedule, sometimes it was essential to image multiple patients in the same day without moving the camera. Since each patient was positioned differently, it was necessary to switch the lens (from 135 mm focal length to 100 mm focal length) to capture a larger field of view which covered the treatment region for every patient. The number of accumulated radiation bursts for each frame of Cherenkov image was changed for some of the patients to investigate how this impacted the possible frame rate. Specifically, for patient 5, a more sensitive PI-MAX4 camera (×10000, 512×512) was used for data acquisition to prove that video rate (frame rate up to 30 fps) was possible by single radiation pulse imaging. Within the scope of patient positioning and movement tracking, these changes in the acquisition procedure will not affect the main conclusions of this work.
Image processing:
All the acquired images for this study were processed in the image processing toolbox of Matlab (Matlab 2013b, MathWorks Inc., Boston, MA). To reduce saturated pixels caused by high energy particles hitting the ICCD directly, a temporal median filter was applied to stacks of Cherenkov images sequentially, with each stack consisting of a number of adjacent frames which was equivalent to 1 sec of acquisition time (32). For example, in the case where the frame rate was approximately 3 fps, with each frame an accumulation of 100 radiation bursts, a stack for the temporal filtering consisted of three frames of Cherenkov images. A spatial bilateral filter (kernel size = 10 pixels × 10 pixels for the PI-MAX3 camera) was then applied to the temporal median filtered frames to further smooth the images with the ability to preserve the edges (33). A summation of all processed frames of Cherenkov images was self-normalized to be the post-processed image for each specific patient at a single imaged treatment fraction. The post-processed image of the first imaged fraction for each patient was overlaid (to the blue channel) to the white light reference image taken at the beginning of the treatment (Fig. 2a).
Figure. 2:
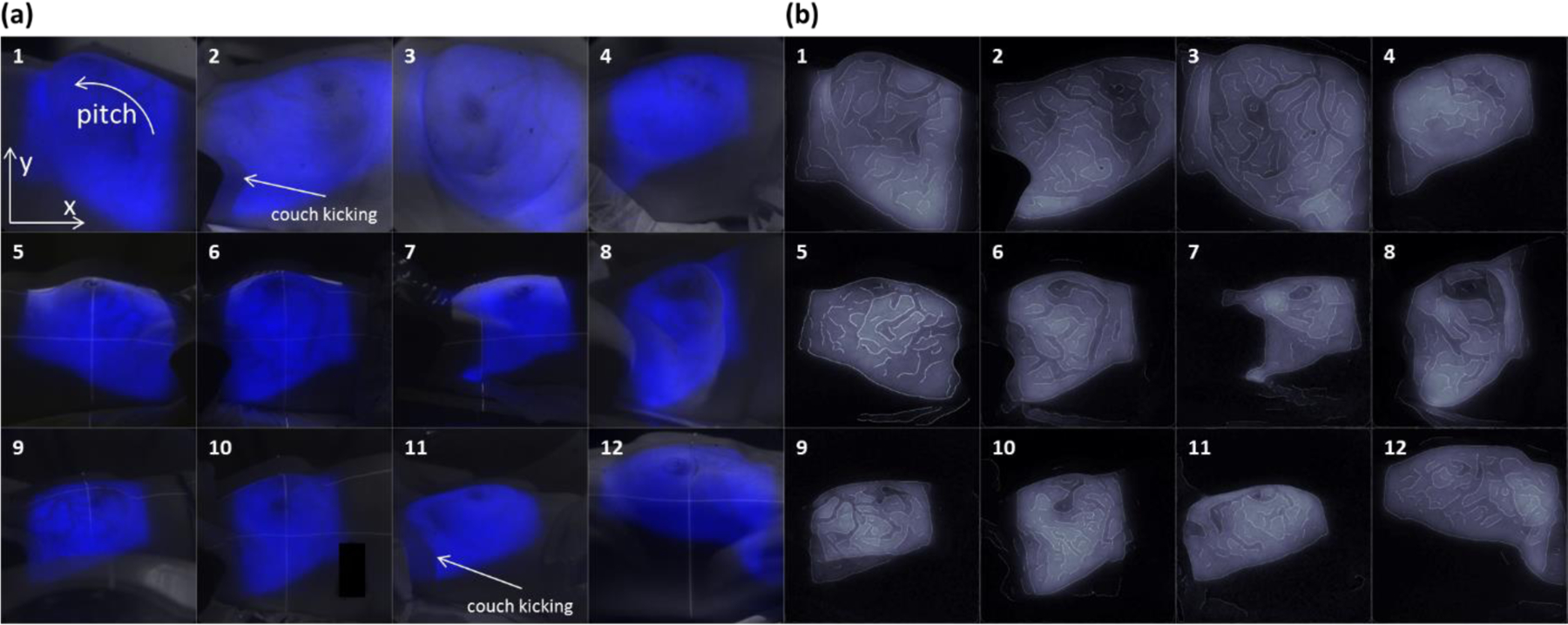
(a) The post-processed Cherenkov image for each patient overlaid with the corresponding white light image. Beam fields, blood vessels and artifacts caused by couch kicking could be clearly observed. (b) Edge enhanced Cherenkov images for each patient. .
Post-processed images for different treatment fractions were rigidly registered to the averaged image by calling the built-in image registration function in Matlab. From the returned registration matrix, shifts in x and y directions and pitch angle rotation with respect to the center of the image, as indicated in Fig. 2a, were retrieved. The Canny edge detection algorithm was applied to enhance edges in post-processed images (34). This method finds edges by looking for local maxima of the gradient, and the gradient was calculated using the derivative of a Gaussian filter. The Canny method then used two thresholds, to detect strong and weak edges, and included the weak edges in the output only if they were connected to strong edges. The kernel size of the Gaussian filter was chosen to be 10 pixels × 10 pixels in this study. Based on statistical features of the input data, the threshold values were calculated automatically and chosen heuristically by the robust built-in edge detection function.
To investigate movement tracking, mainly the respiratory motion, within one treatment fraction for each delivered beam, Cherenkov images acquired for a specific beam within one imaged treatment fraction were temporal median filtered and a bilateral filter smoothed as described above. Each processed frame was rigidly registered to a chosen reference frame. From the registration matrix, shifts in x and y directions with respect to the reference frame were retrieved. The amplitudes of movements (respirations) were calculated as the length of the error vector ([shift in x direction, shift in y direction]). The edge detection was also applied to every processed frame to validate the movement tracking.
Results:
Cherenkov and edge enhanced images:
Figure 2a shows the post-processed Cherenkov image overlaid on the white light image for each patient indicating that the Cherenkov emission from the treatment region was successfully imaged. Note that for patient 2 and 11, their treatment plans included couch kicks (rotation of the treatment coach) among different delivered beams, which can be clearly observed in the composite images (indicated in Fig. 2a). The edge enhanced images are shown in Fig. 2b. For patient 2 and 11, because of the couch kicks, the edge enhancing was done in the Cherenkov image acquired for one beam instead of the composite image of all the treatment beams. The edges of the beam fields appear to be detected. More interestingly, regions with higher absorption such as the major blood vessels near the breast surface were observed in the Cherenkov images as decreases in emitted intensity. These provide a unique pattern for different patients in the edge enhanced images and one which is unique to the biological region being treated.
Patient positioning validation:
Patient positioning errors retrieved from the rigid image registration were shown in Fig. 3. Shifts in x, y directions and pitch angle rotations for all the imaged fractions of each patient (except patient 5) were shown in Fig. 3a, 3b and 3c. For patient 5, the camera (PI-MAX4) with lower resolution (512 × 512) was used, so for consistency of data, the images of patient 5 were not included in Fig. 3. For each patient in this study, the positioning error was well controlled with the aid of the AlignRT system. Statically, shifts in x and y direction were controlled within 2 mm (Fig. 3a and 3b) and the pitch angle rotation was within 1 degree (Fig. 3c). Figure 3d shows the scatter plot of shifts in x and y directions for all imaged fractions with respect to two circles representing the error of 3 and 4 mm. In 95.40% of the imaged fractions, the patient was positioned within the preset error tolerance (3 mm) with few (4.60%) exceeding by no more than 1 mm.
Figure. 3:
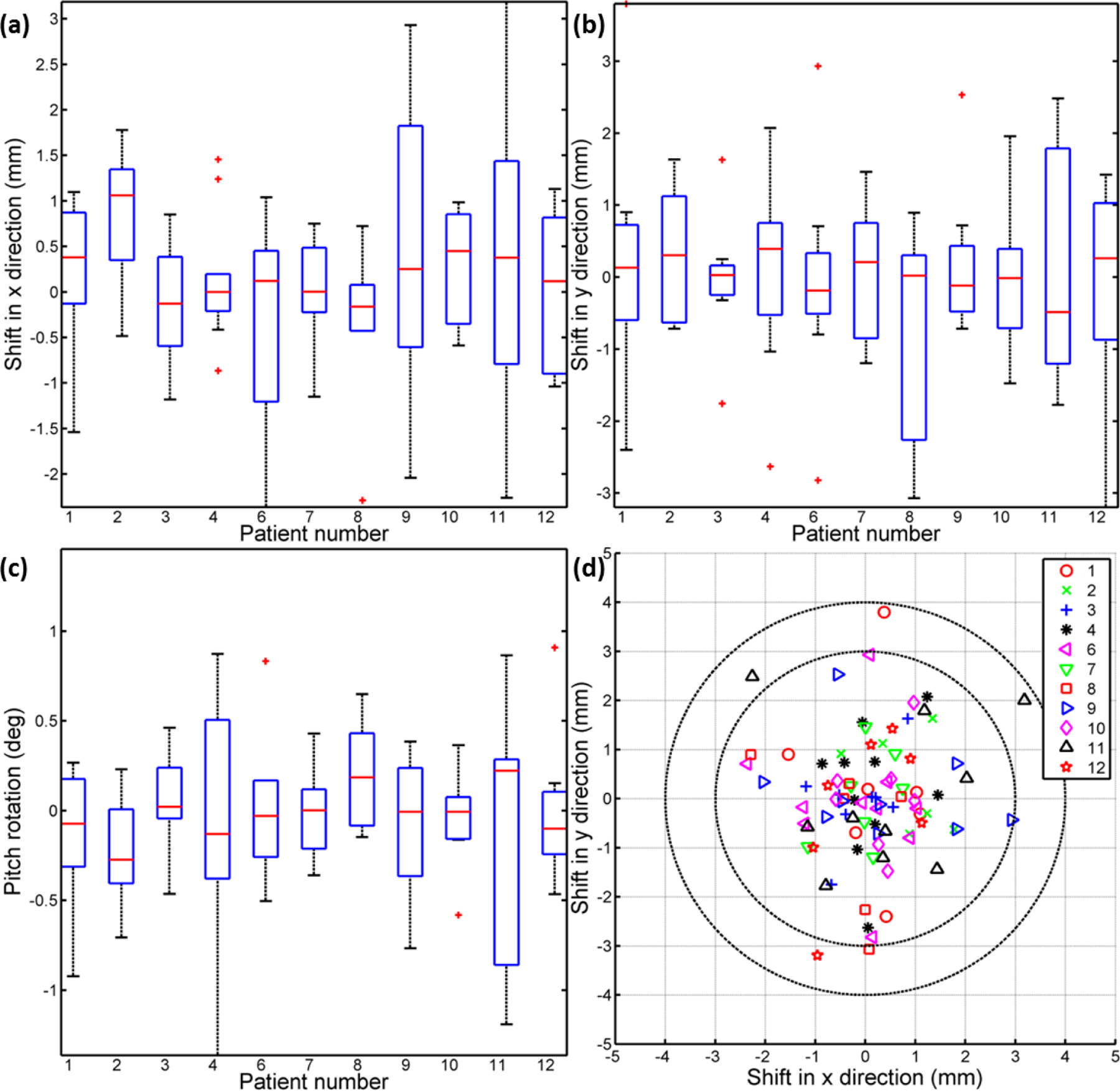
(a-c) Statistical results (shift in x, y direction and pitch angle rotation respectively) of positioning errors retrieved from rigid image registrations for each patient’s imaged treatment fractions. (d) Scatter plot of the positioning error vector in x and y directions, with the comparison with tolerance values preset in the AlignRT system.
Focusing on patient 3, who was required to hold her breath during the treatment (the breath holding was monitored by the RPM system), a case study was conducted to illustrate more details related to patient positioning in Cherenkov and edge enhanced images. The edges of beam field and blood vessels were enhanced in the averaged Cherenkov image (Fig. 4a). Detected edges from the Cherenkov image acquired for individual fractions are shown in Fig. 4b with false colors representing different imaged fractions. The retrieved positional errors for patient 3 are plotted in Fig. 4c, using the same color coding as in Fig. 4b for different fractions. Comparing Fig. 4b and 4c, consistencies exist between the relative shifts of the edges and the corresponding retrieved positioning error. For example, the edges color coded as blue in Fig. 3b showed maximum shift in -x direction, so is the retrieved positioning error in Fig. 3c. Similar results were observed for all the other patients, which suggest that the enhanced edges together with the retrieved positioning errors could be utilized to track position changes.
Figure. 4:
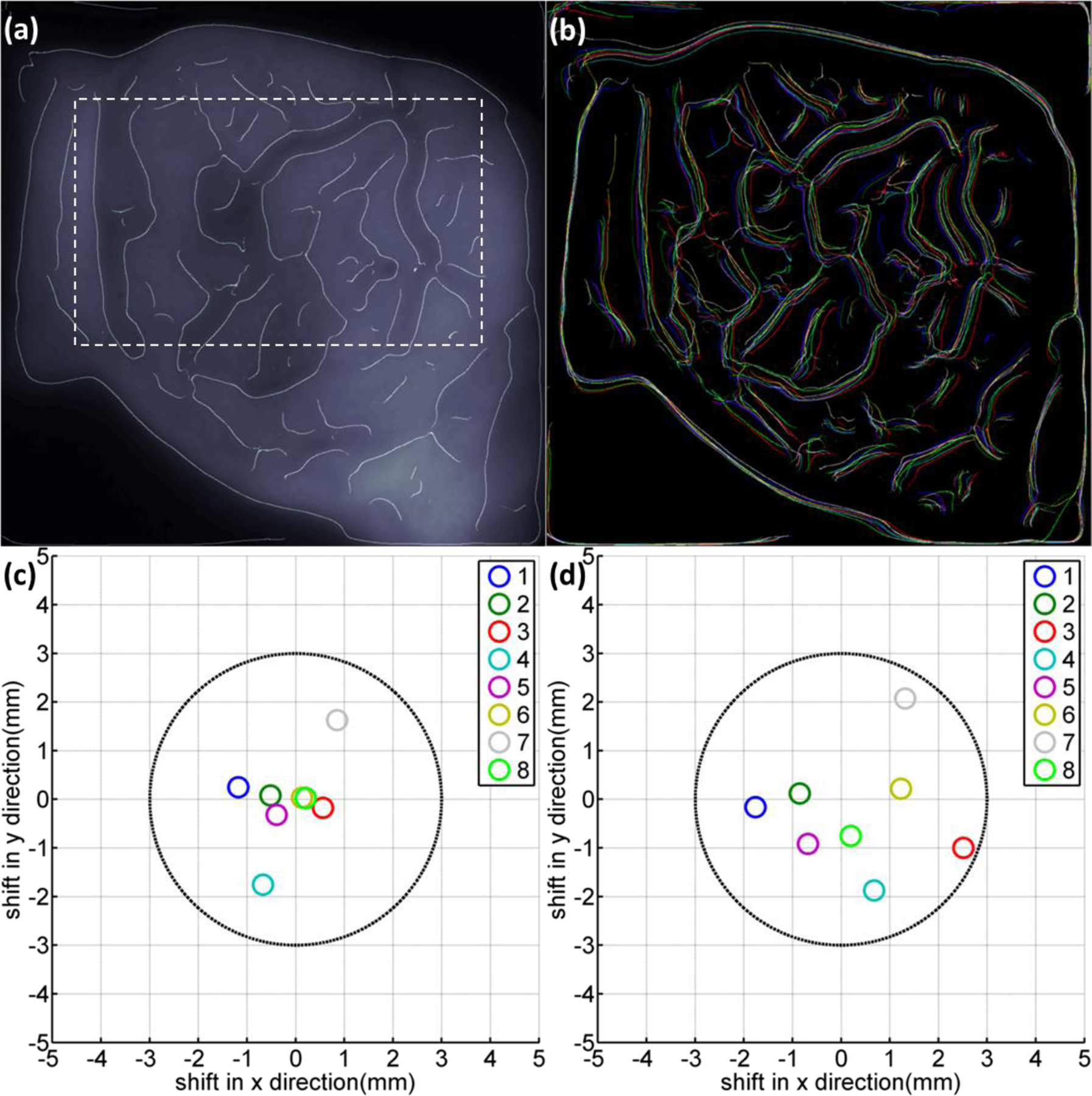
(a) The edge enhanced Cherenkov image of patient 3 with a chosen internal region (indicated by dotted lines) within the treatment beam field. (b) Detected edges in Cherenkov images for patient 3, false colored for different imaged treatment fractions. (c) Retrieved positioning errors for patient 3 from rigid image registrations based on the full frame of images. (d) Retrieved positioning errors for patient 3 from rigid image registrations based on the chosen internal region as indicated in (a) with dotted lines.
In Fig. 4b, the edges of blood vessels showed less overlay with each other than the beam edges, especially in x direction. This could be caused by internal deformation in the treatment region even though the patient was positioned accurately as a rigid body. To validate this, an internal region was indicated in Fig. 4a by a dotted line that was chosen for the images from different fractions. The chosen region was then rigidly registered to the averaged full Cherenkov image. The retrieved positioning errors were plotted in Fig. 4d. Comparing Fig. 4c and 4d, the positioning errors spread out, especially in x direction (agree with Fig. 4b), suggesting that even though the patient can be positioned well as a rigid body, deformations could potentially contribute to positioning errors. The edge enhanced biological features (blood vessels) in Cherenkov image could potentially indicate these deformations and serve as unique biological markers for patient positioning.
Movement (respiration) tracking:
Depending how long a specific treatment beam takes to be delivered, respiration motions during the delivery process could be monitored by Cherenkoscopy. Data acquired during the delivery of specific treatment beams (6MV/RAO for patient 3, 10MV/LPO for patient 1, 6MV/LPO for patient 12 and 6MV/LAO for patient 9) for different beam configurations and acquisition conditions were processed and shown here to illustrate this concept. Edges were detected in every frame of the processed Cherenkov images acquired for each beam. Figures 5a, 5c, 5e and 5g show the averaged edges over all the processed frames for these four beams. Correspondingly, respiratory amplitudes retrieved from image registrations were plotted in Fig. 5b, 5d, 5f and 5h. For patient 3 with a breath hold during the treatment, the edges were relatively sharp in Fig. 5a. The respiratory amplitude was zero during the delivery of the entire beam. For other patients who were breathing freely during the treatment, it can be observed that the averaged edges were blurred (Fig 5c, 5e and 5g), especially for the beam field and blood vessel edges. Depending on the length of the beam and the respiratory frequency of the patient, single (Fig. 5d) and multiple (Fig. 5f and 5h) respiratory periods were monitored. To show the monitored respiratory motions more clearly, additional files (Additional files 1, 2, 3 and 4) for the four beams were included. Similar results were observed for other beams and thus the idea of respiratory monitoring based on Cherenkoscopy is valid for all the patients in this study. Although, limited by the length of most of the treatment beams, only a partial or a single respiratory period, similar to what shows in Fig. 5c, were monitored.
Figure. 5:
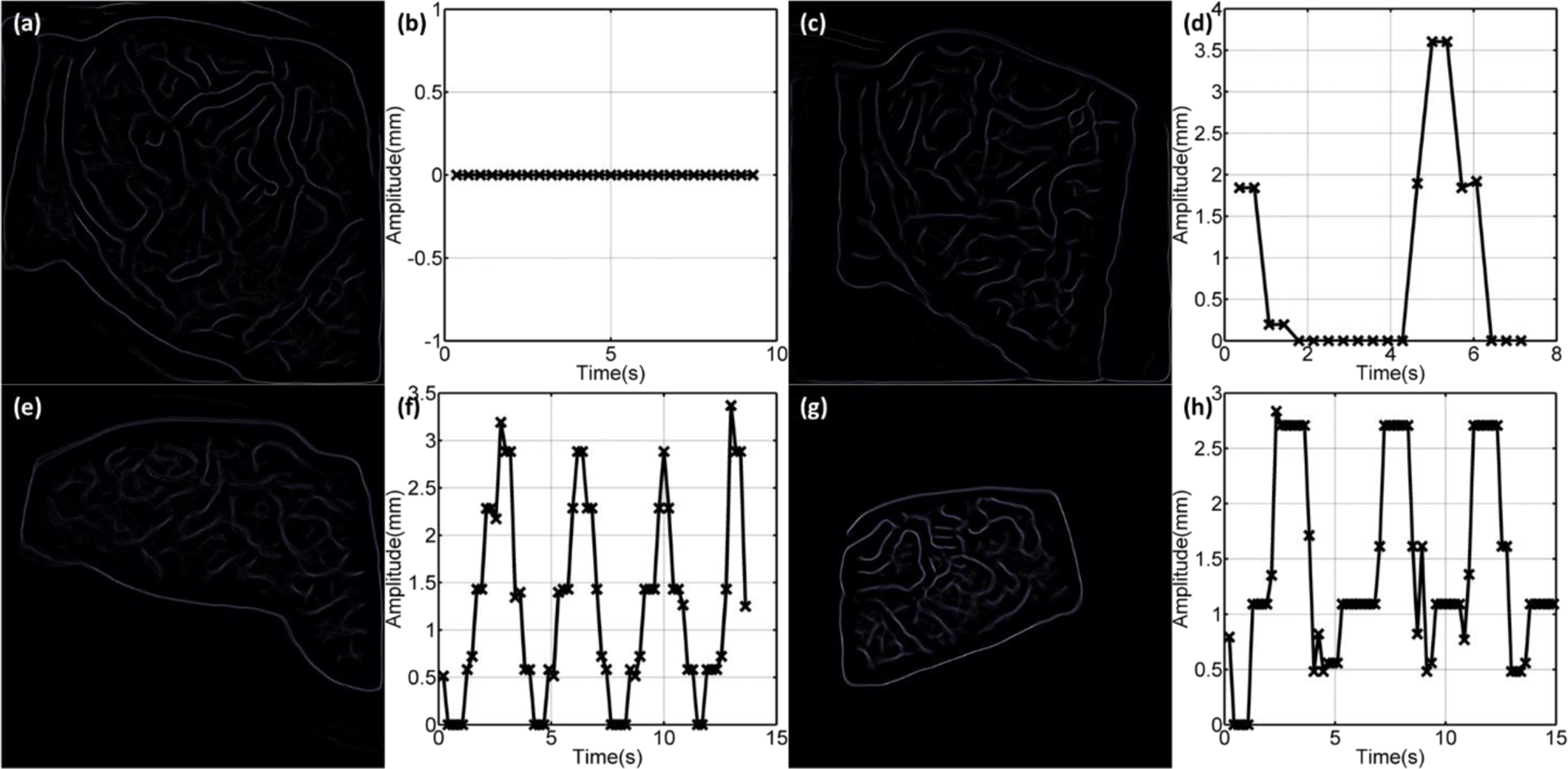
(a) Averaged edges for one treatment beam (6MV/RAO) for a patient (patient 3) treated with a breath hold. (b) Retrieved respiratory amplitude for patient 3 treated with a breath hold. (c, e, g) Averaged edges for patients (patient 1, patient 12 and patient 9) breathed freely during the delivery of treatment beams (10MV/LPO, 6MV/LPO and 6MV/LAO). Blurred beam field and blood vessel edges were observed. (d, f, g) Retrieved respiratory amplitude for patients (6MV/RAO for patient 3, 10MV/LPO for patient 1, 6MV/LPO for patient 12 and 6MV/LAO for patient 9) breathing freely, showing periodic respiratory motions.
Discussion:
In this study, data was acquired in the clinical environment without significantly changing the normal treatment process. For the patient treated with breath holding (patient 3), each frame of Cherenkov image for one treatment beam gives very similar results with consistent input parameters, suggesting that Cherenkoscopy imaging is a robust and relatively straightforward technique for clinical use. The imaging requires nothing more than a gated camera in the treatment room and can provide real time feed of the beam incident upon the tissue to the control room. In addition this real time image stack can be processed to provide patient positional information, as was studied here.
As shown in Fig. 3, the patient positioning errors for each treatment fraction were retrieved from the Cherenkov images. The result was consistent with the tolerance value planned (3 mm in shifts and 3 degree in pitch angle rotation), as preset in the AlignRT system, with just a few outliers exceeding no more than 1 mm. The positioning errors due to deformations and motions (such as respiration) can be recorded by the Cherenkov images which is likely the reason for the few outliers observed (4.60% for shifts and none for pitch angle rotations).
Treatment field edges and blood vessels could be highlighted by applying the edge detection algorithm to the acquired Cherenkov images (Fig. 2b). Similar to the idea of portal imaging, Cherenkov imaging could be acquired in advance of the delivery of the whole treatment in as little as a single pulse from the LINAC. Highlighted edges, especially the ones from the blood vessels, could serve as unique biological markers (Fig. 3), which not only indicate the patient position but also deformations with respect to the treatment field, and vary as the patient shape may vary (due to weight loss, tumor change or skin stretch).
Perhaps the most significant advantage of Cherenkoscopy in the scope of patient positioning and movement tracking is the ability to monitor the patient and the delivered beam field on the patient simultaneously. As shown in Fig. 5 and Additional files 1, 2, 3 and 4, respiratory motions in the beam field were clearly monitored in real time. The patient treated with a breath hold was successfully distinguished from patients who breathed freely, based on the retrieved respiratory amplitude (Fig. 5b, 5d, 5f and 5h). Besides respiration, other motions, such as accidental movements of the patient in the middle of the treatment, could be potentially monitored. Because Cherenkoscopy records the movements and the beam field simultaneously, the effect of the movement on the delivery of beams can be accessed and followed with actions such as suspending the treatment, to ensure the security and quality of the treatment. Although this study focused on the case of post-lumpectomy whole breast radiation therapy, this technique could be extended to other situations (such as radiation treatments for head and neck tumor) without much extensive efforts.
In practice, certain limitations exist in the current stage of this technique. Due to the set-up of the camera, Cherenkov emissions were imaged from limited angles. In this study, either the entrance or exit side of the radiation was imaged. Additional cameras mounted at different positions are necessary to cover a larger imaged solid angle. The frame rate was limited to be approximately 5 fps with the ICCD camera currently used. For this study, it has been shown that 3 to 5 fps is reasonably fast to capture the delivered field and respiratory motions in real time. However, higher frame rates are desirable, especially for treatments with arcs and highly dynamic beam fields, due to the fast motion of the MLCs (such as the treatment for head and neck cancers). For a proof of concept, another camera with the intensifier’s response spectrum more sensitive to the Cherenkov emission from biological tissues (weighted in the red to NIR region because of tissue’s strong scattering and absorption in the blue region) was tested in imaging patient 5. In this case, fewer radiation pulses (single pulse compared to 50 or 100) were accumulated for each frame of images and resulted in acquisition with a higher frame rate close to 30 fps (Additional file 5 and 6). In this study, the AlignRT was only used for patient positioning at the beginning of each treatment fraction. Ideally, it should be kept on to monitor the whole treatment. However, the bright red light projected by the AlignRT system saturated the ICCD camera and thus Cherenkov emissions cannot be imaged. Since the AlignRT system projects pulsed light (approximately 240 Hz based on our measurements) on the patient, one possible way to overcome this problem is to synchronize the ICCD camera to both the radiation pulses and projected light pulses. Detection of Cherenkov emission when the radiation is on and the projected light is off should allow the acquisition of Cherenkov emissions with the AlignRT system on all the time. These issues are currently under investigation and the results will be presented in following studies.
Conclusions:
In conclusion, real time Cherenkoscopy imaging, where both patients and beam fields can be simultaneously imaged, is a novel tool for the purposes of treatment monitoring, patient positioning and movement tracking.
Acknowledgements:
The authors thank the section of Radiation Oncology at Dartmouth-Hitchcock Medical Center, especially the treating radiation therapists and patients, for accommodating our imaging protocol. This work has been financially supported by Pilot Grant Funds from the Norris Cotton Cancer Center, as well as NIH research grant R01CA109558 and R21EB017559.
List of Abbreviations used:
- EBRT
External beam radiation therapy
- LINAC
Linear accelerator
- CTV
Clinical target volume
- TPS
Treatment planning system
- CT
Computed Tomography
- PTV
Planning target volume
- IGRT
Image-guided radiation therapy
- CBCT
Cone beam computed tomography
- ICCD
Intensified charge-coupled device
- emICCD
Electron multiplying charge-coupled device
- MU
Monitor units
- RAO
Right anterior oblique
- LPO
Left posterior oblique
Footnotes
Additional files:
Additional files 1, 2, 3, 4: Videos show the respiratory motions tracked by Cherenkov imaging during specific beams (6MV/RAO for patient 3, 10MV/LPO for patient 1, 6MV/LPO for patient 12 and 6MV/LAO for patient 9) for patients treated with/out (patient 3/patient 1, 9, 12) a breath hold.
Additional files 5, 6: Imaging of Cherenkov emission with single radiation pulse accumulation and frame rate approximately 30 fps.
References:
- 1.National Cancer Institute N, DHHS, Bethesda, MD, http://progressreport.cancer.gov. Cancer Trends Progress Report - 2011/2012 Update. August 2012. [Google Scholar]
- 2.Margalit DN, Chen YH, Catalano PJ, Heckman K, Vivenzio T, Nissen K, et al. Technological advancements and error rates in radiation therapy delivery. International journal of radiation oncology, biology, physics 2011;81(4):e673–9. [DOI] [PubMed] [Google Scholar]
- 3.Verhey LJ, Goitein M, Mcnulty P, Munzenrider JE, Suit HD. Precise Positioning of Patients for Radiation-Therapy. Int J Radiat Oncol 1982;8(2):289–94. [DOI] [PubMed] [Google Scholar]
- 4.Hong TS, Tome WA, Chappell RJ, Chinnaiyan P, Mehta MP, Harari PM. The impact of daily setup variations on head-and-neck intensity-modulated radiation therapy. Int J Radiat Oncol 2005;61(3):779–88. [DOI] [PubMed] [Google Scholar]
- 5.Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA, Wong JW. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol 2003;55(2):392–406. [DOI] [PubMed] [Google Scholar]
- 6.Xing L, Thorndyke B, Schreibmann E, Yang Y, Li TF, Kim GY, et al. Overview of image-guided radiation therapy. Med Dosim 2006;31(2):91–112. [DOI] [PubMed] [Google Scholar]
- 7.Creutzberg CL, Althof VGM, Huizenga H, Visser AG, Levendag PC. Quality Assurance Using Portal Imaging - the Accuracy of Patient Positioning in Irradiation of Breast-Cancer. Int J Radiat Oncol 1993;25(3):529–39. [DOI] [PubMed] [Google Scholar]
- 8.El-Hakim SF, Gerig LH. Patient positioning and monitoring system. Google Patents; 1995.
- 9.Li S, DeWeese T, Movsas B, Liu DZ, Frassica D, Kim J, et al. Initial Validation and Clinical Experience with 3D Optical-Surface-Guided Whole Breast Irradiation of Breast Cancer. Technol Cancer Res T 2012;11(1):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krengli M, Gaiano S, Mones E, Ballare A, Beldi D, Bolchini C, et al. Reproducibility of patient setup by surface image registration system in conformal radiotherapy of prostate cancer. Radiat Oncol 2009;4. [DOI] [PMC free article] [PubMed]
- 11.Gierga DP, Riboldi M, Turcotte JC, Sharp GC, Jiang SB, Taghian AG, et al. Comparison of target registration errors for multiple image-guided techniques in accelerated partial breast irradiation. Int J Radiat Oncol 2008;70(4):1239–46. [DOI] [PubMed] [Google Scholar]
- 12.Schoffel PJ, Harms W, Sroka-Perez G, Schlegel W, Karger CP. Accuracy of a commercial optical 3D surface imaging system for realignment of patients for radiotherapy of the thorax. Phys Med Biol 2007;52(13):3949–63. [DOI] [PubMed] [Google Scholar]
- 13.van Kranen S, van Beek S, Rasch C, van Herk M, Sonke JJ. Setup Uncertainties of Anatomical Sub-Regions in Head-and-Neck Cancer Patients after Offline Cbct Guidance. Int J Radiat Oncol 2009;73(5):1566–73. [DOI] [PubMed] [Google Scholar]
- 14.Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys 2006;33(10):3874–900. [DOI] [PubMed] [Google Scholar]
- 15.Langen KM, Jones DTL. Organ motion and its management. Int J Radiat Oncol 2001;50(1):265–78. [DOI] [PubMed] [Google Scholar]
- 16.Rietzel E, Bert C. Respiratory motion management in particle therapy. Med Phys 2010;37(2):449–60. [DOI] [PubMed] [Google Scholar]
- 17.Kubo HD, Hill BC. Respiration gated radiotherapy treatment: A technical study. Phys Med Biol 1996;41(1):83–91. [DOI] [PubMed] [Google Scholar]
- 18.Zhang PP, Yorke E, Hu YC, Mageras G, Rimner A, Deasy JO. Predictive Treatment Management: Incorporating a Predictive Tumor Response Model Into Robust Prospective Treatment Planning for Non-Small Cell Lung Cancer. Int J Radiat Oncol 2014;88(2):446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan D Developing quality assurance processes for image-guided adaptive radiation therapy. Int J Radiat Oncol 2008;71(1):S28–S32. [DOI] [PubMed] [Google Scholar]
- 20.Yan D, Vicini F, Wong J, Martinez A. Adaptive radiation therapy. Phys Med Biol 1997;42(1):123–32. [DOI] [PubMed] [Google Scholar]
- 21.Cherenkov PA. The spectrum of visible radiation produced by fast electrons. Comptes Rendus De L Academie Des Sciences De L Urss 1938;20:651–5. [Google Scholar]
- 22.Jelley JV. Cerenkov Radiation and Its Applications. Brit J Appl Phys 1955;6(7):227–32. [Google Scholar]
- 23.Axelsson J, Davis SC, Gladstone DJ, Pogue BW. Cerenkov emission induced by external beam radiation stimulates molecular fluorescence. Medical physics 2011;38(7):4127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glaser AK, Davis SC, McClatchy DM, Zhang R, Pogue BW, Gladstone DJ. Projection imaging of photon beams by the Cerenkov effect. Med Phys 2013;40(1):012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaser AK, Davis SC, Voigt WH, Zhang R, Pogue BW, Gladstone DJ. Projection imaging of photon beams using Cerenkov-excited fluorescence. Phys Med Biol 2013;58(3):601–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R, Glaser AK, Gladstone DJ, Fox CJ, Pogue BW. Superficial dosimetry imaging based on Cerenkov emission for external beam radiotherapy with megavoltage x-ray beam. Med Phys 2013;40(10):101914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang R, Fox CJ, Glaser AK, Gladstone DJ, Pogue BW. Superficial dosimetry imaging of Cerenkov emission in electron beam radiotherapy of phantoms. Phys Med Biol 2013;58(16):5477–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang R, Glaser A, Esipova TV, Kanick SC, Davis SC, Vinogradov S, et al. Cerenkov radiation emission and excited luminescence (CREL) sensitivity during external beam radiation therapy: Monte Carlo and tissue oxygenation phantom studies. Biomed Opt Express 2012;3(10):2381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarvis LA, Zhang R, Gladstone DJ, Jiang S, Hitchcock W, Friedman OD, et al. Cherenkov Video Imaging Allows for the First Visualization of Radiation Therapy in Real Time. International journal of radiation oncology, biology, physics 2014. [DOI] [PubMed]
- 30.Glaser AK, Zhang R, Davis SC, Gladstone DJ, Pogue BW. Time-gated Cherenkov emission spectroscopy from linear accelerator irradiation of tissue phantoms. Opt Lett 2012;37(7):1193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang R, Gladstone DJ, Jarvis LA, Strawbridge RR, Jack Hoopes P, Friedman OD, et al. Real-time in vivo Cherenkoscopy imaging during external beam radiation therapy. J Biomed Opt 2013;18(11):110504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Archambault L, Briere TM, Beddar S. Transient noise characterization and filtration in CCD cameras exposed to stray radiation from a medical linear accelerator. Medical physics 2008;35(10):4342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang J, Guo S, Sun Q, Deng Y, Zhou D. Speckle reducing bilateral filter for cattle follicle segmentation. BMC genomics 2010;11 Suppl 2:S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canny J A computational approach to edge detection. IEEE transactions on pattern analysis and machine intelligence 1986;8(6):679–98. [PubMed] [Google Scholar]


